Abstract
Deacidification plays an important role in the conservation of paper-based cultural heritage objects. Herein, a novel approach for the conservation of scale paper-based cultural heritage objects is proposed using a mixture of argon and ethylene oxide (EO-Ar) for the first time. The optimum process conditions for deacidification of ethylene oxide and argon mixture system are determined by orthogonal testing. To evaluate the stabilization effect of paper treated with EO-Ar, the degradation of the mechanical properties (tensile strength, folding endurance and tearing strength tests) of paper after artificial aging was evaluated. The results show that the treated paper had better durability with respect to tensile strength, folding endurance and tearing strength. Additionally, thermal stability, crystallinity and fiber wall thickness increased after EO-Ar treated, which was determined by scanning electron microscope (SEM), diffraction of X-rays (XRD), and thermo gravimetric (TG) analysis. Some compounds, such as polyethylene glycol, organic acids, esters, were detected by GC-MS after treatment with EO-Ar. Two hundred and forty books including acidic, weak acidic and alkaline books were successfully deacidified, resulting in pH values of paper ranges suitable for paper preservation. Finally, a possible mechanism of deacidification of EO-Ar was proposed.
1. Introduction
Cellulose-based paper documents (e.g., manuscripts, calligraphy, paintings, photos) play an important role in historical, cultural and artistic aspects. The natural aging of paper objects has become a widespread problem because of their properties with respect to storage environment [1,2,3]. It is known that cellulose-based paper degradation is mainly caused by acid catalyzed cellulose hydrolysis [4,5,6,7,8], which can destroy the cellulose chain by random cutting of the glycosidic bond [9,10,11]. The acidification degradation of paper documents includes three possible reasons: first, acidic gases (CO2, SO2, HS, NO, NO2, etc.) enriched in the paper lead to acidification and degradation of lignin and hemicelluloses [12,13]; second, papermaking processes introduce acid substances, such as alum, chlorine bleach, gelatin, rosin, etc.; third, the hydrolysis of hemicellulose in the paper releases organic acids. Because of these reasons, the hydrolytic decomposition of the cellulosic material can become autocatalytic, especially in the case of closed books, which do not readily allow the acidic compounds to diffuse away. In order to slow down acidification and deterioration during natural aging, deacidification has been considered as the most important technology for prolongation of an object’s lifetime [14].
Currently, worldwide heritage institutions and archives have conducted deacidification treatment of paper artifacts, for instance the USA, Canada, Germany, France etc. [15]. This includes single-page paper deacidification and large-scale whole book deacidification. Neutralization of acids present on the surface or interior of paper is the main chemical strategy for the deacidification of aged paper. Due to the enhanced alkaline reserve, lower toxicity and moderate price, water-soluble inorganic compounds including Ca(OH)2 and Ca(HCO3)2 have been widely used to treat the aged paper [16,17,18,19]. However, physical changes in the appearance of the paper are usually caused by the drying process of aqueous deacidification. Although MgO nanoparticles dispersed in perfluorinated heptane have been successfully commercialized for paper deacidification due to their good deacidification performance and alkaline reserve [20], they are limitedly employed by paper conservators because of the ineffective system and white sediments on the paper surface [21,22].
Scale deacidification of whole books and volumes of archives has the advantage of saving resources. Mainstream methods are based on alkaline oxides and hydroxides deposited on paper, allowing neutralization of the acid in the paper. Liquid phase deacidification methods including PaperSave and Bookkeeper (USA PTLP, 1985) also provide strategies for deacidification of whole books and volumes of archives [23,24]. Although obvious advantages include a deacidification effect and strong operability, books possessing a certain strength can be soaked in the alkaline dispersions. The resulting effect in certain cases is powdery depositions and bleeding of inks and colors [25]. Gas phase deacidification has good permeability and is potentially suitable for large-scale deacidification. Alkylene oxides and gas-phase parylene polymers have been used many years ago [26,27]. Ethylene oxide (EO) has been widely used to sterilize medical devices. Currently, major hospitals as well as medical device manufacturers still use EO sterilization [28,29,30,31]. EO fumigant is often used in grain and food preservation [32]. Additionally, it can react with both acids and bases due to its unique structure and chemical activity. A treatment system with dry ammonia and ethylene (DAE) was used to deacidify large-size paper-based cultural relics [16]. The stable ethanolamine in situ is formed by introducing two reagents in a vacuum chamber [33]. Although papers were found to have a pH of 8.0–8.7, the dimension was increased by 2% and the initial brightness was decreased [34].
In the present study, a novel approach on the conservation of large-scale cellulose-based cultural heritage collections is proposed using a mixture of EO and argon (EO-Ar) for the first time. Optimum process conditions for deacidification of ethylene oxide and argon mixture system (EO-Ar) were determined by orthogonal tests using pH as a parameter through independently developed deacidification equipment. In order to assess the resistance of paper treated with EO-Ar, the tensile strength, tearing strength and folding endurance were tested to evaluate the mechanical properties of paper after dry heat accelerated aging methods. A comparative study on the microstructure of paper before and after treatment with EO-Ar was characterized by SEM, XRD, and TG analysis. Finally, a mechanism for deacidification of EO-Ar was proposed. Two hundred and forty books, including acidic, weak acidic and alkaline books, were deacidified using EO-Ar.
