The Microstructure and Mechanical and Corrosion Behaviors of Thermally Aged Z3CN20-09M Cast Stainless Steel for Primary Coolant Pipes of Nuclear Power Plants
Abstract
:1. Introduction
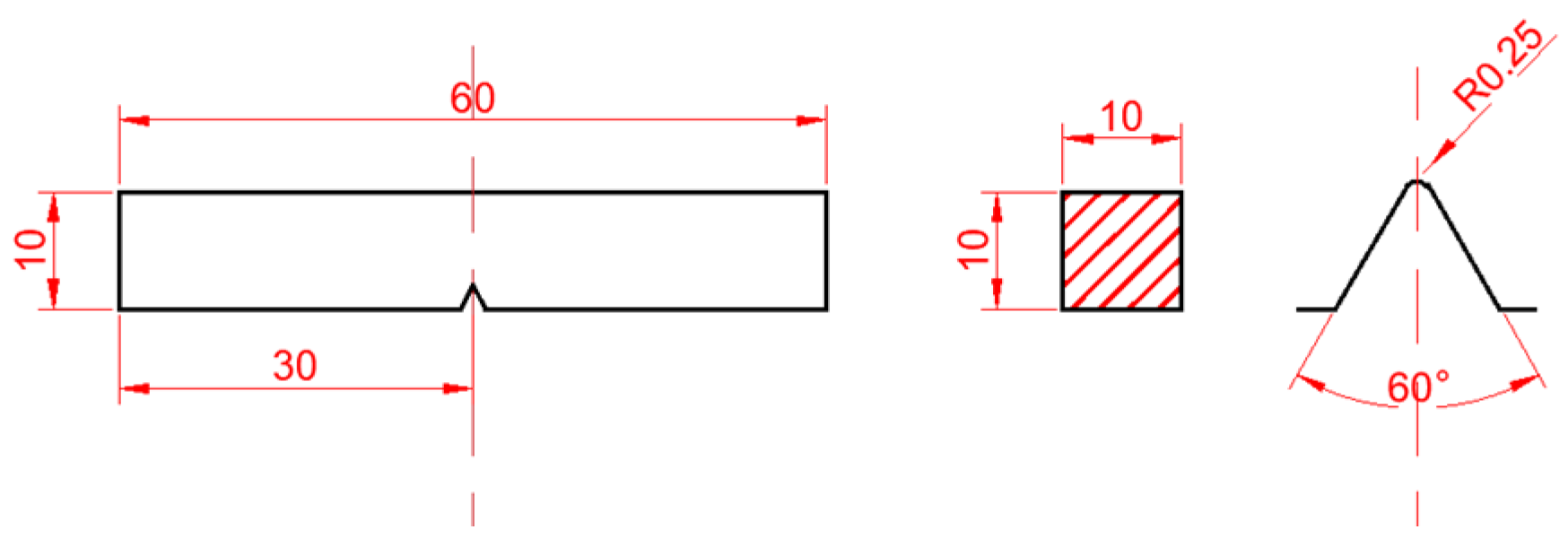
2. Materials and Methods
3. Results and Discussion
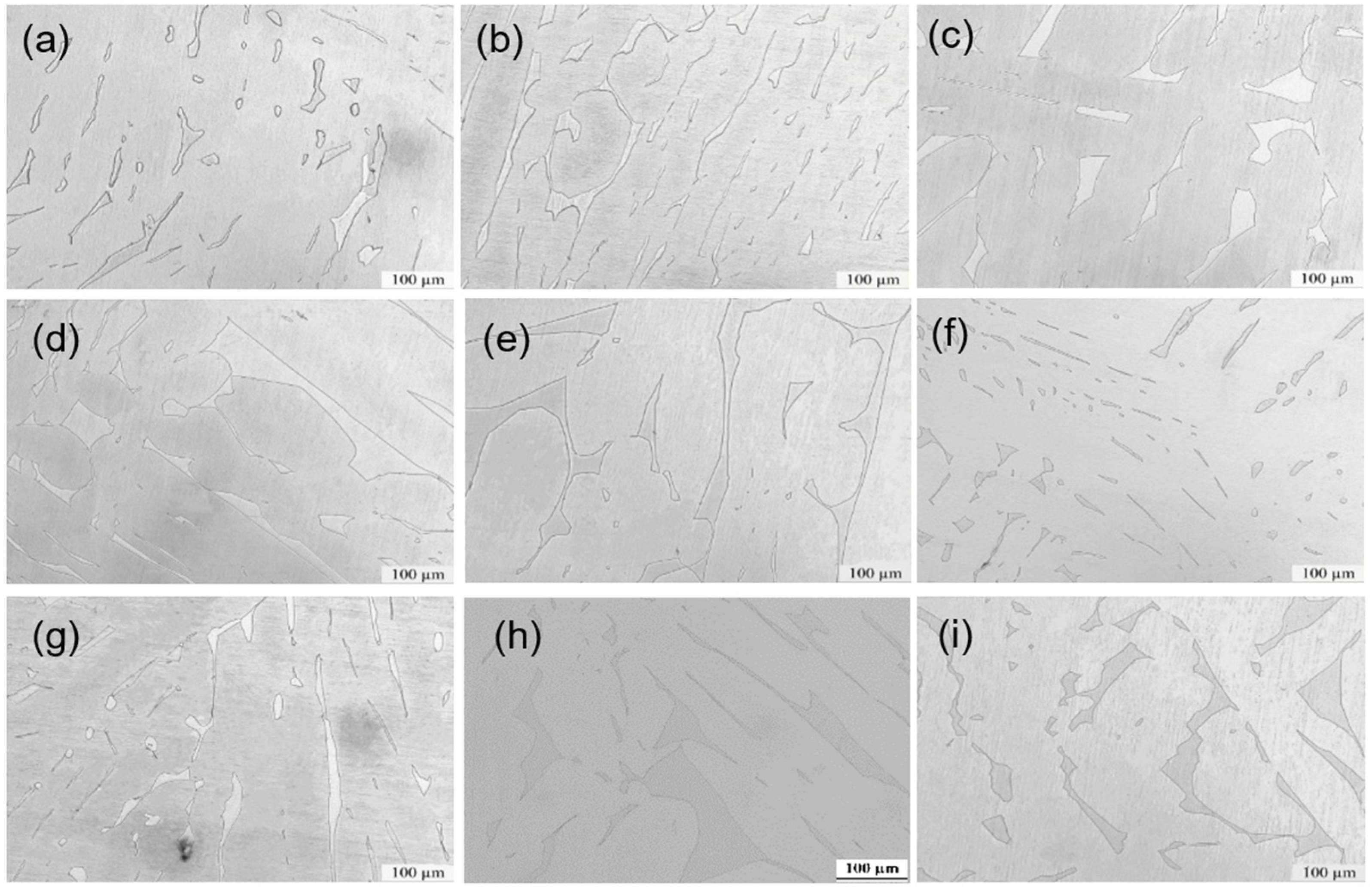
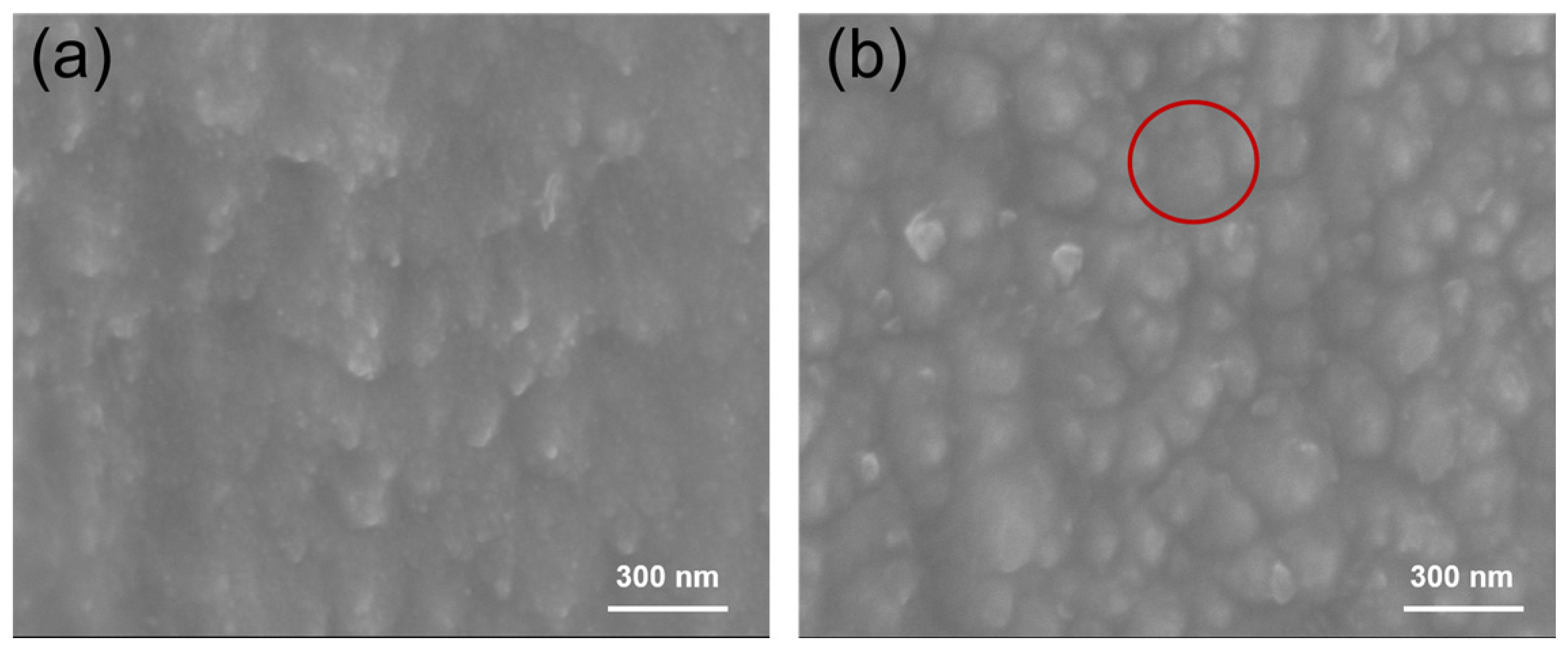
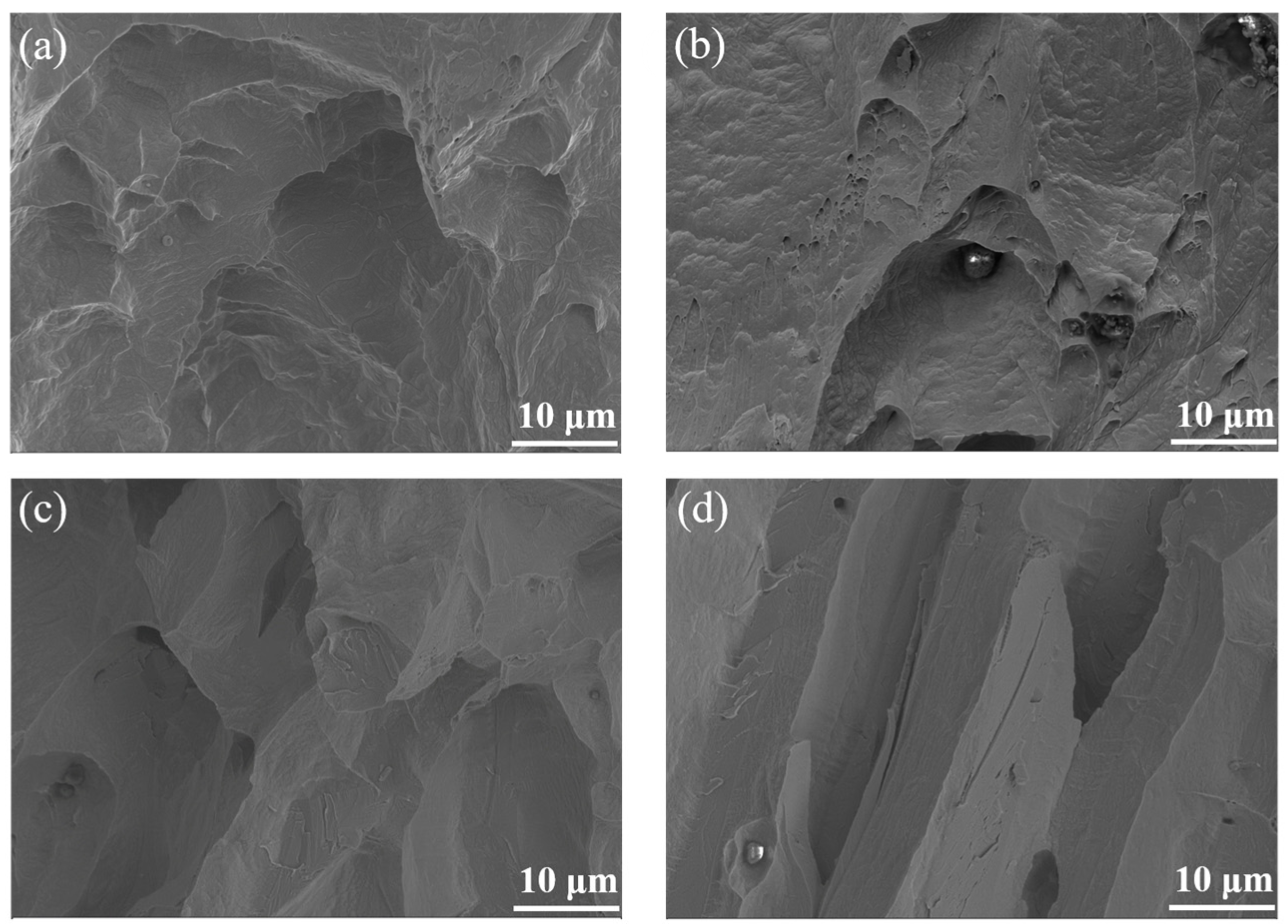
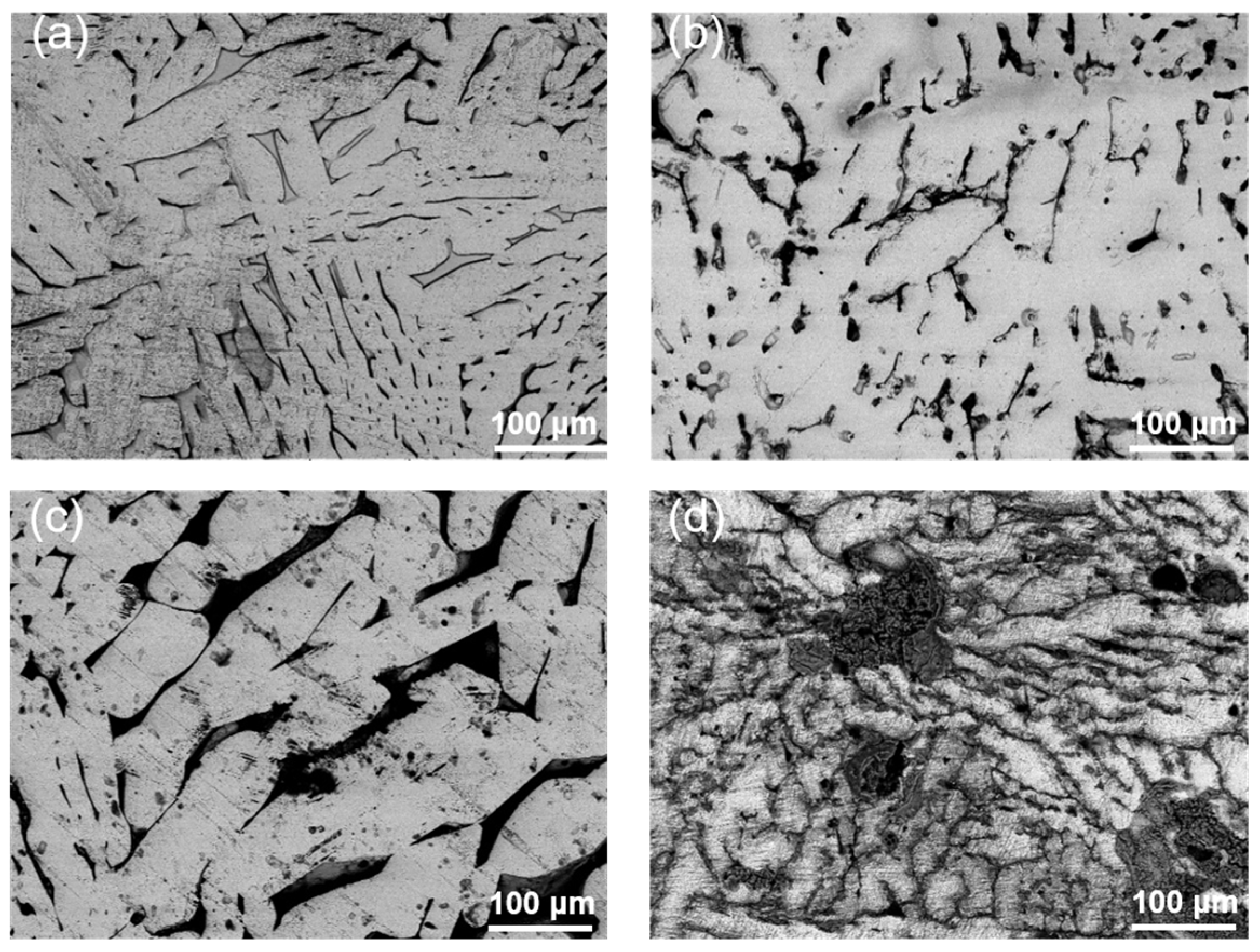
3.1. Microstructure
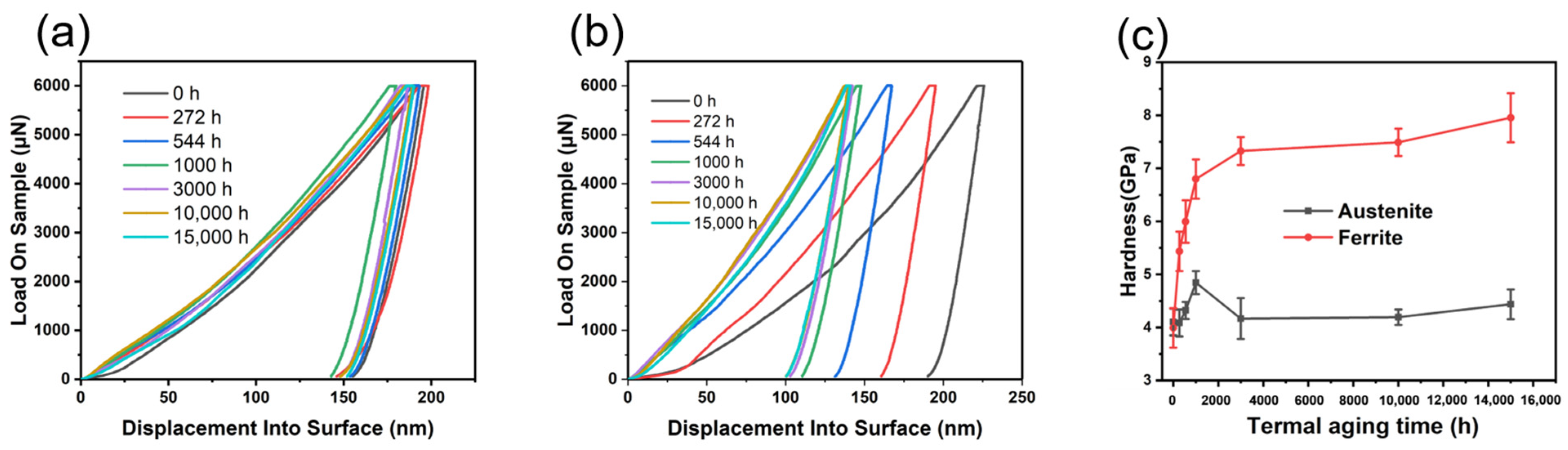
3.2. Mechanical Properties
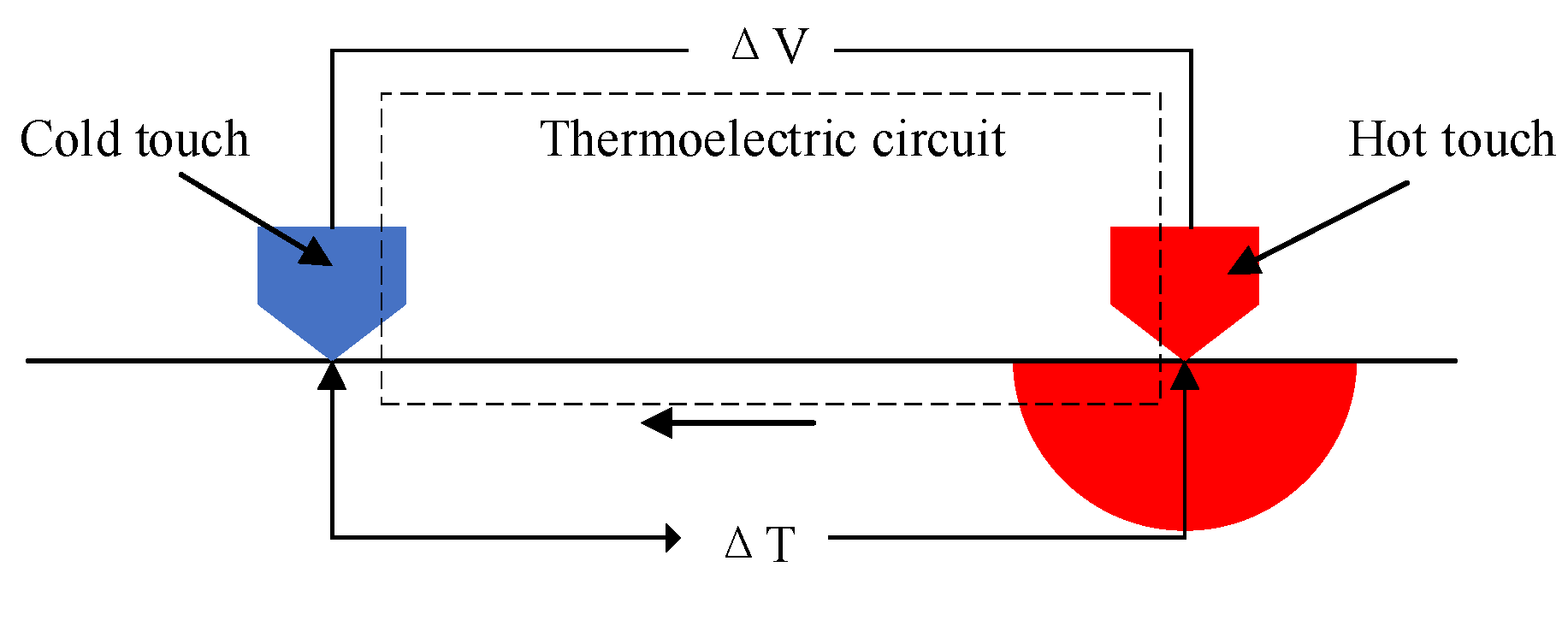
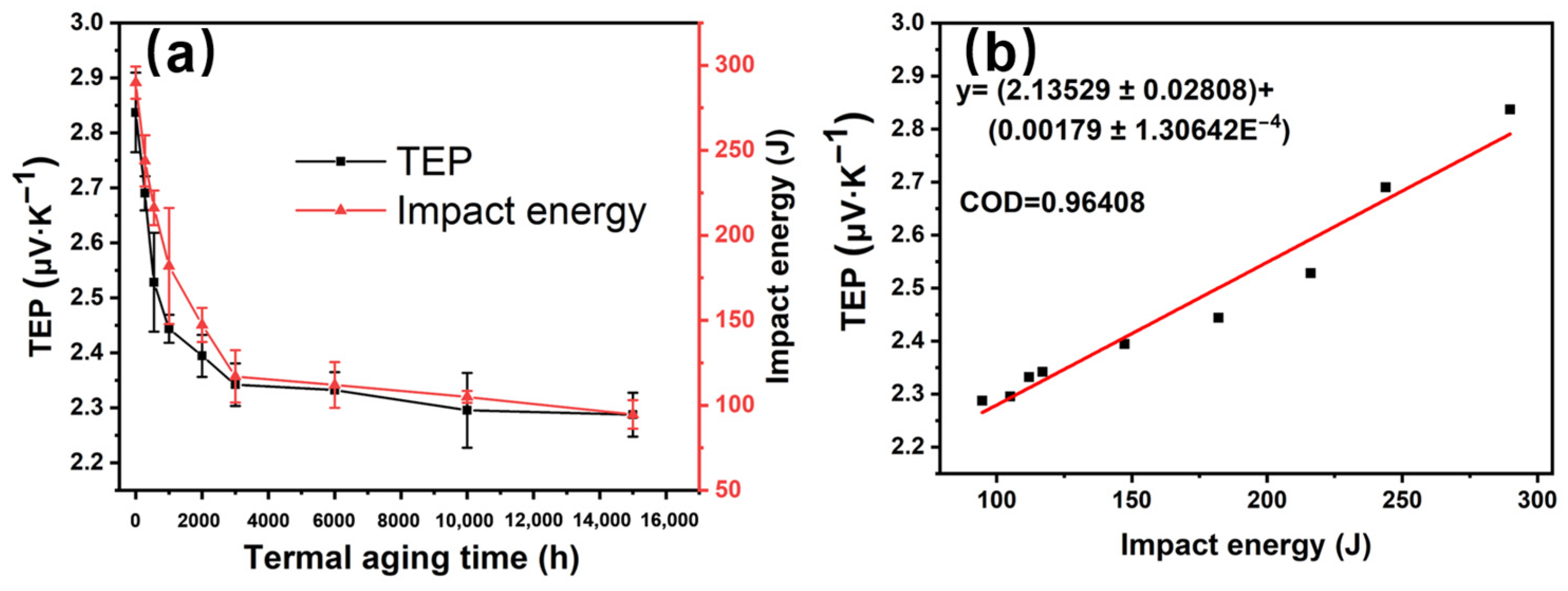
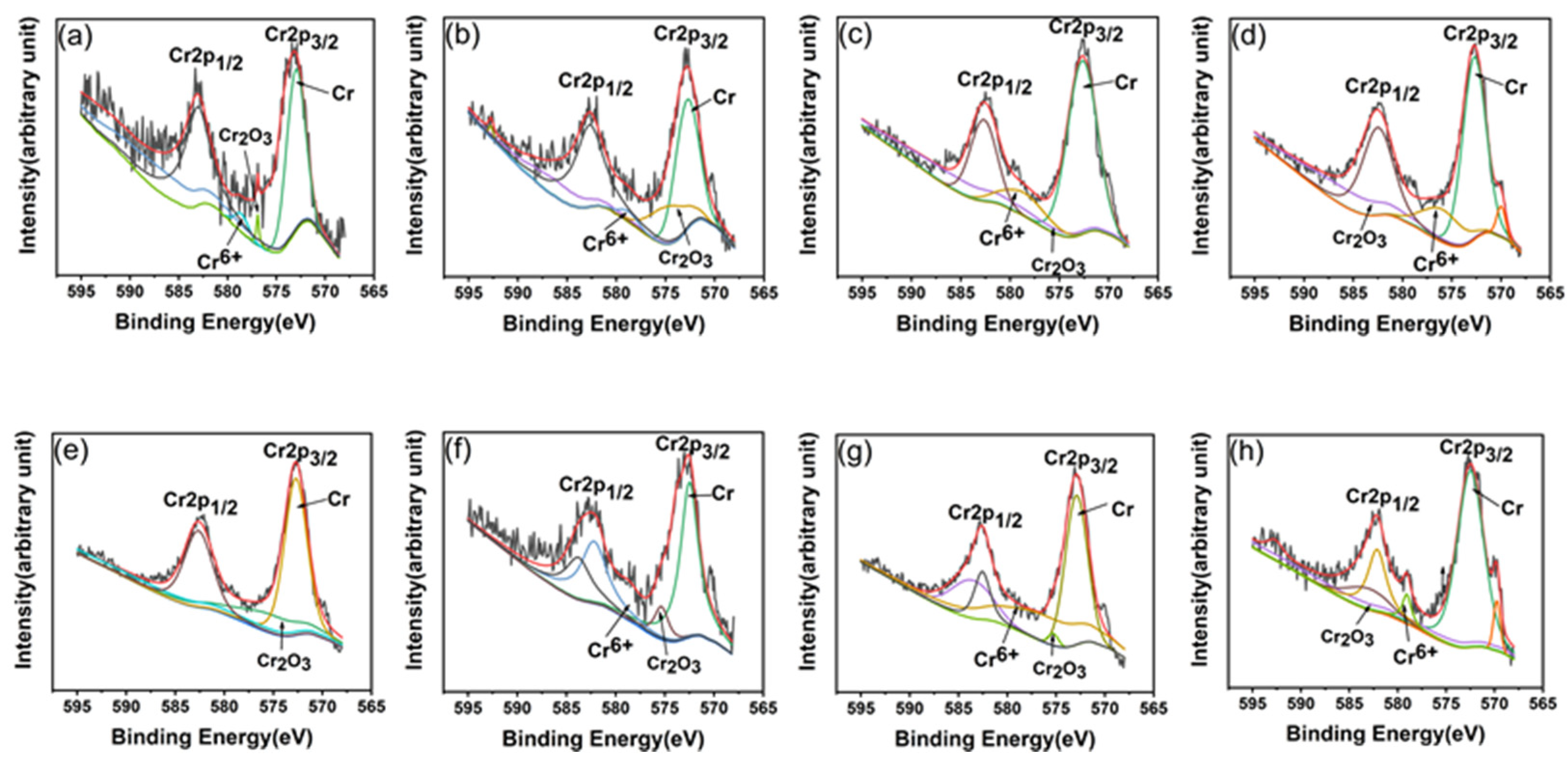
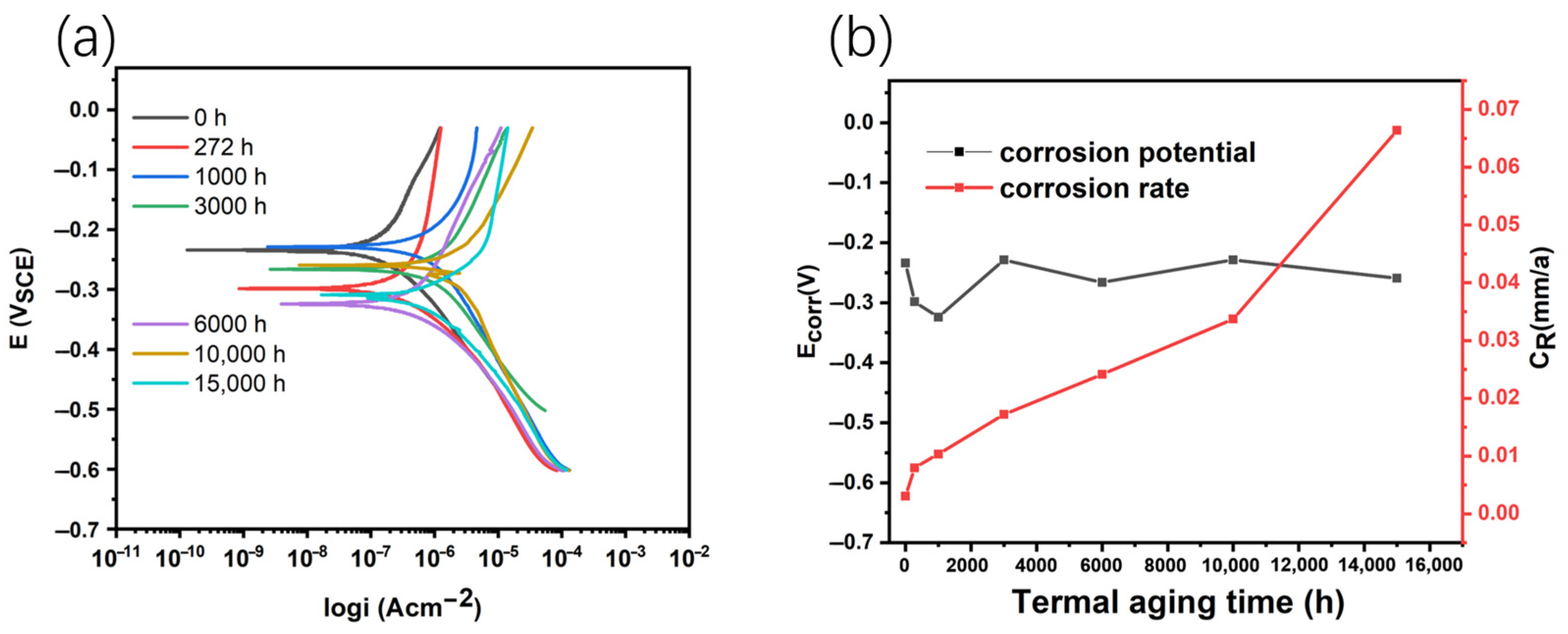
