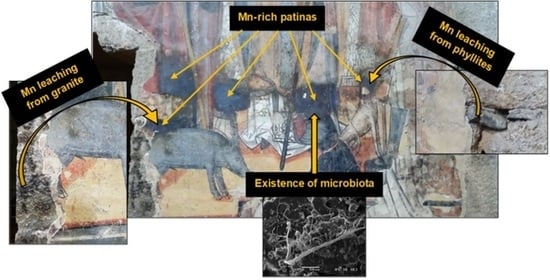
Rare Biogeochemical Phenomenon Associated to Manganese Patinas on Mural Painting and Granite Ashlars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Optical Microscopy
2.3. Micro-Raman Spectroscopy
2.4. X-ray Diffraction (XRD)
2.5. X-ray Fluorescence (XRF)
2.6. Scanning Electron Microscopy (SEM)
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.8. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
3. Results
3.1. Identification of Pigments Used in Mural Painting by Micro-Raman
3.2. Mineralogical Characterisation of Mortars by XRD
3.3. Identification and Chemical Composition of Black-Blue Stains on Mural Painting and Granite Ashlars
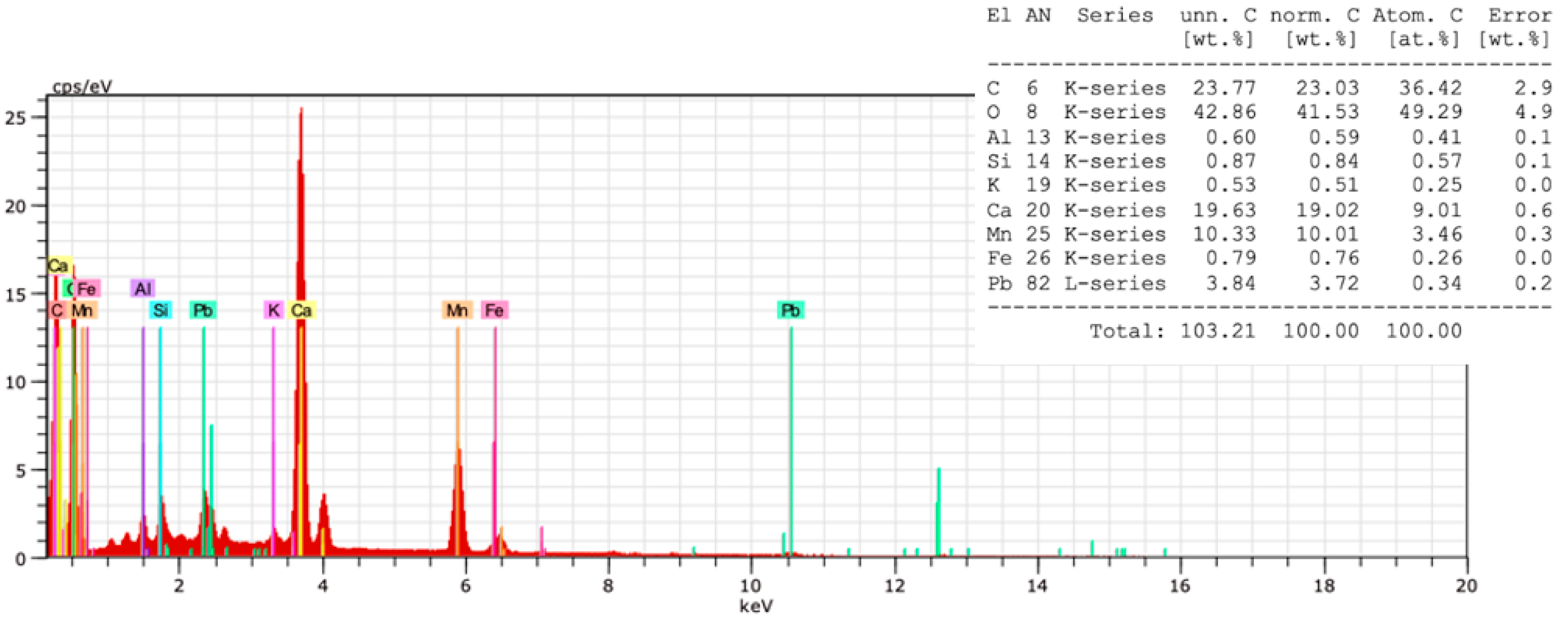
3.3.1. In situ Elemental Analysis by XRF
3.3.2. Oxidation State of Mn by XPS
3.4. Surveying Mn Sources by ICP-MS
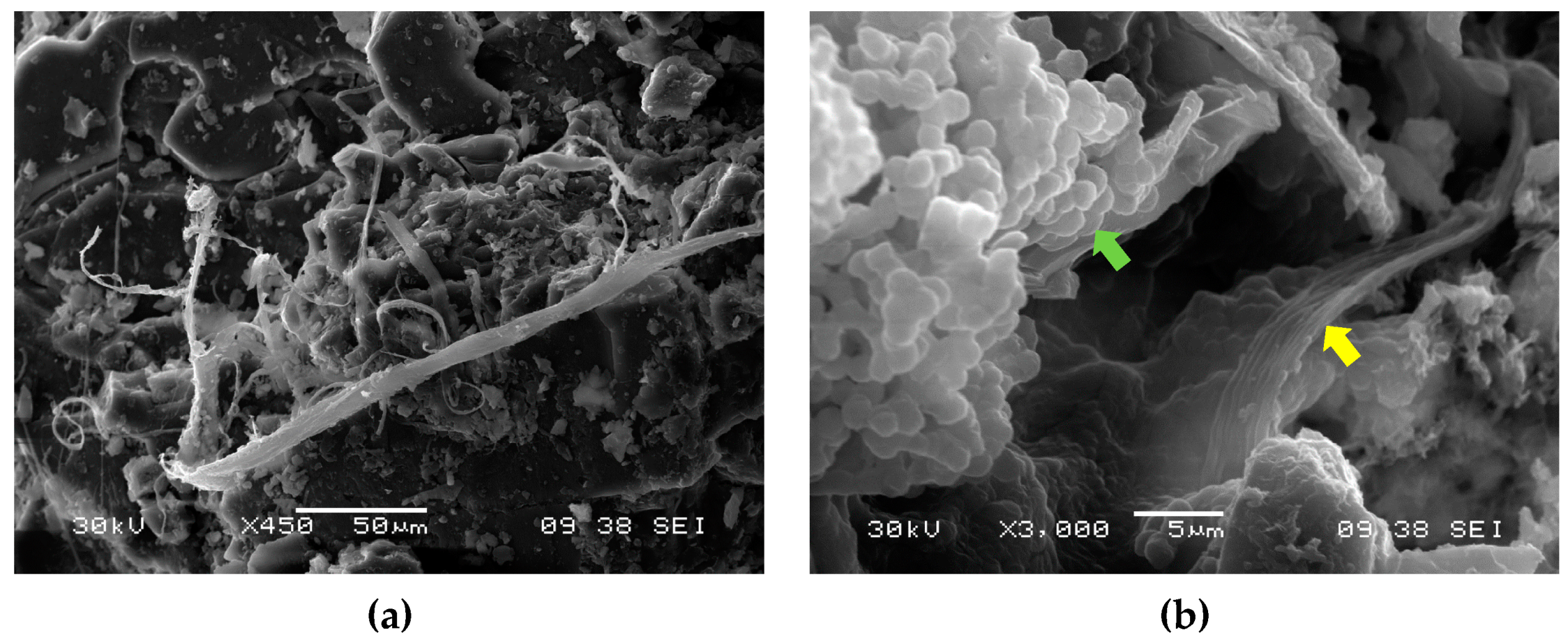
3.5. Microbiological Evidence by SEM and SEM-EDX
4. Discussion
4.1. Mn Leaching from Building Materials
4.2. Hypothetical Mechanism Formation of Mn-Rich Patina
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derudi, M.; Gelosa, S.; Sliepcevich, A.; Cattaneo, A.; Cavallo, D.; Rota, R.; Nano, G. Environments., emission of air pollutants from burning candles with different composition in indoor. Environ. Sci. Pollut. Res. 2013, 21, 4320–4330. [Google Scholar] [CrossRef]
- Perez-Monserrat, M.E.; Varas-Muriel, J.M.; de Buergo, A.M.; Fort, R. Black layers of decay and color patterns on heritage limestone as markers of environmental change. Geosciences 2016, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Pozo-Antonio, J.S.; Papanikolaou, A.; Philippidis, A.; Melessanaki, K.; Rivas, T.; Pouli, P. Cleaning of gypsum-rich black crusts on granite using a dual wavelength Q-Switched Nd:YAG laser. Constr. Build. Mater. 2019, 226, 721–733. [Google Scholar] [CrossRef]
- Prieto, B.; Silva, B.; Aira, N.; Álvarez, L. Toward a definition of a bioreceptivity index for granitic rocks: Perception of the change in appearance of the rock. Int. Biodeterior. Biodegrad. 2006, 58, 150–154. [Google Scholar] [CrossRef]
- Guillitte, O. Bioreceptivity: A new concept for building ecology studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Crispim, C.A.; Gaylarde, C.C.; Gaylarde, P.M. Biofilms on church walls in Porto Alegre, RS, Brazil, with special attention to cyanobacteria. Int. Biodeterior. Biodegradation 2004, 54, 121–124. [Google Scholar] [CrossRef]