2. Materials and Methods
2.1. Paper Samples
Selected paper samples used in this study are shown in Table 1. Sample 1 stands for the acid paper archives. Sample 2 refers to the weak acid paper archives. Sample 3 represents the alkaline paper cultural relics. Sample 4 represents extremely acidic paper. Sample 5 represents a weak acidic book. Sample 6 stands for an alkaline book.

Table 1.
Selected paper samples used in this study.
2.2. Conservation Procedure and Characterization Methods
2.2.1. Optimization of Deacidification Conditions
Deacidification equipment consisted of a deacidification box, inflation system, control system, vacuum system, exhaust degradation system, safety system and other parts. The four main factors including pretreatment humidity, gas ratio, temperature and time were considered in the optimization of deacidification conditions, and the corresponding results are summarized in Table 2. The orthogonal tests were applied to optimize the process deacidification conditions of EO-Ar system. The pH value of sample 1 was 3.82, and it is selected as the test index. Four factors and three levels orthogonal experimental table were used in the experiment. All pH values were averages of 10 valid tests.

Table 2.
Factor level table.
2.2.2. Accelerated Aging Test
The samples were kept at 105 °C for artificial accelerated aging (72 ± 0.75 h) based on the standard ISO 5630-1:1991 [35,36].
2.2.3. Mechanical Strength Test
The tensile test was performed according to the standard ISO 1924-2:2008 [37]. Samples were cut into 15 mm × 180 mm strips and stretched at a uniform rate of 20 mm/min until fracture. The folding resistance was measured according to BS-ISO-5626-1993 [38]. Samples were cut into 15 mm × 150 mm strips. All mechanical tests were repeated at least 20 times, and the corresponding values were computed as average ± 1 standard deviation.
2.2.4. pH Test
Based on the standard ISO 6588-1:2012 [39], the cold-water extraction method was employed to measure the pH of samples 1, 2, and 3. Samples were cut into pieces (<5 mm2), and 2 g sample was accurately weighed in 250 mL beaker. 100 mL distilled water was added and allowed to soak for 1 h, during which the flask was shaken at least once within this time. Seven Compact S210-K pH meter (Mettler Toledo International Trading Co., LTD, Shanghai, China) with a InLab® Surface electrode was used to non-destructively measure pH of the extracted solution of sample 1, 2, and 3 to determine pH of paper samples.
2.2.5. Thermal Gravimetric (TG)
The thermal stability of the treated and untreated samples was measured by using a Q600 thermal gravimetric analyzer (TA Instruments-Waters LLC, New Castle, DE, USA). Initial weight of the sample was 3 mg, with nitrogen flow of 10 mL/min, heating rate of 10 °C/min, and temperature range of 40–600 °C.
2.2.6. X-ray Diffraction (XRD)
Smart Lab (9) high resolution X-ray diffraction system (Nippon science Co., Ltd, Tokyo, Japan) was used for the test, where Cu_K-beta was used to generate X-rays, 45 kV, 200 mA, step angle 0.01, reflection angle 2θ, range 10°–50°. XRD crystallinity index Cr.I of the fiber was calculated according to the diffraction intensity [40,41].
I002 refers to XRD peak strength of cellulose crystallization zone when 2θ ranges from 22° to 23°. Iam represents the amorphous region of cellulose when 2θ is around 18°.
2.2.7. Field Emission Scanning Electron Microscope (FE-SEM)
The samples were soaked in water, followed by being frozen in liquid nitrogen for 1 min observing fiber cross-sectional. The frozen paper was broken along the longitudinal direction perpendicular to the paper, then dried at 60 °C for 2 h. The surface of samples was sprayed gold uniformly for 60 s by using an ion sputtering instrument. SU-8020 field emission scanning electron microscope (Hitachi hi-tech Co., Ltd, Tokyo, Japan) was employed with acceleration voltage of 1 kV.
2.2.8. GC-MS Analysis
Next, 2 g treated and untreated sample edges were chopped and placed in a narrow mouth bottle, and 20 mL chromatographic methanol was added with ultrasonic oscillation (40 KHz) for 1 h. The extracted liquid was filtered by using 0.45 µm needle filter, then it was evaporated to 2 mL employing a rotary evaporation. Chromatographic column (hp-5 ms capillary, 30.0 m, 0.32 mm, 0.25 ms) was used, and the temperature was controlled at 80 °C in the column box. The temperature of the injection port was 250 °C, and the heating procedure was maintained at 80 °C for 2 min. The purge and column flow rates were 3.0 and 1.97 mL/min, respectively. Electron bombardment (EI) ion source temperature was 230 °C, and interface temperature was 250 °C with 30–600 m/z.