3.3. Corrosion Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chopra, O.K.; Chung, H.M. Aging of cast duplex stainless steels in lwr systems. Nucl. Eng. Des. 1985, 89, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wang, W.; Qiang, W.; Shu, G. Mechanical properties and kinetics of thermally aged z3cn20.09m cast duplex stainless steel. Int. J. Miner. 2018, 25, 1148–1155. [Google Scholar] [CrossRef]
- Park, C.; Kwon, H. Effects of aging at 475 °C on corrosion properties of tungsten-containing duplex stainless steels. Corros. Sci. 2002, 44, 2817–2830. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Makhlouf, K.; Sidhom, H.; Philibert, J. Effects of ageing conditions on the precipitates evolution, chromium depletion and intergranular corrosion susceptibility of aisi 316l: Experimental and modeling results. Mater. Sci. Eng. A 2004, 372, 98–108. [Google Scholar] [CrossRef]
- Murty, K.L.; Ramaswamy, K. 1-Overview of Ageing and Degradation Issues in Light Water Reactors (LWRs). In Materials Ageing and Degradation in Light Water Reactors; Murty, K.L., Ed.; Woodhead Publishing Series in Energy: Cambridge, UK, 2013; pp. 3–69. [Google Scholar]
- Lach, T.G.; Frazier, W.E.; Wang, J.; Devaraj, A.B.; Thak, S. Precipitation-site competition in duplex stainless steels: Cu clusters vs. spinodal decomposition interfaces as nucleation sites during thermal aging. Acta Mater. 2020, 196, 456–469. [Google Scholar] [CrossRef]
- Guo, E.; Wang, M.; Jing, T.; Chawla, N. Temperature-dependent mechanical properties of an austenitic–ferritic stainless steel studied by in situ tensile loading in a scanning electron microscope (SEM). Mater. Sci. Eng. A 2013, 580, 159–168. [Google Scholar] [CrossRef]
