The Effect of Polytetrafluoroethylene (PTFE) Particles on Microstructural and Tribological Properties of Electroless Ni-P+PTFE Duplex Coatings Developed for Geothermal Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Surface Preparation
2.2. Bath Preparation and Electroless Ni-P+PTFE Composite Coating
2.3. Testing and Characterisation of Coatings
3. Results
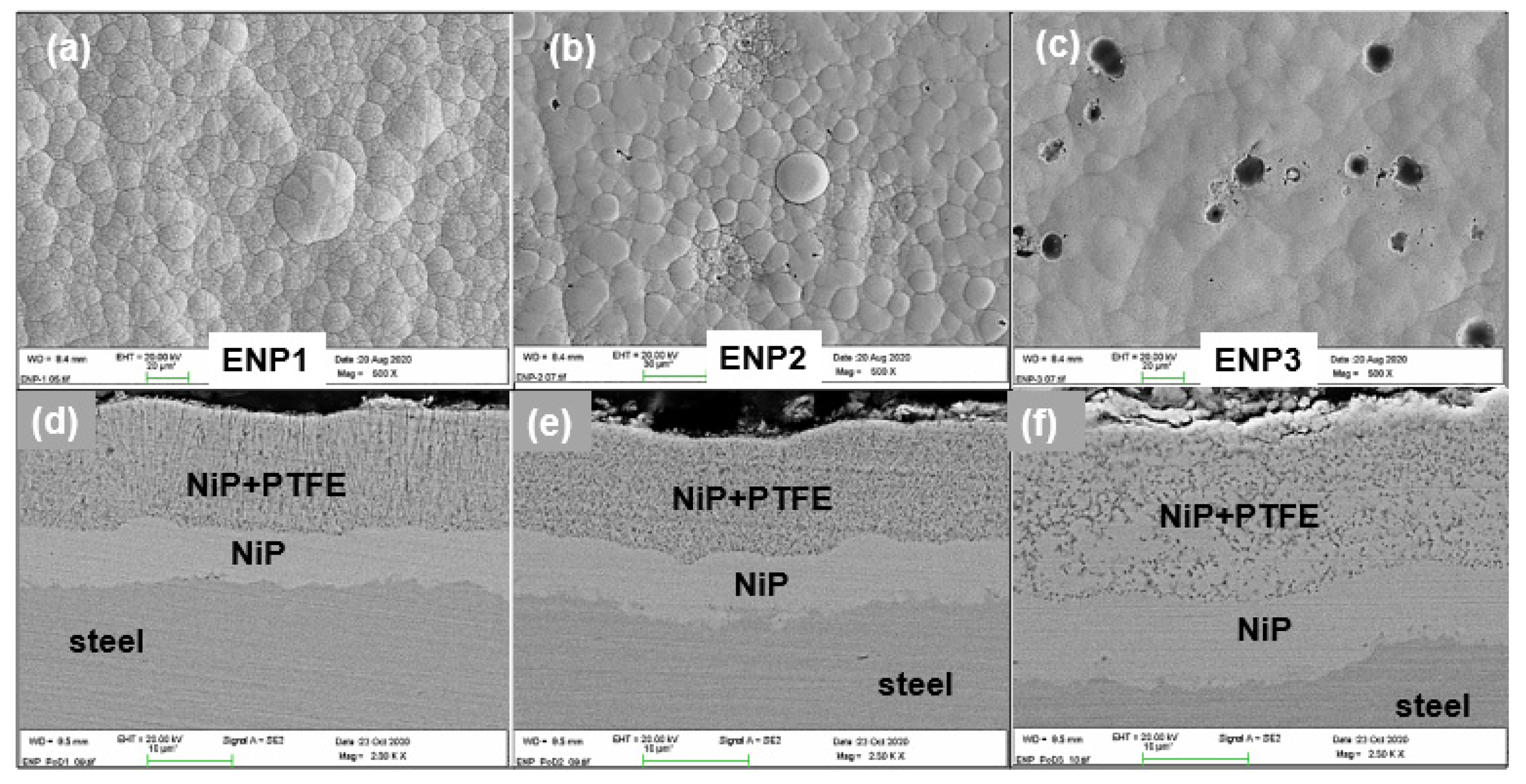