2.2.9. Chromatic Values Test
The L* a* b* values of treated and untreated pigment samples before and after aging were determined by X-Rite VS450 non-contact spectrophotometer (X-Rite, Incorporated, Grand rapids, MI, USA). The test conditions were: D65 light source, 0°/45° double angle illumination, 12 mm light spot and 0–150% reflection between 400–700 nm measured wavelength.
2.3. Application of Large-Scale Deacidification Technology
After that, 240 books including samples 4, 5 and 6 were posed into deacidification box using optimal deacidification conditions. The pH value distribution was determined.
3. Results and Discussion
3.1. Optimization of Deacidification Conditions
Table 3 shows the orthogonal test results. According to the principle of the highest pH of the test index, Ki and i (Ki is the sum of the same level test index of all factors, i is the average of the test index of the same level of each factor) were compared, and found the optimal combination of A3, B2, C2 and D3. Optimal conditions for deacidification involving pretreatment of the sample were 24 h under 80% RH and at 40 °C, and then vacuumed to less than 10 mmHg. EO-Ar (volume ratio 7:3) were filled into the tank with the rate of 2 mmHg/min. The temperature was maintained at 40 °C for 10 h. In the following measurements, the same optimal deacidification conditions are also applied to measure pH values, mechanical properties, chromatic aberrations, etc. Additionally, an R value indicates a significant order of factors with A > B > D > C, indicating that the pretreatment humidity is the most important factor influencing an EO-Ar deacidification system.

Table 3.
Optimization of deacidification conditions.
3.2. Effect of pH
Figure 1 presents the comparison of pH values for paper samples treated and untreated with EO-Ar. After EO-Ar deacidification treatment, the pH of samples 1, 2 and 3 increased from 3.82, 5.57 and 8.85 to 8.09, 8.44, and 9.63, respectively, which is a safe value for paper materials [42]. Hence, EO-Ar treatment will not increase high pH values of alkaline paper compared to traditional deacidification method using deposits of alkaline reserve. The results indicate that for a whole book with mixed acid and base, the deacidification process can save resources without disassembly. In addition, after 3 years of tracking, paper acidity can still be maintained.
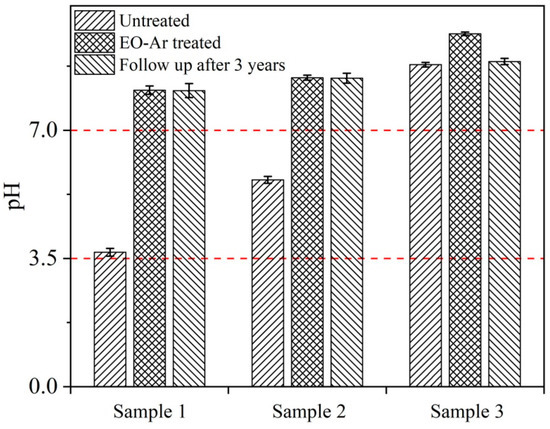
Figure 1.
Comparison of pH values for paper samples (sample 1 is groundwood printing papers; sample 2 is ingrain paper; sample 3 is xuan paper) treated and untreated with EO-Ar.
3.3. Effect on Mechanical Properties
The tensile strength, tearing resistance and folding endurance of untreated and treated paper before and after accelerated aging tests are shown in Figure 2. The tensile strength, tearing resistance and folding endurance at cross directions (CD) of treated sample 1 increased by 10.6%, 2.4% and 2.6%, and at mechanical directions (MD) increased by 11.1%, 9.6% and 19.5%, respectively. Samples 2 and 3 exhibited similar trends. The tensile strength of untreated samples at cross directions (CD) decreased by 66.9% after being dry heat aged, while treated samples decreased by 38.3%. The folding endurance of untreated samples at cross directions (CD) decreased by 67.1% after being dry heat aged, while treated samples decreased by 34.5%. Samples 2 and 3 exhibited similar trends, suggesting that the aging resistance of paper is improved after EO-Ar treatment.
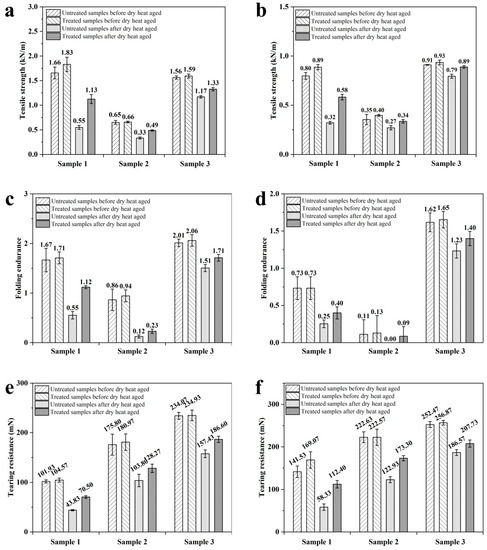
Figure 2.