- Dakal, T.; Cameotra, S. Microbially induced deterioration of architectural heritages: Routes and mechanisms involved. Environ. Sci. Eur. 2012, 24, 36. [Google Scholar] [CrossRef] [Green Version]
- Gaylarde, L.H.G.; Morton, C.C. Deteriogenic biofilms on buildings and their control: A review. Biofouling 1999, 14, 59–74. [Google Scholar] [CrossRef]
- Ortega-Morales, P.M.; Guezennec, O.; Hernández-Duque, J.; Gaylarde, G.; Gaylarde, C.C. Phototrophic biofilms on ancient mayan buildings in Yucatan, Mexico. Curr. Microbiol. 2000, 40, 81–85. [Google Scholar] [CrossRef]
- Cappitelli, F.; Abbruscato, P.; Foladori, P.; Zanardini, E.; Ranalli, G.; Principi, P.; Villa, F.; Polo, A.; Sorlini, C. Detection and elimination of cyanobacteria from frescoes: The case of the St. Brizio Chapel (Orvieto Cathedral, Italy). Microb. Ecol. 2009, 57, 633–639. [Google Scholar] [CrossRef]
- Wichert, J. Slate as Dimension Stone—Origin, Standards, Properties, Mining and Deposits, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- La Russa, M.F.; Fermo, P.; Comite, V.; Belfiore, C.M.; Barca, D.; Cerioni, A.; de Santis, M.; Barbagallo, L.F.; Ricca, M.; Ruffolo, S.A. The Oceanus statue of the Fontana di Trevi (Rome): The analysis of black crust as a tool to investigate the urban air pollution and its impact on the stone degradation. Sci. Total Environ. 2017, 593, 297–309. [Google Scholar] [CrossRef]
- Gaylarde, C.; Baptista-Neto, J.A.; Tabasco-Novelo, C.; Ortega-Morales, O. Weathering of granitic gneiss: A geochemical and microbiological study in the polluted sub-tropical city of Rio de Janeiro. Sci. Total Environ. 2018, 644, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Municchia, A.C.; Bartoli, F.; Bernardini, S.; Caneva, G.; della Ventura, G.; Ricci, M.; Suy, B.; Sodo, A. Characterization of an unusual black patina on the Neang Khmau temple (archaeological Khmer area, Cambodia): A multidisciplinary approach. J. Raman Spectrosc. 2016, 47, 1467–1472. [Google Scholar] [CrossRef]
- Ogawa, A.; Celikkol-Aydin, S.; Gaylarde, C.; Baptista-Neto, J.; Beech, I. Microbial communities on painted wet and dry external surfaces of a historic fortress in Niterói, Brazil. Int. Biodeterior. Biodegrad. 2017, 123, 164–173. [Google Scholar] [CrossRef]
- Alonso, L.; Creuzé-des-Châtelliers, C.; Trabac, T.; Dubost, A.; Moënne-Loccoz, Y.; Pommier, T. Rock substrate rather than black stain alterations drives microbial community structure in the passage of Lascaux Cave. Microbiome 2018, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Marvasi, M.; Donnarumma, F.; Frandi, A.; Mastromei, G.; Sterflinger, K.; Tiano, P.; Perito, B. Black microcolonial fungi as deteriogens of two famous marble statues in Florence, Italy. Int. Biodeterior. Biodegradation 2012, 68, 36–44. [Google Scholar] [CrossRef]