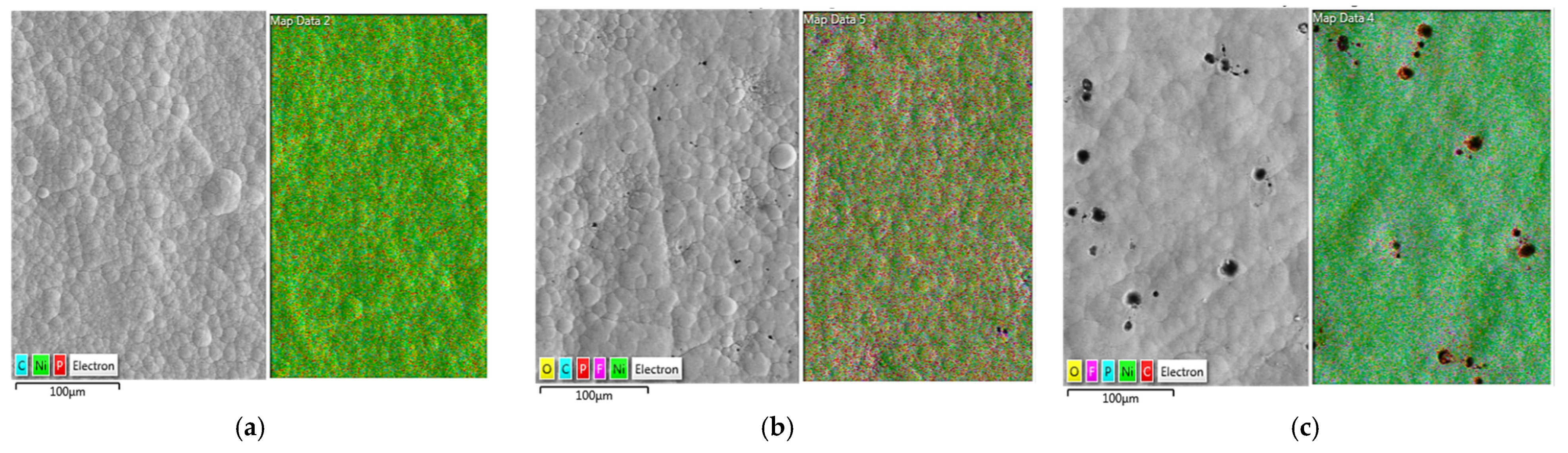
3.1. Morphology and Structure of the Duplex Coatings
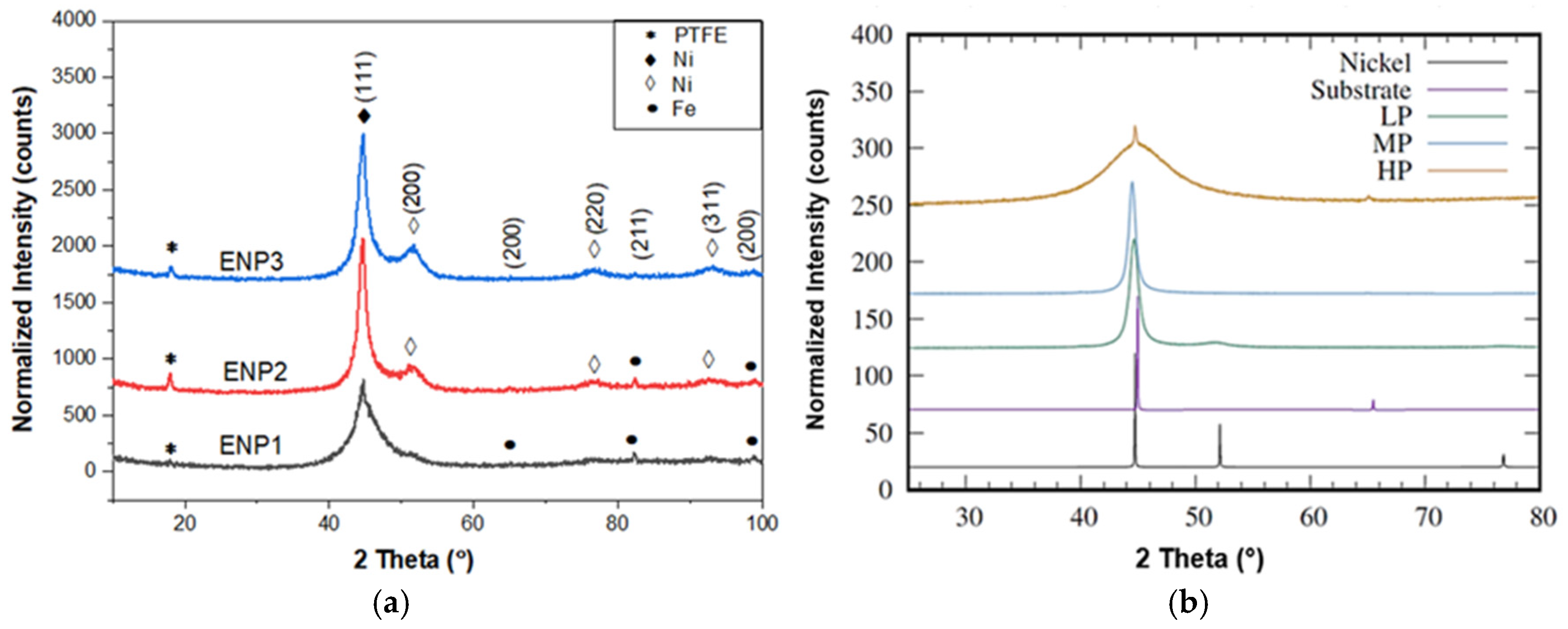
3.2. Phase Composition of the Coatings
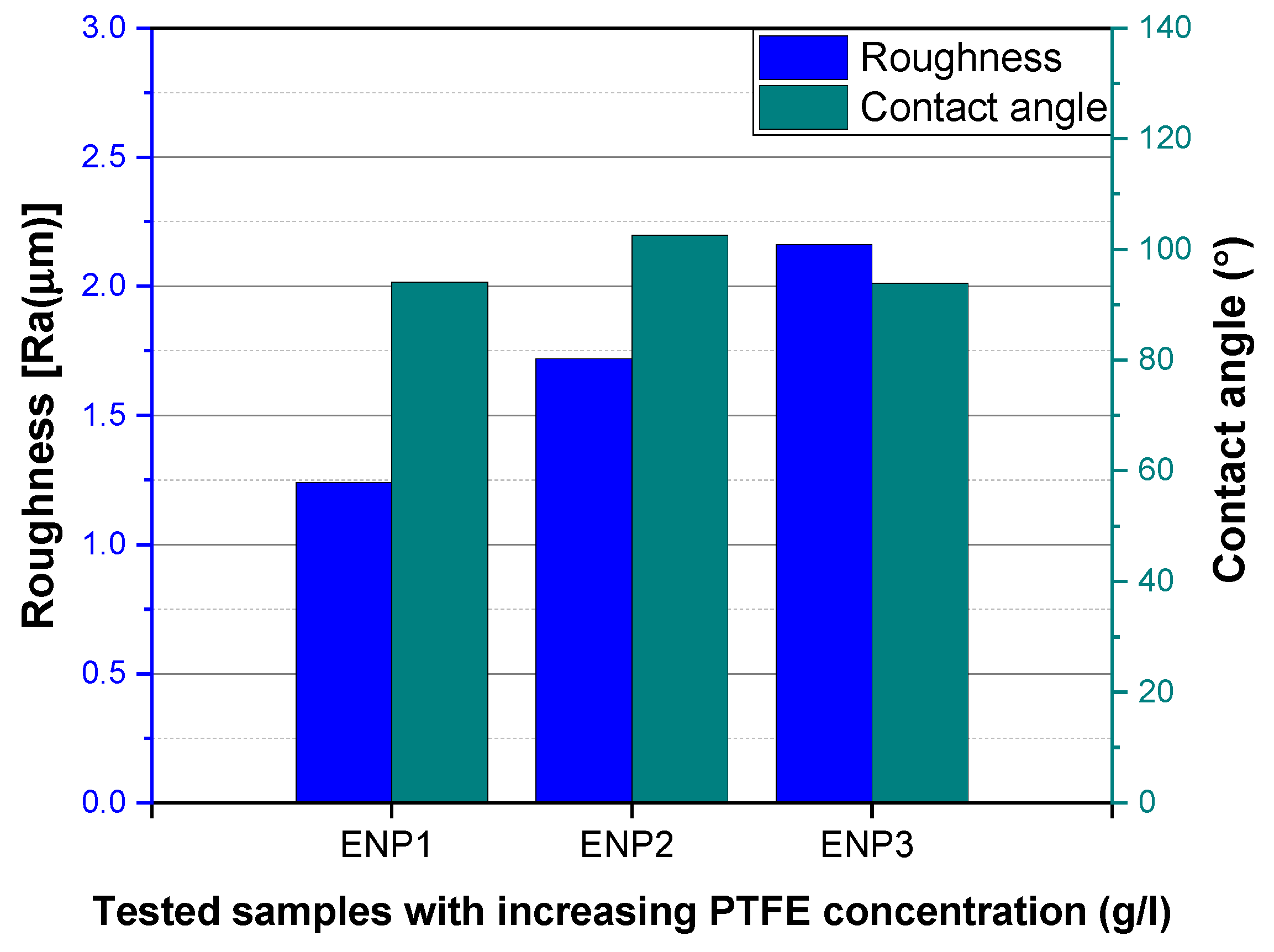
3.3. Surface Roughness and Water Contact Angle (WCA) Analysis of the Coatings
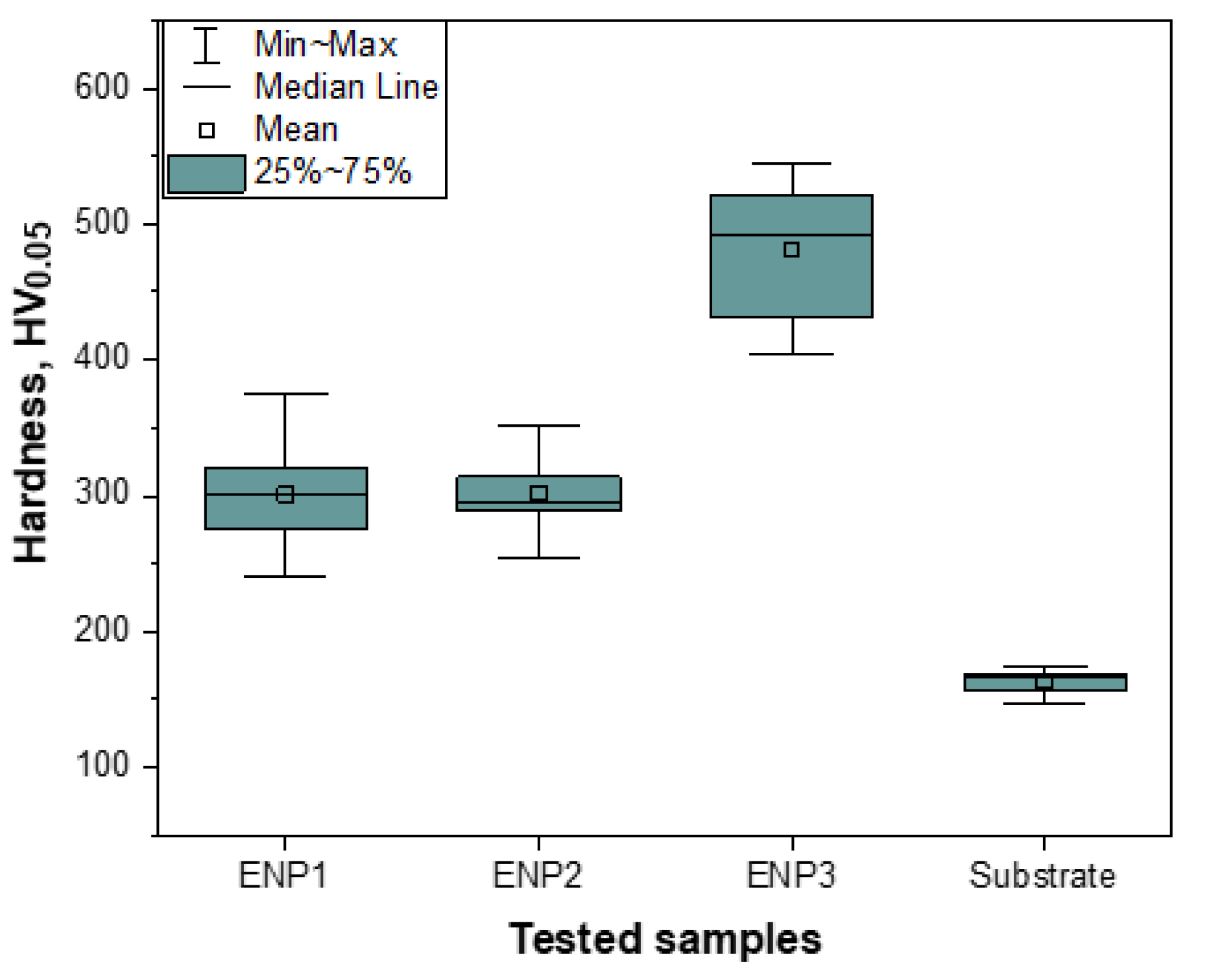
3.4. Microhardness Analysis of the Coatings
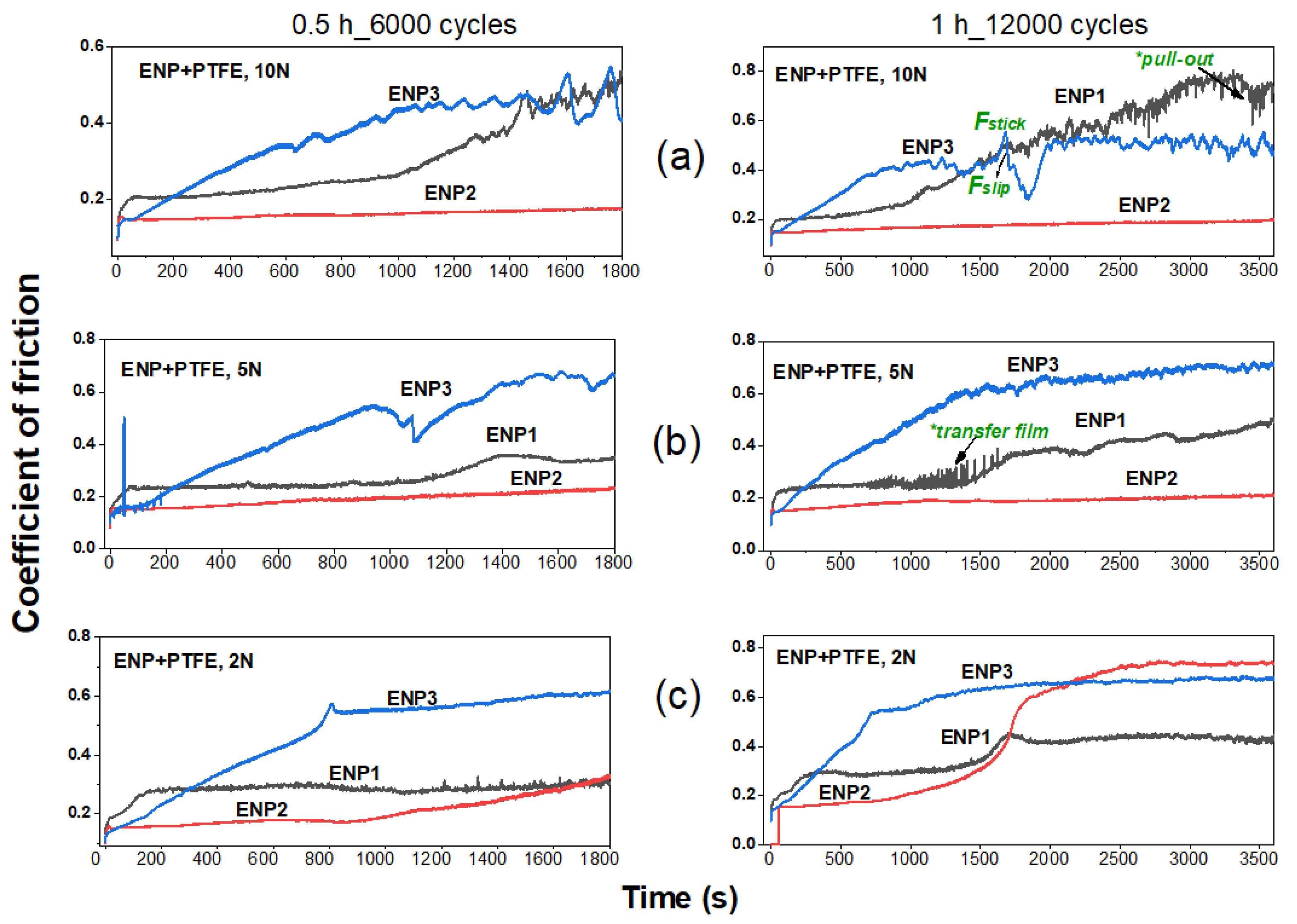