Mechanical strength of treated and untreated paper samples before and after accelerated aging tests: MD of tensile strength (a); CD of tensile strength (b); MD of folding endurance (c); CD of folding endurance (d); MD of tearing resistance (e); CD of tearing resistance (f).
3.4. The Chromatic Aberration of Paper Samples Treated with EO-Ar
The chromatic aberration of paper samples treated with EO-Ar was tested. The effect of the EO-Ar on papers was measured by using the CIE L* a* b* system as shown in Table 4. L* represents the lightness, which ranges from 0 to 100; 0 is the blackest, 100 is the whitest. If a* is positive, the color tends to be red, and if a* is negative, the color tends to be green. If b* is positive, the color tends to be yellow, and if b* is negative, the color tends to be blue. From Table 4, it can be seen that the color changes of samples are around 1.0, indicating that the EO-Ar treatment did not influence the paper color. The visible light reflection curve of the samples were tested by using the American X-rite VS450 spectrophotometer as shown in Figure S1.

Table 4.
Colorimetric coordinates of paper samples before and after EO-Ar treatment.
3.5. Characterization of Morphology and Chemical Composition
Figure 3a,b shows the SEM images of sample 1 treated and untreated with EO-Ar after aging. It can be seen that surface morphology between treated and untreated samples is almost identical. Figure 3c,d shows the paper fiber section; the inner wall of the fiber cavity had a significant change after accelerated aging. Compared with the inner wall of untreated paper (fiber displays cracking and tilting with flake shedding), it became smooth and round after EO-Ar treatment. The fiber wall thickness of EO-Ar treated and untreated paper was ca. 2.24 and 1.5 μm, respectively. Combined with the results presented in Figure 2, it can be speculated that the reaction products are attached to the paper fiber network structure, where increased fiber wall thickness can increase the mechanical strength of the paper and prevent possible degradation due to temperature and humidity. The hydrophobic properties of the surface of the treated and untreated paper samples were studied through contact angle measurements (Figure S2). The results show that EO-Ar treatment did not affect the hydrophobic properties of papers.
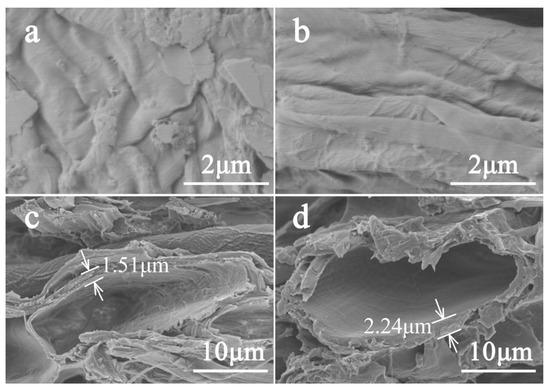
Figure 3.
SEM images of sample 1 before (a) and after (b) treatment with EO-Ar after aging × 20,000, (c) and (d) the inner wall of untreated and treated fibroblast cavity × 3500.
TG and DTG curves of samples 1, 2 and 3 treated and untreated with EO-Ar are displayed in Figure 4. The sample 1 thermal decomposition temperature increased from 297 to 315 °C after EO-Ar treatment (Figure 4a,b). The initial extrapolated decomposition temperature of sample 1 treated with EO-Ar was 315 °C, and thermal decomposition ended at ca. 359 °C (Figure 4b). A similar trend occurred in sample 2 and 3 (Figure 4c–f). The initial extrapolated decomposition temperature of untreated sample 2 was 255 °C, and finally ended at ca. 404 °C in Figure 4c. In comparison with sample 2, TG curve of sample 1 had a significant mass loss at ca. 270 °C due to ingrain paper being slightly acidic. The reason might be ascribed to the existence of some lignin pectin. Figure 4e,f show TG and DTG curves of alkaline handmade rice paper, the initial extrapolated decomposition temperature of untreated sample 3 was 323 °C, and thermal decomposition ended at ca. 372 °C (Figure 4e). The initial extrapolated decomposition temperature of EO-Ar treated sample 3 was 332 °C, and thermal decomposition ended at ca. 373 °C (Figure 4d). The results show that the thermal stability of acidic, weak acid and alkaline paper is improved after EO-Ar treatment.
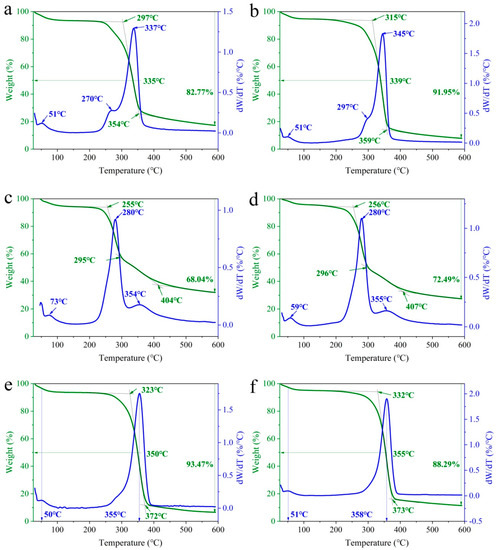
Figure 4.