- González-Gómez, W.S.; Quintana, P.; Gómez-Cornelio, S.; García-Solis, C.; Sierra-Fernandez, A.; Ortega-Morales, O.; De la Rosa-García, S.C. Calcium oxalates in biofilms on limestone walls of Maya buildings in Chichén Itzá, Mexico. Environ. Earth Sci. 2018, 77, 230. [Google Scholar] [CrossRef]
- Sert, K.; Sterflinger, H. A new Coniosporium species from historical marble monuments. Mycol. Prog. 2009, 9, 353–359. [Google Scholar] [CrossRef]
- Abdel-Haliem, M.E.F.; Sakr, A.A.; Ali, M.F.; Ghaly, M.F.; Sohlenkamp, C. Characterization of Streptomyces isolates causing colour changes of mural paintings in ancient Egyptian tombs. Microbiol. Res. 2013, 168, 428–437. [Google Scholar] [CrossRef]
- Mosquera, S.; Benítez, M.; Perry, D. Pore structure in mortars applied on restoration: Effect on properties relevant to decay of granite buildings. Cem. Concr. Res. 2002, 32, 1883–1888. [Google Scholar] [CrossRef] [Green Version]
- Pozo-Antonio, J.S.; Pereira MF, C.; Rocha CS, A.; Puente, I.; Figueiredo, C. Comparative study of deterioration forms on nearby granitic bridges from an urban setting in the NW Iberian Peninsula. Geomorphology 2016, 274, 11–30. [Google Scholar] [CrossRef]
- Sanmartín, B.; Sanjurjo-Sánchez, P.; Prieto, J. Covering layers on granite buildings of northwestern Iberian Peninsula: When observable characteristics and lab characterization do not match. Coatings 2020, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Coccato, P.; Moens, A.; Vandenabeele, L. On the stability of mediaeval inorganic pigments: A literature review of the effect of climate, material selection, biological activity, analysis and conservation treatments. Herit. Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- de Ferri, G.; Mazzini, L.; Vallotto, F.; Pojana, D. In situ non-invasive characterization of pigments and alteration products on the masonry altar of S. Maria ad Undas (Idro, Italy). Archaeol. Anthropol. Sci. 2019, 11, 609–625. [Google Scholar] [CrossRef]
- Vagnini, C.; Vivani, M.; Viscuso, R.; Favazza, E.; Brunetti, M.; Sgamellotti, B.; Miliani, A. Investigation on the process of lead white blackening by Raman spectroscopy, XRD and other methods: Study of Cimabue’s paintings in Assisi. Vib. Spectrosc. 2018, 98, 41–49. [Google Scholar] [CrossRef]