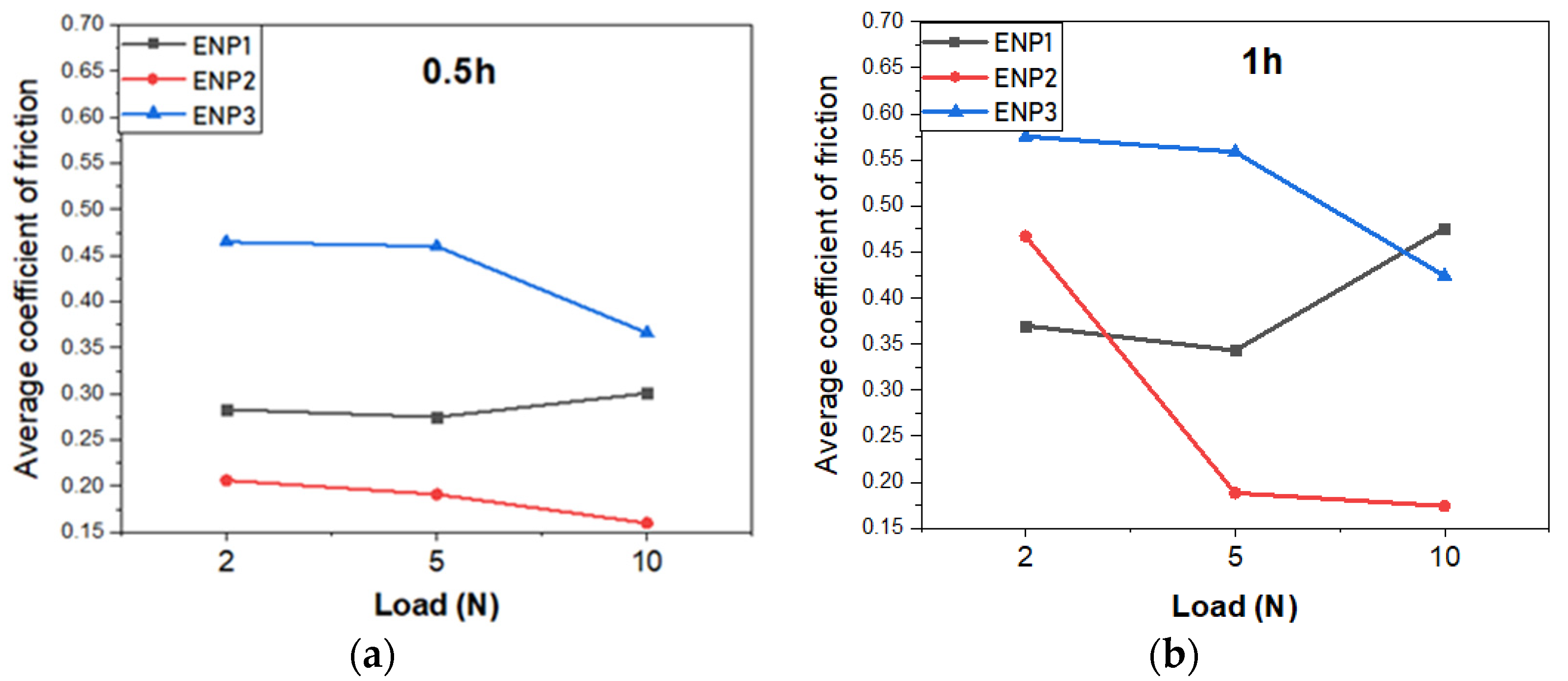
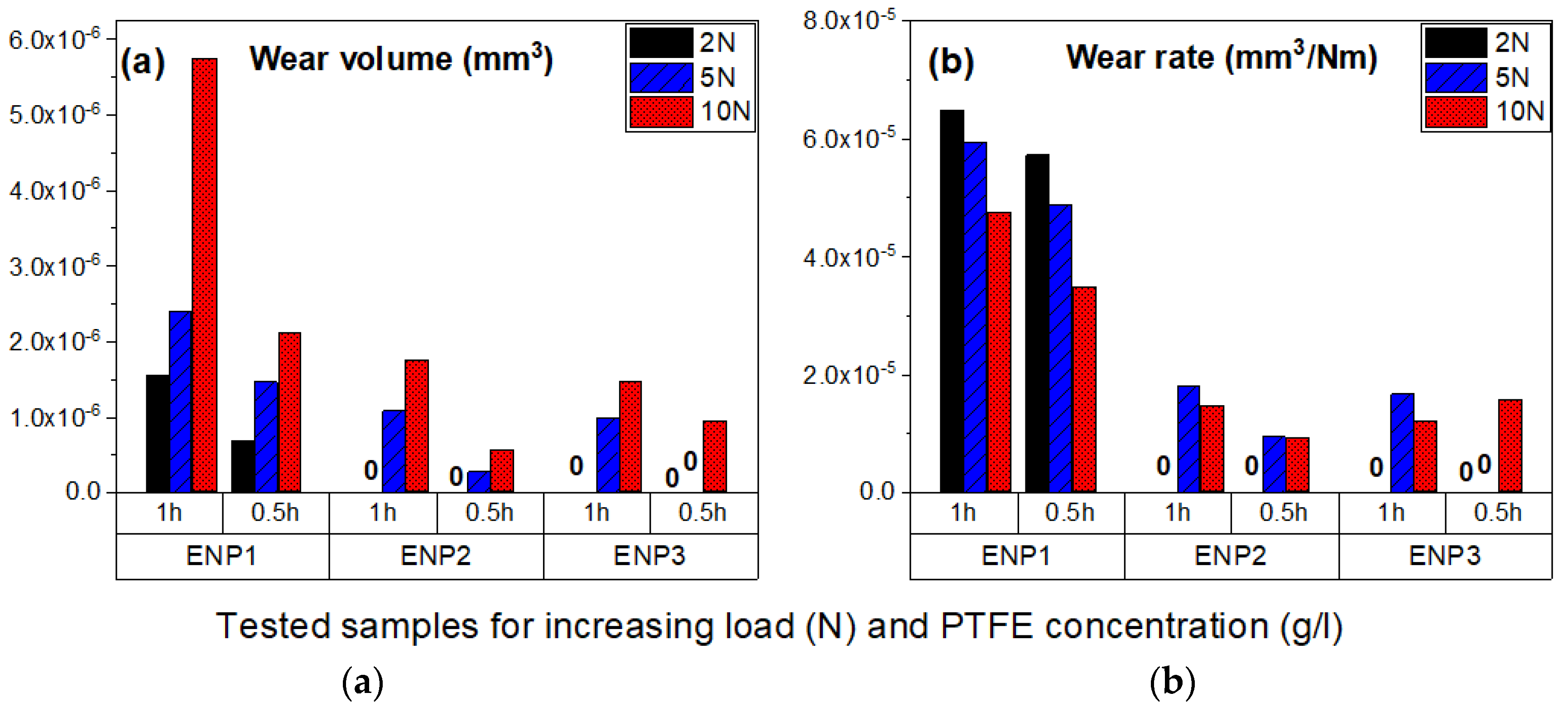
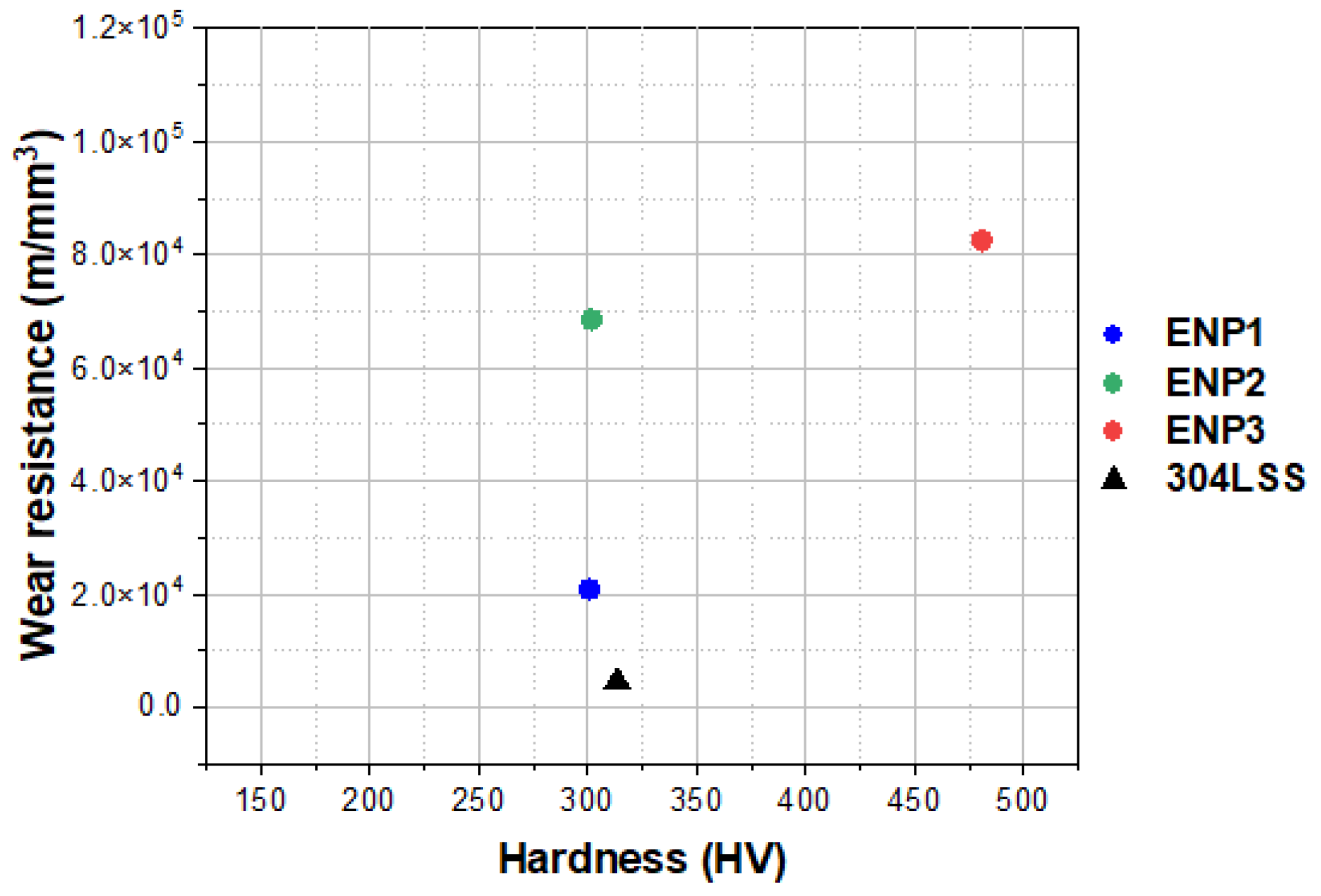
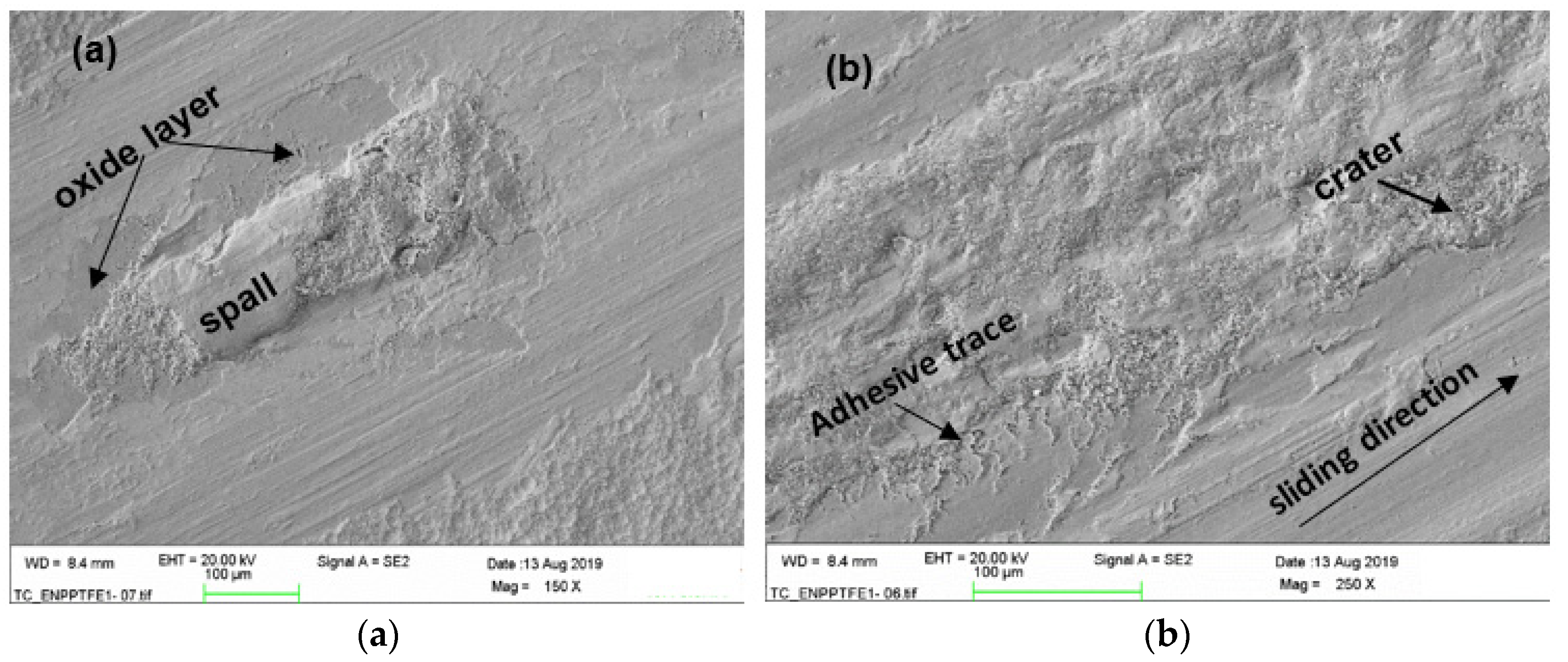
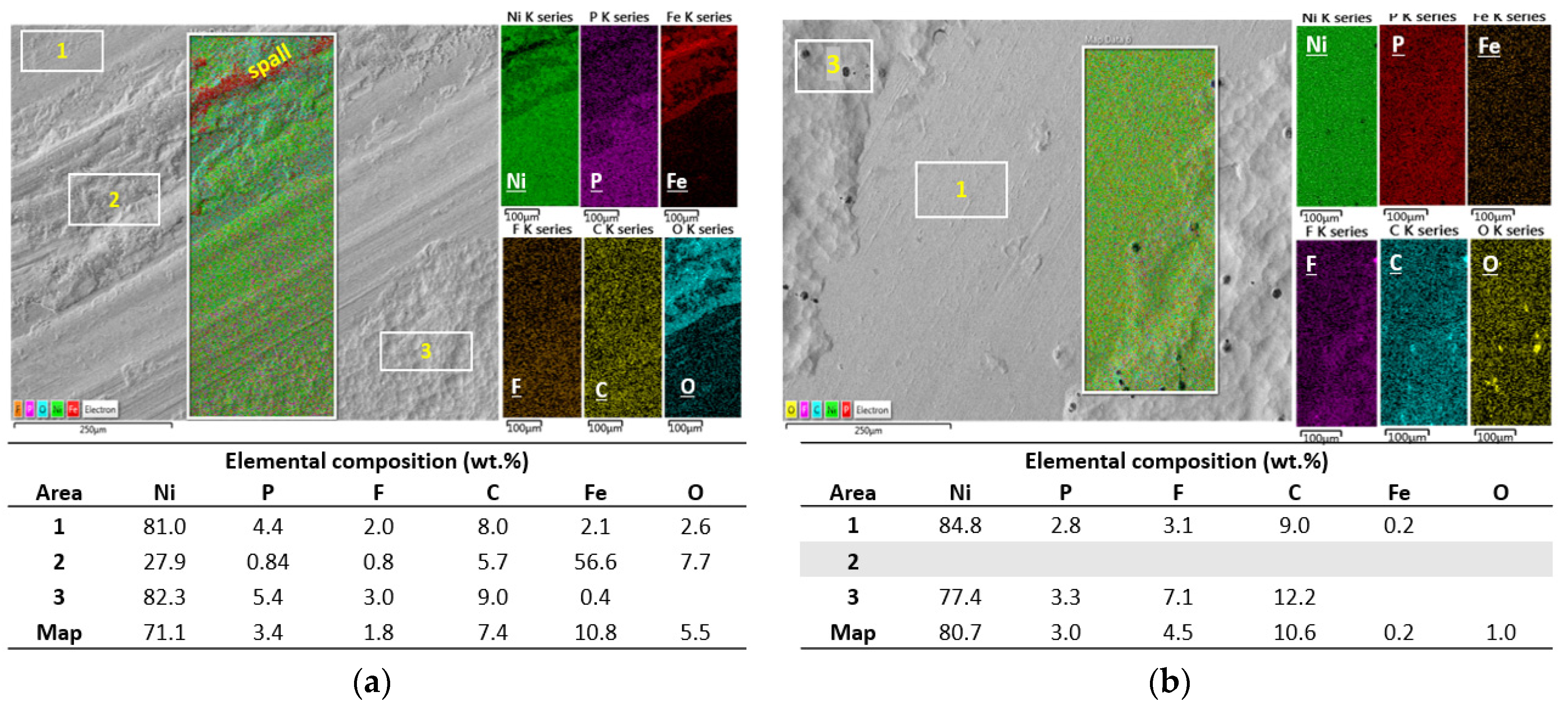
3.5. Tribological Properties
4. Discussion
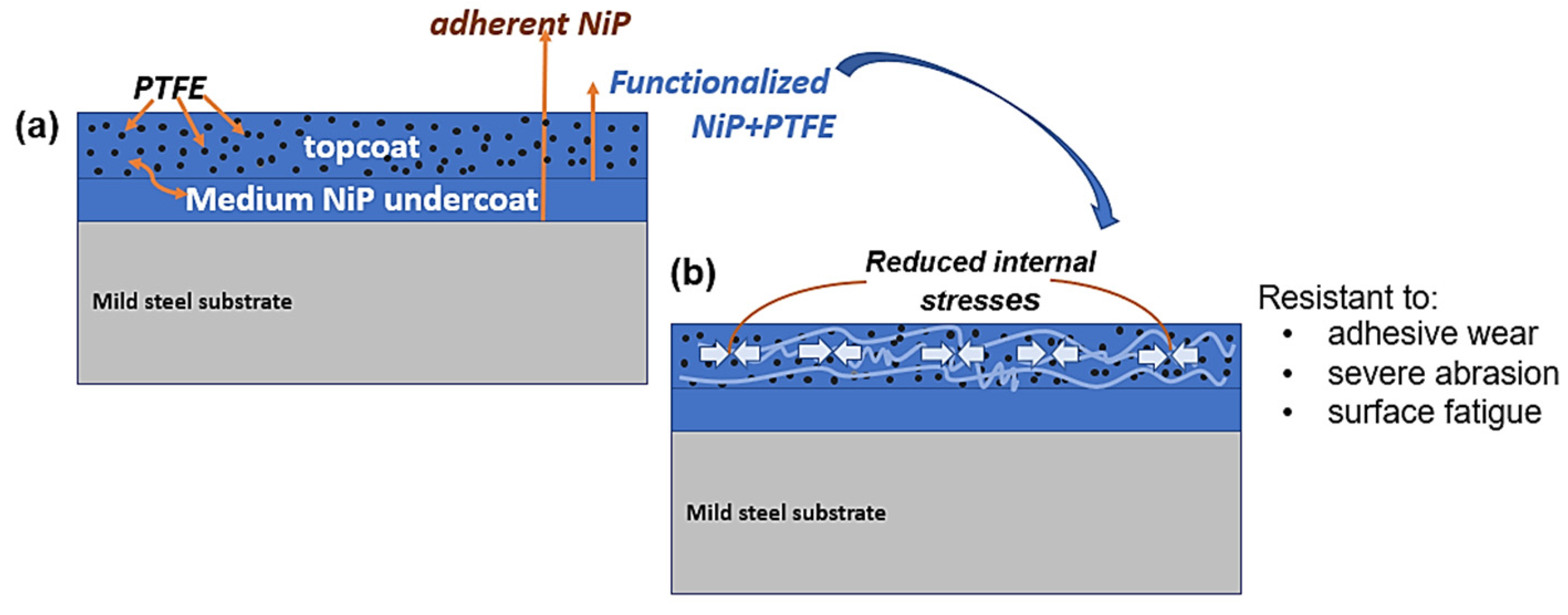
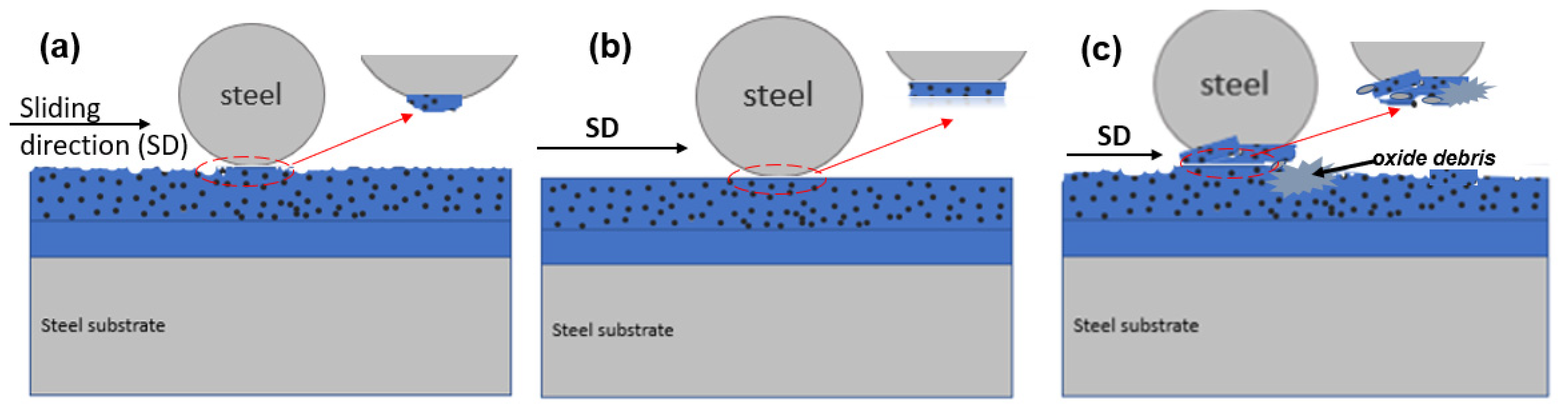
Lubrication and Wear Mechanism
- (1)
- the smoothing by plastic deformation from gradual abrasion of the Ni-P+PTFE top layer and
- (2)
- the fatigue wear of only ENP1 due to high internal stresses via spalling [31].
5. Conclusions
- SEM/EDS and XRD analyses demonstrated the presence of PTFE particles. Significant changes in the coatings’ morphology and microstructure were observed to improve grain size, hydrophobicity, arithmetic average surface roughness, and hardness with PTFE addition. In the ENP solution, the ideal amount for PTFE homogenization was 10 g/L in this work.
- In the tribological testing, the PTFE was responsible for the lubricating properties of the coatings. Independent of the coating composition and structure, PTFE positively influenced the formation of a robust transfer film eliminating stick-slip, high volume loss and adhesion in the tribo-contact. However, this property is a function of PTFE dispersion. In comparison to the reference 304 L SS, the ENP2 found a steady friction value of 0.17 with a 79% increase in lubricity at the highest test load and cycles.
- The sporadic hardness values measured in the ENP3 coating confirmed the clustering effect of PTFE particles and affected steady-state friction-wear conditions. The cycles to steady-state friction occurred at the highest load and sliding cycles.