TG of paper samples untreated and treated with EO-Ar. (a,b) untreated and treated sample 1 with EO-Ar, (c,d) untreated and treated sample 2 with EO-Ar, (e,f) untreated and treated sample 3 with EO-Ar.
XRD patterns of paper samples untreated and treated with EO-Ar are presented in Figure 5. Typical diffraction peaks are observed at 2θ = 15.15°–15.74°, which stands for the projection of the plane (). The peak at 2θ = 16.32°–16.53° represents the projection of the plane (110) [43], and the typical diffraction peaks at 2θ = 22.48°–22.69° correspond to the (200) crystallographic plane of cellulose I [44]. As shown in Figure 5a, several sharp intense peaks correspond to the peaks of talc powder, which commonly serve as one additive to improve the paper’s printability. Compared with the crystallinity index of untreated samples after artificial accelerated aging, it increased after being treated with EO-Ar (Figure 5a–c). It is concluded that crystallinity of EO-Ar treatment was enhanced, which is beneficial to improve durability.
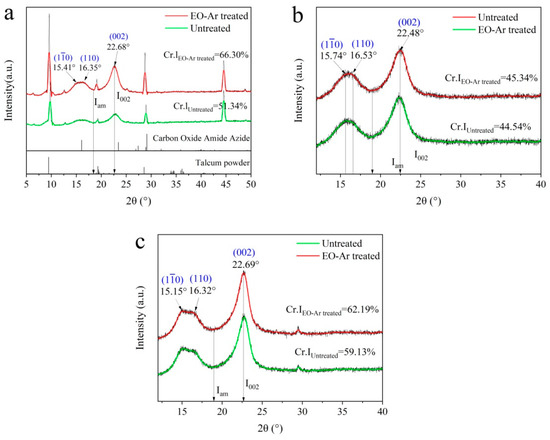
Figure 5.
XRD patterns of paper samples untreated and EO-Ar treated after artificial accelerated aging. (a) sample 1, (b) sample 2, (c) sample 3.
The methanol extract of paper after EO-Ar treatment was analyzed by GC-MS, and the corresponding results are listed in Table 5. As shown in Table 5, the severely aged paper contained some organic acids. The reason is mainly because oxidation or photooxidation of anhydrous glucose unit leads to the formation of organic acids [45,46]. The hydrolysis of cellulose promotes cellulose β-1,4-glycoside bond cleavage in the present of acid, which leads to the mechanical strength decreasing of the paper. Different molecular weight polyethylene glycol (PEG) and esters were detected after the paper was treated with EO-Ar (Table 6). PEG is formed by ring-opening polymerization of EO, while esters are formed via esterification reaction between PEG and different acids. The acetic acid in Table 5 may correspond to the ethylene glycol acetate in Table 6. The main reason may be that the free acid in the paper caused EO to ring and form esters with organic acids [47]. In combination with Figure 1, it may be due to the increase in H value of paper after EO-Ar treatment.

Table 5.
Analysis of methanol extract for untreated sample 4.

Table 6.
Analysis of methanol extract for treated sample 4.
3.6. Application of Scale Deacidification
The pH of entire books before and after treatment with EO-Ar is presented in Figure 6a,b. As shown in Figure 6a, 240 books including acidic, weak acid and alkaline paper were treated with EO-Ar at the same time. The average pH value of each book was concentrated at 3–5 before treatment, while it increased to 7–9 after treatment with EO-Ar, which is generally considered as suitable parameters to preserve paper. Hence, alkaline paper was not significantly enhanced after EO-Ar treatment, compared to traditional deacidification method using deposits of alkaline reserve. As shown in Figure 6b, the pH of different page numbers for sample 4 were ca. 3.5, where pH increased to 7.0 after EO-Ar treatment, and the pH was similar for every 100 pages. Samples 5 and 6 also exhibited comparable deacidification effects. This indicates that EO-Ar had strong permeability throughout the whole book, creating an even distribution of deacidification for every page of the book. Therefore, the developed method can be successfully utilized to generate evenly distributed pH for entire acidic, weak acidic and alkaline books, reaching a suitable pH range for paper preservation, while not influencing book thickness. We attach great importance to the safety of ethylene oxide in the experimental processes as shown in Figures S3–S7 (e.g., deacidification equipment, deacidifying gas, human health).
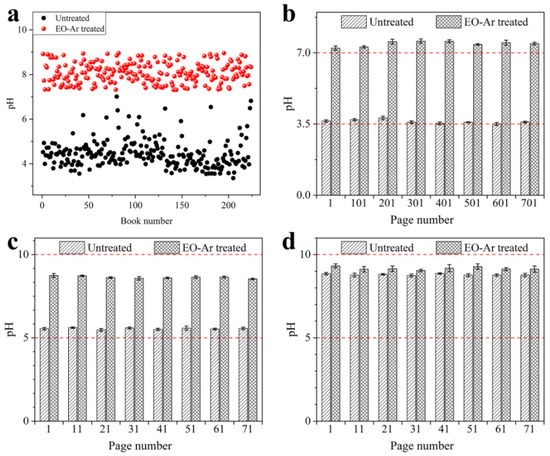
Figure 6.
pH of the entire book before and after treatment with EO-Ar: 240 books (a); different page numbers for sample 4 (b); different page numbers for sample 5 (c); different page numbers for sample 6 (d).