- Yamada, T.; Okano, S.; Kuwano, H. Mechanical property and microstructural change by thermal aging of SCS14A cast duplex stainless steel. J. Nucl. Mater. 2006, 350, 47–55. [Google Scholar] [CrossRef]
- Tucker, J.D.; Miller, M.K.; Young, G.A. Assessment of thermal embrittlement in duplex stainless steels 2003 and 2205 for nuclear power applications. Acta Mater. 2015, 87, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, Y.; Zhang, H.; Li, S.; Zheng, K.; Xue, F.; Wang, X. Microstructure evolution and impact fracture behaviors of Z3CN20-09M stainless steels after long-term thermal aging. J. Nucl. Mater. 2013, 433, 41–49. [Google Scholar] [CrossRef]
- Xue, F.; Wang, Z.; Xue, F.; Wang, Z.; Shu, G.; Yu, W.; Shi, H.; Ti, W. Thermal aging effect on Z3CN20.09M cast duplex stainless steel. Nucl. Eng. Des. 2009, 239, 2217–2223. [Google Scholar] [CrossRef]
- Yu, W.; Liu, Z.; Fan, M.; Gao, H.; Liu, E.; Chen, M.; Xue, F.; Chen, X. The effect of constraint on fracture properties of Z3CN20.09M after accelerated thermal aging. Int. J. Press. Vessel. Pip. 2021, 190, 104294. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, T.; Xin, L.; Han, Y.; Lu, Y.; Shoji, T. Thermal aging behaviors of duplex stainless steels used in nuclear power plant: A review. J. Nucl. Mater. 2020, 544, 152693. [Google Scholar] [CrossRef]