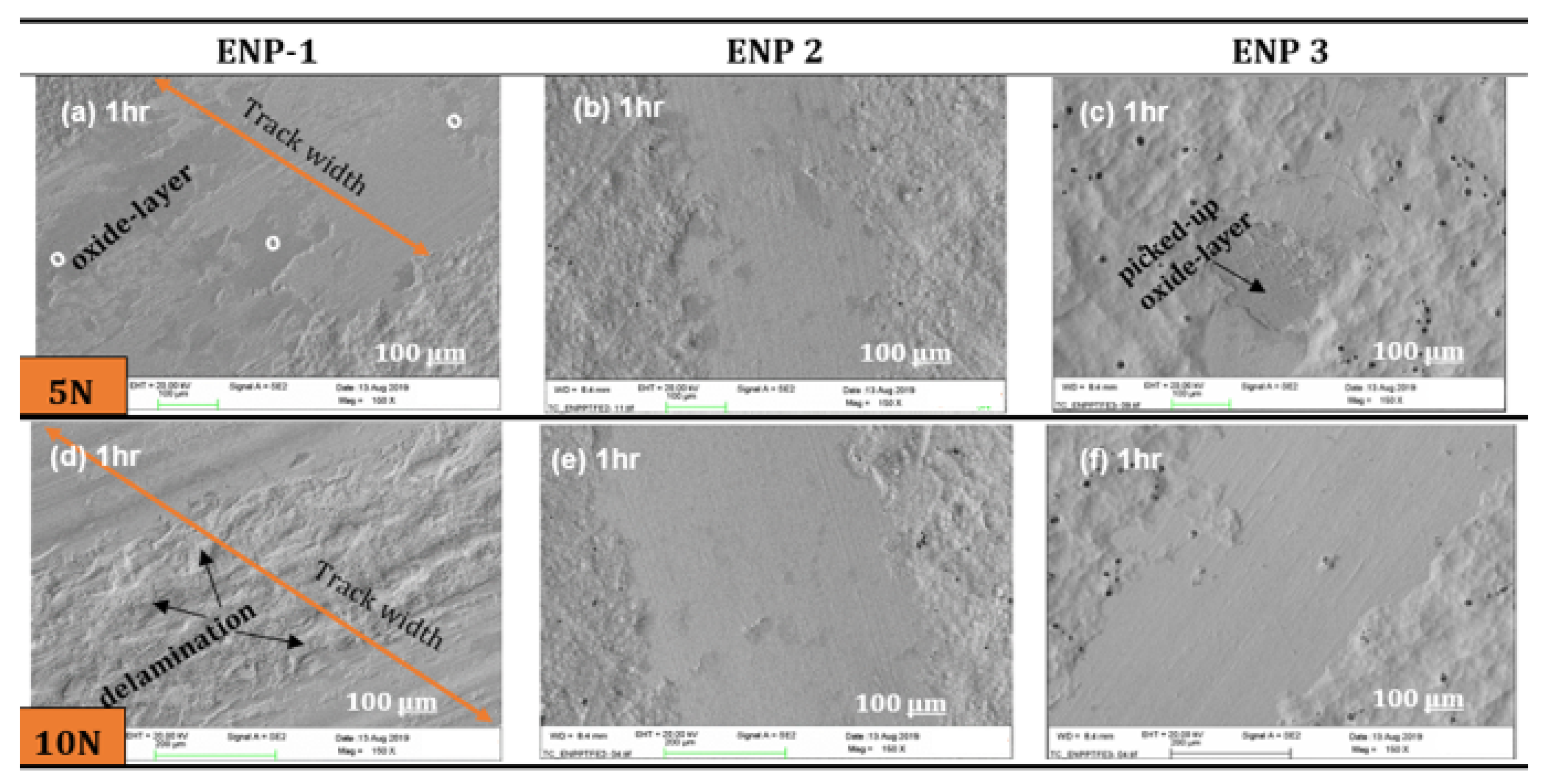
- The worn zones of the coatings displayed smooth surfaces from fine abrasion or scratching, however, at higher loads and sliding cycles, ENP1 coating failed by galling and fatigue wear.
- Finally, the coating with dispersed PTFE reduced surface energy by more than 100% compared to the substrate. Thus, the combination of low friction and low hydrophobicity suggests that the duplex coatings present good candidacy for drag, scaling and corrosion mitigation in heating and cooling units (e.g., heat exchangers) used in geothermal power plants. Furthermore, ENP2 (10 g/L PTFE) is optimum for both low friction and wear applications at low contact load, while ENP3 (15 g/L PTFE) has desirable wear resistances but compromised lubrication under dry contact sliding (due to non-uniform dispersion and agglomeration of PTFE in the matrix).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchtík, M.; Kosár, P.; Wasserbauer, J.; Tkacz, J.; Doležal, P. Characterization of Electroless Ni–P Coating Prepared on a Wrought ZE10 Magnesium Alloy. Coatings 2018, 8, 96. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloy. Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Narayanan, T.S.N.S.; Seshadri, S.K. Electroless Ni–P composite coatings. J. Appl. Electrochem. 2003, 33, 807–816. [Google Scholar] [CrossRef]
- Krick, B.A.; Ewin, J.J.; Blackman, G.S.; Junk, C.P.; Sawyer, W.G. Environmental dependence of ultra-low wear behavior of polytetrafluoroethylene (PTFE) and alumina composites suggests tribochemical mechanisms. Tribol. Int. 2012, 51, 42–46. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, S.K. Tribology of electroless nickel coatings—A review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- Gutsev, D.; Antonov, M.; Hussainova, I.; Grigoriev, A.Y. Effect of SiO2 and PTFE additives on dry sliding of NiP electroless coating. Tribol. Int. 2013, 65, 295–302. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Ramamoorthy, B. Effect of reducing agent and nano Al2O3 particles on the properties of electroless Ni–P coating. Appl. Surf. Sci. 2014, 307, 654–660. [Google Scholar] [CrossRef]
- Tang, A.; Wang, M.; Huang, W.; Wang, X. Composition design of Ni–nano-Al2O3–PTFE coatings and their tribological characteristics. Surf. Coatings Technol. 2015, 282, 121–128. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P.; Davim, J.P. Comparative study of tribological behavior of electroless Ni-B, Ni-B-Mo, and Ni-B-W coatings at room and high temperatures. Lubricants 2018, 6, 67. [Google Scholar] [CrossRef]
- Zhou, Y.-R.; Zhang, S.; Nie, L.-L.; Zhu, Z.-J.; Zhang, J.-Q.; Cao, F.-H.; Zhang, J.-X. Electrodeposition and corrosion resistance of Ni–P–TiN composite coating on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 2976–2987. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Seshadri, S.K. Synthesis and corrosion behavior of electroless Ni-P-Si3N4 composite coatings. J. Mater. Sci. Lett. 1998, 17, 1297–1299. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Ghorbani, M. Wear and corrosion properties of electroless nickel composite coatings with PTFE and/or MoS2 particles. J. Coat. Technol. Res. 2011, 8, 527–533. [Google Scholar] [CrossRef]
- Zhang, S. State-of-the-art of polymer tribology. Tribol. Int. 1998, 31, 49–60. [Google Scholar] [CrossRef]
- Keogh, W.; Boakye, G.O.; Neville, A.; Olsen, J.H.; Charpentier, T. Lead Sulfide (PbS) Scale Behavior and Deposition as a Function of Polymeric Sulfide Inhibitor Concentration in Multiphase. In Proceedings of the International Corrosion Conference and Expo Series 2018, Phoenix, ZA, USA, 15–19 April 2018. [Google Scholar]
- Rahmati, H.; Mahboobi, F. Studying the Effect of the Concentration of PTFE Nanoparticles on the Tribological Behavior of Ni-P-PTFE Composite Coatings. Iran. J. Oil Gas Sci. Technol. 2015, 4, 67–75. [Google Scholar]
- Tajbakhsh, M.; Yaghobizadeh, O.; Nia, M.F. Investigation of the physical and mechanical properties of Ni–P and Ni–P–PTFE nanocomposite coatings deposited on aluminum alloy 7023. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2019, 233, 94–103. [Google Scholar] [CrossRef]
- Kinoshita, H.; Yonezawa, S.; Kim, J.; Kawai, M.; Takashima, M.; Tsukatani, T. Electroless Nickel-plating on PTFE fine particles. J. Fluor. Chem. 2008, 129, 416–423. [Google Scholar] [CrossRef]
- Balaji, R.; Pushpavanam, M.; Kumar, K.Y.; Subramanian, K. Electrodeposition of bronze–PTFE composite coatings and study on their tribological characteristics. Surf. Coat. Technol. 2006, 201, 3205–3211. [Google Scholar] [CrossRef]
- Sahoo, P. Tribological performance of electroless Ni-P coatings composite. In Materials and Surface Engineering; Woodhead Publishing: Swanston Cambridge, UK, 2012; pp. 163–205. [Google Scholar]