According to the results of Figure 1 and Figure 3, and Table 4 and Table 5, the possible mechanism of EO-Ar treatment on paper fibers is shown in Figure 7. The aged paper contained some organic acids that originated from the oxidation or photooxidation of an anhydrous glucose unit. EO-Ar could permeate the paper and react with organic acids or free acids. Different molecular weight polyethylene glycol (PEG) and esters were detected after the paper was treated with EO-Ar. PEG is formed by ring-opening polymerization of EO in the condition of acids, while esters are formed via an esterification reaction between PEG and different acids. The PEG and esters may have attached to the fiber, which increased the thickness of the fiber wall as well as its strength.
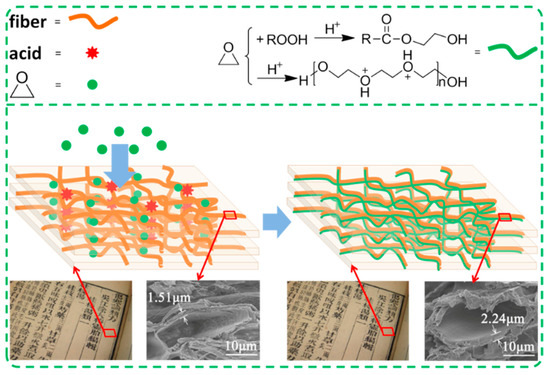
Figure 7.
Possible mechanism of EO-Ar treatment on paper fibers.
4. Conclusions
In summary, a novel deacidification method for bulk books was investigated using a mixture of EO and Ar for the first time. The optimum process conditions for deacidification of EO-Ar were determined by orthogonal tests using independently developed deacidification equipment. In order to evaluate the resistance of paper after EO-Ar treatment, the tensile strength, tearing strength and folding endurance were measured to assess the mechanical properties of paper after artificial aging by using dry heat accelerated aging methods. Paper treated with EO-Ar displayed superior durability to untreated paper. A comparative study was conducted focusing on the microstructure of paper before and after EO-Ar treatment through characterization by SEM, XRD, and TG analysis. The results show that thermal stability, crystallinity and fiber wall thickness increased after EO-Ar treatment. Different molecular weights PEG and esters were detected by GC-MS after being treated with EO-Ar. Two hundred and forty books, including whole acidic, weak acidic and alkaline books were successfully deacidified to create pH ranges suitable for paper preservation, while not affecting book thickness. The proposed process was simple, avoiding disassembling the entire book, and not time consuming or causing consumption of material resources. In the future, it will be possible to reduce the time required with various techniques in order to accelerate the treatment for large-scale cellulose-based cultural heritage. Finally, the possible mechanism of deacidification of EO-Ar was proposed.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/coatings11080973/s1, Figure S1: The visible light reflection curve of samples (a): Sample 1, (b): Sample 2, (c): Sample 3, (d): Sample 4, (e): Sample 5, (f): Sample 6, Figure S2: Diagram of exhaust gas degradation system, Figure S3: Small gas explosion test device, Figure S4: Schematic diagram of experimental apparatus in mice, Figure S5: Mouse lung tissue section (a): control groups × 400, (b): experimental groups × 400, (c): control groups × 100, (d): experimental groups × 100; p: pneumonocyte; rbc: red blood cells; ia: intercellular area; pa: pulmonary alveoli, Figure S6: Mouse liver tissue section (a): control groups × 400, (b): experimental groups × 400, (c): control groups × 100, (d): experimental groups × 100; h: hepatocyte; rbc: red blood cells; ia: inter-cellular area; bv: blood vessel, Figure S7: Water contact angles of samples before and after EO-Ar treatment.