- Cotte, M.; Susini, M.; Metrich, J.; Moscato, N.; Gratziu, A.; Bertagnini, C.; Pagano, A. Blackening of pompeian cinnabar paintings: X-ray microspectroscopy analysis. Anal. Chem. 2006, 78, 7484–7492. [Google Scholar] [CrossRef] [PubMed]
- Costantini, J.; Lottici, I.; Bersani, P.; Pontiroli, D.; Casoli, D.; Castro, A.; Madariaga, K. Darkening of lead- and iron-based pigments on late Gothic Italian wall paintings: Energy dispersive X-ray fluorescence, μ-Raman, and powder X-ray diffraction analyses for diagnosis: Presence of β-PbO2 (plattnerite) and α-PbO2 (scrutinyite). J. Raman Spectrosc. 2020, 51, 680–692. [Google Scholar] [CrossRef]
- Gaylarde, P.; Ortega-Morales, C.; Bartolo-Pérez, B. Biogenic black crusts on buildings in unpolluted environments. Curr. Microbiol. 2007, 54, 162–166. [Google Scholar] [CrossRef]
- Mihajlovski, P.; Seyer, A.; Benamara, D.; Bousta, H.; di Martino, F. An overview of techniques for the characterization and quantification of microbial colonization on stone monuments. Ann. Microbiol. 2015, 65, 1243–1255. [Google Scholar] [CrossRef] [Green Version]
- Lian BChen YZhu, L.; Yang, R. Effect of microbial weathering on carbonate rocks. Earth Sci. Front. 2008, 15, 90–99. [Google Scholar] [CrossRef]
- Caetano, J. O Marão E As Oficinas De Pintura Mural Nos Séculos XV E XVI; Aparição: Lisbon, Portugal, 2001. [Google Scholar]
- Sahoo, J.; Kausar, P.; Lee, F.; Han, J. Facile fabrication of silver nanoparticle embedded CaCO3 microspheres via microalgae-templated CO2 biomineralization: Application in antimicrobial paint development. RSC Adv. 2014, 4, 32562–32569. [Google Scholar] [CrossRef]
- Gulzar, S.; Burg, J.P. Preliminary investigation of late Mughal period wall paintings from historic monuments of Begumpura, Lahore. Front. Archit. Res. 2018, 7, 465–472. [Google Scholar] [CrossRef]
- Ferrazza, P.; Valls, L.P.; Zamorano, M.C.; Barber, G.; Radvan, D.; Chelmus, R.; Ratoiu, A.; Ghervase, L.; Cortea, L.; Ortiz, I. Multidisciplinary approach applied to the diagnosis of the facade of the Arciprestal Church of Santa María de Morella (Castellón, Spain). Scanning 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sujith, P.P.; Bharathi, P.A.L. Manganese oxidation by bacteria: Biogeochemical aspects. Progress Mol. Subcell. Biol. 2011, 52, 49–76. [Google Scholar]
- Santelli, C.M.; Webb, S.M.; Dohnalkova, A.C.; Hansel, C.M. Diversity of Mn oxides produced by Mn(II)-oxidizing fungi. Geochim. Cosmochim. Acta 2011, 75, 2762–2776. [Google Scholar] [CrossRef]
- Grote, G.; Krumbein, W.E. Microbial precipitation of manganese by bacteria and fungi from desert rock and rock varnish. Geomicrobiol. J. 1992, 10, 49–57. [Google Scholar] [CrossRef]