- Sheu, H.H.; Jian, S.Y.; Lin, M.H.; Hsu, C.I.; Hou, K.H.; der Ger, M. Electroless Ni-P/PTFE self-lubricating composite thin films applied for medium-carbon steel substrate. Int. J. Electrochem. Sci. 2017, 12, 5464–5482. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Hu, X.; Liu, F. Corrosion resistance properties of electroless nickel composite coatings. Electrochimica Acta 2004, 49, 4313–4319. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Electroless Ni-P-PTFE-Al2O3 dispersion nanocomposite coating for corrosion and wear resistance. J. Mater. Eng. Perform. 2014, 23, 142–151. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Corrosion and wear resistance study of Ni-P and Ni-P-PTFE nanocomposite coatings. Cent. Eur. J. Eng. 2011, 1, 234–243. [Google Scholar]
- American Society for Testing and Materials. ASTM G99-05, Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus; American Society for Testing and Materials: Philadelphia, PA, USA, 2010. [Google Scholar]
- Liu, Y.; Zhao, Q. Study of PTFE content and anti-corrosion properties of electroless Ni-P-PTFE coatings. Plat. Surf. Finish. 2004, 91, 48–51. [Google Scholar]
- Srinivasan, K.N.; John, S. Studies on electroless nickel–PTFE composite coatings. Surf. Eng. 2005, 21, 156–160. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Abdullah, A.M.; Hassan, M.K.; Mohamed, A.M.; Wang, C.; Jarjoura, G.; Farhat, Z. Synthesis, Characterization, and Application of Novel Ni-P-Carbon Nitride Nanocomposites. Coatings 2018, 8, 37. [Google Scholar] [CrossRef]
- Chen, S.Y.; Shi, Y.; Fan, H.; Liang, J.; Liu, C.S.; Sun, K. Synthesis of Ni-P-PTFE-nano-Al2O3 composite plating coating on 45 steel by electroless plating. J. Compos. Mater. 2012, 46, 1405–1416. [Google Scholar]
- Jiang, W.; Shen, L.; Wang, Z.; Wang, K.; Xu, M.; Tian, Z. Wear resistance of a Ni-PTFE composite coating strengthened with nano-SiC particles. Mater. Res. Express 2019, 6, 096443. [Google Scholar] [CrossRef]
- Blau, P. Effects of Tribosystem Variables on Friction. In Nondestructive Evaluation; CRC Press: Boca Raton, FL, USA, 2008; pp. 269–313. [Google Scholar]
- Ramalho, A.; Miranda, J. Friction and wear of electroless NiP and NiP+PTFE coatings. Wear 2005, 259, 828–834. [Google Scholar] [CrossRef]
- Hutchings, I.; Gee, M.; Santner, E. Friction and Wear. In Springer Handbook of Materials Measurement Methods, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 685–710. [Google Scholar]
- Taheri, R.; Oguocha, I.; Yannacopoulos, S. The tribological characteristics of electroless NiP coatings. Wear 2001, 249, 389–396. [Google Scholar] [CrossRef]

| Plating Method | Undercoat Ni-P * | Topcoat Ni-P+PTFE * | Sample ID | |
|---|---|---|---|---|
| P Content (wt%) | P (wt%) | PTFE (g/L) | ||
| Bath 1 | medium | Medium | 5 | ENP1 |
| Bath 2 | Medium | Medium | 10 | ENP2 |
| Bath 3 | Medium | Medium | 15 | ENP3 |
| Chemical | Range |
|---|---|
| Nickel sulphate (NiSO4 × 6H2O) | 0.1–0.11 M |
| Sodium hypophosphite (NaH2PO2 × H2O) | 0.19–0.28 M |
| Sodium citrate (C6H5Na3O7 × 2H2O) | 0.035 M |
| Acetic acid/ammonium hydroxide (NH4OH/CH₃COOH) | pH~5.5–6.2 |
| Surface Analysis Ni-P+PTFE | Ni | P | C | F | ||||
|---|---|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | wt% | at% | |
| ENP1 | 81.8 | 54.9 | 5.3 | 6.7 | 9.8 | 32.0 | 3.1 | 6.4 |
| ENP2 | 76.7 | 46.6 | 3.2 | 3.7 | 10.9 | 32.5 | 9.2 | 17.2 |
| ENP3 | 72.3 | 38.8 | 3.0 | 3.0 | 17.8 | 46.7 | 6.9 | 11.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppong Boakye, G.; Ormsdóttir, A.M.; Gunnarsson, B.G.; Irukuvarghula, S.; Khan, R.; Karlsdóttir, S.N. The Effect of Polytetrafluoroethylene (PTFE) Particles on Microstructural and Tribological Properties of Electroless Ni-P+PTFE Duplex Coatings Developed for Geothermal Applications. Coatings 2021, 11, 670. https://doi.org/10.3390/coatings11060670
Oppong Boakye G, Ormsdóttir AM, Gunnarsson BG, Irukuvarghula S, Khan R, Karlsdóttir SN. The Effect of Polytetrafluoroethylene (PTFE) Particles on Microstructural and Tribological Properties of Electroless Ni-P+PTFE Duplex Coatings Developed for Geothermal Applications. Coatings. 2021; 11(6):670. https://doi.org/10.3390/coatings11060670
Chicago/Turabian StyleOppong Boakye, Gifty, Arna María Ormsdóttir, Baldur Geir Gunnarsson, Sandeep Irukuvarghula, Raja Khan, and Sigrún Nanna Karlsdóttir. 2021. "The Effect of Polytetrafluoroethylene (PTFE) Particles on Microstructural and Tribological Properties of Electroless Ni-P+PTFE Duplex Coatings Developed for Geothermal Applications" Coatings 11, no. 6: 670. https://doi.org/10.3390/coatings11060670
APA StyleOppong Boakye, G., Ormsdóttir, A. M., Gunnarsson, B. G., Irukuvarghula, S., Khan, R., & Karlsdóttir, S. N. (2021). The Effect of Polytetrafluoroethylene (PTFE) Particles on Microstructural and Tribological Properties of Electroless Ni-P+PTFE Duplex Coatings Developed for Geothermal Applications. Coatings, 11(6), 670. https://doi.org/10.3390/coatings11060670