Author Contributions
Y.Q. and Z.J. contributions equally. Data curation, Z.J., Y.Z., Y.W., G.Z. and X.C.; formal analysis, Y.Q. and Z.J.; funding acquisition, Z.J. and Y.L.; investigation, H.X.; project administration, Y.L.; supervision, Y.L.; validation, Z.J.; writing—original draft, Z.J.; writing—review and editing, Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 22002080), the Key Research and Development Program of Shaanxi Province, China (Grant No. 2021GY-172), Fundamental Research Funds for the Central Universities (Grant No. GK 202103060), and the Science and Technology Project of Xi’an, China (Grant No. 2020KJRC0014).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Yuhu Li for setting up the framework of the entire project. Thanks for the funding support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, C.; Huang, Y.; Zhang, H.; Ye, Z.; Liu, P.; Wang, S.; Zhang, Y.; Tang, Y. Selectively functionalized zeolite NaY composite materials for high-efficiency multiple protection of paper relics. Ind. Eng. Chem. Res. 2020, 59, 11196–11205. [Google Scholar] [CrossRef]
- Bukovský, V. The influence of light on ageing of newsprint paper. Restaurator 2000, 21, 55–76. [Google Scholar] [CrossRef]
- Havermans, J. Effects of air pollutants on the accelerated ageing of cellulose-based materials. Restaurator 1995, 16, 209–233. [Google Scholar] [CrossRef]
- Area, M.C.; Cheradame, H. Paper aging and degradation: Recent findings and research methods. BioResources 2011, 6, 5307–5337. [Google Scholar]
- Bukovsky, V.; Trnková, M. The influence of secondary chromophores on the light induced oxidation of paper part II: The influence of light on groundwood paper. Restaurator 2003, 24, 18–35. [Google Scholar] [CrossRef]
- Chamberlain, D. Anion mediation of aluminium-catalysed degradation of paper. Polym. Degrad. Stabil. 2007, 92, 1417–1420. [Google Scholar] [CrossRef]
- Gehlen, M.H. Approximate solution of the autocatalytic hydrolysis of cellulose. Cellulose 2009, 16, 1069–1073. [Google Scholar] [CrossRef]
- Carter, H.A. The chemistry of paper preservation: Part 1. the aging of paper and conservation techniques. J. Chem. Educ. 1996, 73, 417–425. [Google Scholar] [CrossRef]
- Ahn, K.; Hennniges, U.; Banik, G.; Potthast, A. Is cellulose degradation due to β-elimination processes a threat in mass deacidification of library books? Cellulose 2012, 19, 1149–1159. [Google Scholar] [CrossRef]
- Carter, H.A. The Chemistry of Paper Preservation: Part 2. The yellowing of paper and conservation bleaching. J. Chem. Educ. 1996, 73, 1068–1076. [Google Scholar] [CrossRef]
- Calvini, P. Comments on the article “On the degradation evolution equations of cellulose” by Hongzhi Ding and Zhongdong Wang. Cellulose 2007, 15, 225–228. [Google Scholar] [CrossRef]
- Matisová-Rychlá, L.; Rychlý, J.; Ebringerová, A.; Csomorová, K.; Malovíková, A. Chemiluminescence accompanying the oxidation of hemicelluloses. Polym. Degrad. Stabil. 2008, 93, 1674–1680. [Google Scholar] [CrossRef]
- Lojewski, T.; Miskowiec, P.; Molenda, M.; Lubanska, A.; Lojewska, J. Artificial versus natural ageing of paper. Water role in degradation mechanisms. Appl. Phys. A 2010, 100, 625–633. [Google Scholar] [CrossRef]
- Mihram, D. Paper deacidification: A bibliographic survey. Part II. Restaurator 1986, 7, 2. [Google Scholar] [CrossRef]
- Holik, D.I.H. Book and Paper Preservation; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; Chapter 13. [Google Scholar]
- Baty, J.W.; Maitland, C.L.; Minter, W.; Hubbe, M.A.; Jordan-Mowery, S.K. Deacidification for the conservation and preservation of paper-based works: A review. BioResources 2010, 5, 1955–2023. [Google Scholar] [CrossRef]
- Helmut, B. Aqueous deacidification-with calcium or with magnesium? Restaurator 1998, 19, 1–40. [Google Scholar]
- Baty, J.W.; Sinnott, M.L. The kinetics of the spontaneous, proton-and AlIII-catalysed hydrolysis of 1,5-anhydrocellobiitol models for cellulose depolymerization in paper aging and alkaline pulping, and a benchmark for cellulase efficiency. Can. J. Chem. 2005, 83, 1516–1524. [Google Scholar] [CrossRef]
- Botti, L.; Mantovani, O.; Orrù, M.A.; Ruggiero, D. The effect of sodium and calcium ions in the deacidification of paper: A chemo-physical study using thermal analysis. Restaurator 2006, 27, 9–23. [Google Scholar] [CrossRef]
- Giorgi, R.; Dei, L.; Ceccato, M.; Schettino, C.; Baglioni, P. Nanotechnologies for conservation of cultural heritage: Paper and canvas deacidification. Langmuir 2002, 21, 8198–8203. [Google Scholar] [CrossRef]
- Banik, G. Mass deacidification technology in Germany and its quality control. Restaurator 2005, 26, 63–75. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Smith, R.D.; Zou, X.; Katuscak, S.; Potthast, A.; Ahn, K. Deacidification of acidic books and paper by means of non-aqueous dispersions of alkaline particles: A review focusing on completeness of the reaction. BioResources 2017, 12, 4410–4477. [Google Scholar] [CrossRef]
- Bookkeeper Deacidification. Preservation Technologies. Available online: https://ptlp.com/en/bookkeeper/tools-guide-lines/faq/ (accessed on 28 June 2021).