- Cahyani, M.; Murase, V.R.; Ishibashi, J.; Asakawa, E.; Kimura, S. Phylogenetic positions of Mn2+-oxidizing bacteria and fungi isolated from Mn nodules in rice field subsoils. Biol. Fertil. Soils 2009, 45, 337–346. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.; Zhang, Z.; Chen, Z.; Liu, H.; Ali, J.; Liu, M.; Li, F. Population structure of manganese-oxidizing bacteria in stratified soils and properties of manganese oxide aggregates under manganese–complex medium enrichment. PLoS ONE 2013, 8, e73778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerrato, J.M.; Falkinham, J.O.; Dietrich, A.M.; Knocke, W.R.; McKinney, C.W.; Pruden, A. Manganese-oxidizing and -reducing microorganisms isolated from biofilms in chlorinated drinking water systems. Water Res. 2010, 44, 3935–3945. [Google Scholar] [CrossRef]
- Piazza, N.; Casalini, A.C.; Pacini, L.; Sanguinetti, V.A.; Ottado, G.; Gottig, J. Environmental bacteria involved in Manganese(II) oxidation and removal from groundwater. Front. Microbiol. 2019, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ruiz, E.B.; Cooper, M.; Fastner, J.; Szewzyk, U. Manganese-oxidizing bacteria isolated from natural and technical systems remove cylindrospermopsin. Chemosphere 2020, 238, 124625. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.; Oguejiofor, C.; Zühlke, D.; Barreto, C.C.; Wünsche, C.; Riedel, K.; Kuramae, E.E. Impact of different trace elements on the growth and proteome of two strains of Granulicella, class ‘Acidobacteriia’. Front. Microbiol. 2020, 11, 1227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fu, C. Manganese-oxidizing microbes and biogenic manganese oxides: Characterization, Mn(II) oxidation mechanism and environmental relevance. Rev. Environ. Sci. Bio/Technol. 2020, 19, 489–507. [Google Scholar] [CrossRef]
- Zeiner, C.A.; Purvine, S.O.; Zink, E.; Wu, S.; Paša-Tolić, L.; Chaput, D.L.; Santelli, C.M.; Hansel, C.M. Mechanisms of manganese (II) oxidation by filamentous ascomycete fungi vary with species and time as a function of secretome composition. Front. Microbiol. 2021, 12, 81. [Google Scholar] [CrossRef]
- Nealson, K.H. The Manganese-Oxidizing Bacteria. In The Prokaryotes; Springer: New York, NY, USA, 2006. [Google Scholar]
- Greene, A.C.; Madgwick, J.C. Heterotrophic manganese-oxidizing bacteria from Groote Eylandt, Australia. Geomicrobiol. J. 1988, 6, 119–127. [Google Scholar] [CrossRef]