- Viherkoski, M.; Huttunen-Saarivirta, E.; Isotahdon, E.; Uusitalo, M.; Tiainen, T.; Kuokkala, V.T. The effect of aging on heat-resistant cast stainless steels. Mater. Sci. Eng. A 2014, 589, 189–198. [Google Scholar] [CrossRef]
- Hamaoka, T.; Nomoto, A.; Nishida, K.; Dohi, K.; Soneda, N. Accurate determination of the number density of g-phase precipitates in thermally aged duplex stainless steel. Philos. Mag. 2012, 92, 2716–2732. [Google Scholar] [CrossRef]
- Erneman, J.; Schwind, M.; Andrén, H.O.; Nilsson, J.O.; Wilson, A.; Ågren, J. The evolution of primary and secondary niobium carbonitrides in AISI 347 stainless steel during manufacturing and long-term ageing. Acta Mater. 2006, 54, 67–76. [Google Scholar] [CrossRef]
- Du, D.; Chen, K.; Yu, L.; Lu, H.; Zhang, L.; Shi, X.; Xu, X. SCC crack growth rate of cold worked 316l stainless steel in PWR environment. J. Nucl. Mater. 2015, 456, 228–234. [Google Scholar] [CrossRef]
- Gutiérrez-Vargas, G.; Ruiz, A.; Kim, J.-Y.; López-Morelos, V.H.; Ambriz, R.R. Evaluation of thermal embrittlement in 2507 super duplex stainless steel using thermoelectric power. Nucl. Eng. Technol. 2019, 51, 1816–1821. [Google Scholar] [CrossRef]
- Xie, X.; Ning, D.; Chen, B.; Lu, S.; Sun, J. Stress corrosion cracking behavior of cold-drawn 316 austenitic stainless steels in simulated PWR environment. Corros. Sci. 2016, 112, 576–584. [Google Scholar] [CrossRef]
- Chopra, O.K.; Sather, A. Initial Assessment of the Mechanisms and Significance of Low-Temperature Embrittlement of Cast Stainless Steels in LWR Systems; United States Nuclear Regulatory Commission: Rockville, MD, USA, 1990; pp. 6–8.