- Zervos, S.; Alexopoulou, I. Paper conservation methods: A literature review. Cellulose 2015, 22, 2859–2897. [Google Scholar] [CrossRef]
- Pauk, S. The bookkeeper mass deacidification process—some effects on 20th century library material. Abbey Newsletter. 1996, 20, 50–54. [Google Scholar]
- Smith, R.D. Preserving Cellulose Materials through Treatment with Alkylene Oxides. U.S. Patent 3,676,055, 11 July 1972. [Google Scholar]
- Humphrey, B.J. Paper strengthening with gas-phase parylene polymers: Practical considerations. Restaurator 1990, 11, 48–68. [Google Scholar] [CrossRef]
- Hideharu, S. Ethylene oxide gas sterilization of medical devices. Biocontrol Sci. 2017, 22, 1–9. [Google Scholar]
- Muscarella, L.F. Use of Ethylene-oxide gas sterilisation to terminate multidrug-resistant bacterial outbreaks linked to duodenoscopes. RMD Open 2019, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deschamp, D. Use of Ethylene oxide in medical and surgical sterilisation. Evaluation of the occupational risk of opacification of the lens. Br. Med. J. 1988, 2, 446–452. [Google Scholar]
- Mendes, G.C.C.; Brandão, T.R.S.; Silva, C.L.M. Ethylene oxide sterilization of medical devices: A review. Am. J. Infect. Control 2007, 35, 574–581. [Google Scholar] [CrossRef]
- BackE, E.A.; Cotton, R.T.; Ellington, G.W. Ethylene oxide as a fumigant for food and other commodities. J. Econ. Entomol. 1930, 23, 226–231. [Google Scholar] [CrossRef]
- Okayama, T.; Gotoh, T.; Oye, R. Degradation Control of Book Paper by Ammonia-ethylene Oxide Treatment. In Proceedings of the 1994 Pulp and Paper Research Conference Proceedings, Tokyo, Japan, 20–24 June 1994; pp. 132–135. [Google Scholar]
- Win, K.R.; Okayama, T. Mass Deacidification Treatments of Acidic Bamboo Paper. Fiber 2012, 68, 143–148. [Google Scholar] [CrossRef][Green Version]
- Havlínová, B.; Katuščák, S.; Petrovičová, M.; Maková, A.; Brezová, V. A study of mechanical properties of papers exposed to various methods of accelerated ageing. Part I. The effect of heat and humidity on original wood-pulp oapers. J. Cult. Herit. 2009, 10, 222–231. [Google Scholar] [CrossRef]
- Paper and Board—Accelerated Ageing—Part 1: Dry Heat Treatment at 105 Degrees C; ISO 5630-1:1991; ISO: Geneva, Switzerland, 1991.
- Paper and Board—Determination of Tensile Properties—Part 2: Constant Rate of Elongation Method (20 mm/min); ISO 1924-2-2008; ISO: Geneva, Switzerland, 2008.
- Paper—Determination of Folding Endurance; BS ISO 5626:1993; BS: London, UK, 1994.
- Paper, Board and Pulps—Determination of pH of Aqueous Extracts—Part 1: Cold Extraction; ISO 6588-1:2012; ISO: Geneva, Switzerland, 2012.
- Causin, V.; Marega, C.; Marigo, A.; Casamassima, R.; Peluso, G.; Ripani, L. Forensic differentiation of paper by x-ray diffraction and infrared spectroscopy. Forensic Sci. Int. 2010, 197, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Perez-Rodriguez, J.L.; Espejo, T.; Franquelo, M.L.; Castaing, J.; Walter, P. Characterization of illuminated manuscripts by laboratory-made portable XRD and micro-XRD systems. Anal. Bioanal. Chem. 2009, 395, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Information and Documentation—Paper for Documents—Requirements for Permanence; ISO 9706:1994; ISO: Geneva, Switzerland, 1994.
- Santos, S.M.; Carbajo, J.M.; Quintana, E.; Ibarra, D.; Gomez, N.; Ladero, M.; Eugenio, M.E.; Villar, J.C. Characterization of purifified bacterial cellulose focused on its use on paper restoration. Carbohyd. Polym. 2015, 116, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Cao, L.; Wang, B. Facile cellulose dissolution without heating in [C4mim] [CH3COO]/DMF solvent. Carbohyd. Polym. 2015, 137, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.R.; Alonso, M.; Berhardt, R.J. Alkoxylated Fatty Esters and Derivatives from Natural Oil Metathesis. U.S. Patent 878972B2, 2 January 2014. [Google Scholar]
- Allen, D.R.; Bernhardt, R.J.; Brown, A. Hard Surface Cleaners Based on Compositions Derived from Natural Oil Metathesis. U.S. Patent 057612 B2, 5 April 2016. [Google Scholar]
- Wurm, F.; Nieberle, J.; Frey, H. Double-hydrophilic linear-hyperbranched block copolymers based on poly(ethylene oxide) and poly(glycerol). Macromolecules 2016, 41, 1909–1911. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).