- Takano, K.; Itoh, Y.; Ogino, T.; Kurosawa, K.; Sasaki, K. Phylogenetic analysis of manganese-oxidizing fungi isolated from manganese-rich aquatic environments in Hokkaido, Japan. Limnology 2006, 7, 219–223. [Google Scholar] [CrossRef]
- de la Torre, M.A.; Gomez-Alarcon, G. Manganese and iron oxidation by fungi isolated from building stone. Microb. Ecol. 1994, 27, 177–188. [Google Scholar] [CrossRef]
- Timonin, M.I.; Illman, W.I.; Hartgerink, T. Oxidation of manganous salts of manganese by soil fungi. Can. J. Microbiol. 1972, 18, 793–799. [Google Scholar] [CrossRef]
- Gounot, A.-M. Microbial oxidation and reduction of manganese: Consequences in groundwater and applications. FEMS Microbiol. Rev. 1994, 14, 339–349. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi as Geologic Agents. Geomicrobiol. J. 2000, 17, 97–124. [Google Scholar] [CrossRef]
- O’Brien, P.F.; Bell, E.; Santamaria, S.P.; Boyland, P.; Cooper, T.P. Role of mortars in the decay of granite. Sci. Total Environ. 1995, 167, 103–110. [Google Scholar] [CrossRef]
- Brás, A.; Valença, A.; Faria, P. Performance-based methods for masonry building rehabilitation using innovative leaching and hygrothermal risk analyses. Sustain. Cities Soc. 2017, 28, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Dromgoole, E.L.; Walter, L.M. Iron and manganese incorporation into calcite: Effects of growth kinetics, temperature and solution chemistry. Chem. Geol. 1990, 81, 311–336. [Google Scholar] [CrossRef]
- Osinubi, K.J.; Yohanna, P.; Eberemu, A.O. Cement modification of tropical black clay using iron ore tailings as admixture. Transp. Geotech. 2015, 5, 35–49. [Google Scholar] [CrossRef]
- Gupta, S.; Rao, A. Weathering indices and their applicability for crystalline rocks. Bull. Eng. Geol. Environ. 2001, 60, 201–221. [Google Scholar] [CrossRef]
- Sun, G.; Kitchaev, W.; Kramer, D.; Ceder, D. Non-equilibrium crystallization pathways of manganese oxides in aqueous solution. Nat. Commun. 2019, 10, 573. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.; Gateman, S.; Stephens, S.; Lacasse, L.; Schulz, R.; Mauzeroll, R. Pourbaix diagrams as a simple route to first principles corrosion simulation. J. Electrochem. Soc. 2019, 166, C3186–C3192. [Google Scholar] [CrossRef]
- Reis, A.; Parker, A.; Alencoão, A. Storage and origin of metals in active stream sediments from mountainous rivers: A case study in the River Douro basin (North Portugal). Appl. Geochem. 2014, 44, 69–79. [Google Scholar] [CrossRef]



| Element | Unit | GR1 | GR2 | X1 | X2 | J1 | J2 | Arg1 | Arg2 |
|---|---|---|---|---|---|---|---|---|---|