- Chung, H.M.; Leax, T.R. Embrittlement of laboratory and reactor aged CF3, CF8, and CF8M duplex stainless steels. Mater. Sci. Technol. 1990, 6, 249–262. [Google Scholar] [CrossRef]
- Nakajima, Y.; Uno, Y.; Suzuki, M. Fracture toughness behaviour of service-exposed type 321 stainless steel at room and elevated temperature under normal and low straining rates. Eng. Fract. Mech. 1989, 33, 295–307. [Google Scholar] [CrossRef]
- Coste, J.F.; Leborgne, J.M.; Massoud, J.P.; Borrelly, R. Development of A Portable Device for Thermoelectrical Power Measurement—Application to the Inspection of Duplex Stainless Steel Components. In Review of Progress in Quantitative Nondestructive Evaluation; Thompson, D.O., Chimenti, D.E., Eds.; Springer US: Boston, MA, USA, 1999; Volume 18, pp. 2095–2102. [Google Scholar]
- Perez, M.; Massardier, V.; Kleber, X. Thermoelectric power applied to metallurgy: Principle and recent applications. Int. J. Mater. Res. 2009, 100, 1461–1466. [Google Scholar] [CrossRef] [Green Version]
- Saillet, S.; Le Delliou, P. Prediction of J-R curves and thermoelectric power evolution of cast austenitic stainless steels after very long-term aging (200,000 h) at temperatures below 350 °C. J. Nucl. Mater. 2020, 540, 152328. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Raja, V.S.; Tewari, R.; Dey, G.K. Low Temperature thermal ageing embrittlement of austenitic stainless steel welds and its electrochemical assessment. Corros. Sci. 2012, 54, 278–290. [Google Scholar] [CrossRef]
- Shiao, J.J.; Tsai, C.H.; Kai, J.J.; Huang, J.H. Aging embrittlement and lattice image analysis in a Fe-Cr-Ni duplex stainless steel aged at 400 °C. J. Nucl. Mater. 1994, 217, 269–278. [Google Scholar] [CrossRef]
- Yang, M.; King Daniel, J.M.; Postugar, I.; Wen, Y.; Luan, J.; Kuhn, B.; Jiao, Z.; Wang, C.; Wenman, M.R.; Liu, X. Precipitation behavior in G-phase strengthened ferritic stainless steels. Acta Mater. 2021, 205, 116542. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, X.; Xue, F. G-Phase precipitation in duplex stainless steels after long-term thermal aging: A high-resolution transmission electron microscopy study. J. Nucl. Mater. 2014, 452, 382–388. [Google Scholar] [CrossRef]
- Baylac, G.; Grandemange, J.M. The French code RCC-M: Design and construction rules for the mechanical components of PWR nuclear islands. Nucl. Eng. Des. 1991, 129, 239–254. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kameda, J.; Nagai, Y.; Toyama, T.; Nishiyama, Y.; Onizawa, K. Study on microstructural changes in thermally-aged stainless steel weld-overlay cladding of nuclear reactor pressure vessels by atom probe tomography. J. Nucl. Mater. 2011, 415, 198–204. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. A guide to polarisation curve interpretation: Deconstruction of experimental curves typical of the Fe/H2O/H+/O2 corrosion system. Corros. Sci. 2005, 47, 2125–2156. [Google Scholar] [CrossRef]
- Draźić, D.M.; Vascic, V. The Inflection point on the polarization curve and its use in corrosion rate measurements. Corros. Sci. 1985, 25, 483–491. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.; Dong, F.; Yang, B.; Li, S.; Yu, X. Pitting behavior of thermally aged Z3CN20.09M cast stainless steel for primary coolant pipe of nuclear power plant. Eng. Fail. Anal. 2018, 83, 1–8. [Google Scholar] [CrossRef]



| Element | C | Cr | Ni | Si | Mn | S | P | N | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Designed Composition | 0.03 | 20.19 | 8.92 | 1.27 | 1.13 | 0.01 | 0.02 | 0.03 | Bal. |
| Measured Composition | 0.03 | 20.96 | 9.31 | 1.03 | 0.94 | 0.02 | 0.01 | 0.04 | Bal. |
| Thermal Aging Time(h) | 0 | 272 | 1000 | 3000 | 6000 | 10,000 | 15,000 |
|---|---|---|---|---|---|---|---|
| Corrosion current(A/cm2) | 2.4941 × 10−7 | 6.4936 × 10−7 | 8.4454 × 10−7 | 1.4058 × 10−6 | 1.9713 × 10−6 | 2.7516 × 10−6 | 5.4181 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, F.; Shi, F.; Zhang, C.; Zheng, Q.; Yi, D.; Li, X.; Li, Y. The Microstructure and Mechanical and Corrosion Behaviors of Thermally Aged Z3CN20-09M Cast Stainless Steel for Primary Coolant Pipes of Nuclear Power Plants. Coatings 2021, 11, 870. https://doi.org/10.3390/coatings11080870
Xue F, Shi F, Zhang C, Zheng Q, Yi D, Li X, Li Y. The Microstructure and Mechanical and Corrosion Behaviors of Thermally Aged Z3CN20-09M Cast Stainless Steel for Primary Coolant Pipes of Nuclear Power Plants. Coatings. 2021; 11(8):870. https://doi.org/10.3390/coatings11080870
Chicago/Turabian StyleXue, Fei, Fangjie Shi, Chuangju Zhang, Qiaoling Zheng, Dawei Yi, Xiuqing Li, and Yefei Li. 2021. "The Microstructure and Mechanical and Corrosion Behaviors of Thermally Aged Z3CN20-09M Cast Stainless Steel for Primary Coolant Pipes of Nuclear Power Plants" Coatings 11, no. 8: 870. https://doi.org/10.3390/coatings11080870
APA StyleXue, F., Shi, F., Zhang, C., Zheng, Q., Yi, D., Li, X., & Li, Y. (2021). The Microstructure and Mechanical and Corrosion Behaviors of Thermally Aged Z3CN20-09M Cast Stainless Steel for Primary Coolant Pipes of Nuclear Power Plants. Coatings, 11(8), 870. https://doi.org/10.3390/coatings11080870