| Al | mg·kg−1 | 3537 | 9353 | 32,146 | 50,488 | 45,229 | 34,819 | 4295 | 8517 |
| As | 19.24 | 4.76 | 41.62 | 35.17 | 37.08 | 43.39 | 11.01 | 9.91 | |
| Ba | 26 | 13 | 129 | 118 | 163 | 77 | 40 | 89 | |
| Ca | 1175 | 2400 | 1103 | 12,136 | 5131 | 8711 | 231,486 | 195,166 | |
| K | 1119 | 2049 | 13,307 | 7547 | 13,566 | 14,015 | 3501 | 6278 | |
| Co | 0.58 | 1.37 | 8.62 | 12.87 | 126.46 | 28.46 | 3.85 | 5.02 | |
| Cr | 7.23 | 5.01 | 70.65 | 33.68 | 59.37 | 55.13 | 10.44 | 15.02 | |
| Cu | 10.17 | 3.38 | 31.46 | 15.20 | 23.12 | 27.05 | 94.54 | 13.60 | |
| Fe | 3522 | 9874 | 48,730 | 35,833 | 30,674 | 32,733 | 4449 | 7945 | |
| Mg | 343 | 1140 | 18,186 | 2703 | 10,705 | 10,646 | 2987 | 4868 | |
| Mn | 39 | 141 | 427 | 203 | 1347 | 379 | 149 | 1068 | |
| Na | 203 | 199 | 181 | 836 | 603 | 3370 | 1552 | 2140 | |
| Ni | 3.10 | 3.48 | 35.97 | 17.74 | 42.00 | 24.87 | 10.82 | 14.66 | |
| P | 504 | 1187 | 429 | 2480 | 700 | 542 | 240 | 528 | |
| Pb | 15.9 | 8.5 | 8.4 | 11.7 | 29.0 | 8.0 | 270.5 | 70.7 | |
| Sr | 8.0 | 5.3 | 13.3 | 29.9 | 18.4 | 31.1 | 217.3 | 1005.4 | |
| U | 6.05 | 10.12 | 1.15 | 1.91 | 2.93 | 5.83 | 0.89 | 2.26 | |
| Zn | 32.62 | 95.31 | 101.38 | 67.98 | 705.87 | 122.34 | 40.93 | 37.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, B.; Marco, A.; Freire-Lista, D.M.; Durães, N.; Silvestre-Albero, J.; da Silva, E.F.; Vieira, E.; Pintado, M.; Moreira, P.R. Rare Biogeochemical Phenomenon Associated to Manganese Patinas on Mural Painting and Granite Ashlars. Coatings 2021, 11, 917. https://doi.org/10.3390/coatings11080917
Campos B, Marco A, Freire-Lista DM, Durães N, Silvestre-Albero J, da Silva EF, Vieira E, Pintado M, Moreira PR. Rare Biogeochemical Phenomenon Associated to Manganese Patinas on Mural Painting and Granite Ashlars. Coatings. 2021; 11(8):917. https://doi.org/10.3390/coatings11080917
Chicago/Turabian StyleCampos, Bruno, Alexandra Marco, David M. Freire-Lista, Nuno Durães, Joaquin Silvestre-Albero, Eduardo Ferreira da Silva, Eduarda Vieira, Manuela Pintado, and Patrícia R. Moreira. 2021. "Rare Biogeochemical Phenomenon Associated to Manganese Patinas on Mural Painting and Granite Ashlars" Coatings 11, no. 8: 917. https://doi.org/10.3390/coatings11080917
APA StyleCampos, B., Marco, A., Freire-Lista, D. M., Durães, N., Silvestre-Albero, J., da Silva, E. F., Vieira, E., Pintado, M., & Moreira, P. R. (2021). Rare Biogeochemical Phenomenon Associated to Manganese Patinas on Mural Painting and Granite Ashlars. Coatings, 11(8), 917. https://doi.org/10.3390/coatings11080917