Pulsed Laser Deposition of Epitaxial Non-Doped PbTiO3 Thin Films from PbO–TiO2 Mosaic Targets
Abstract
1. Introduction
2. Experiments
2.1. Fabrication and Characterization of Targets
2.2. Thin Films Growth
2.3. Characterization of Films
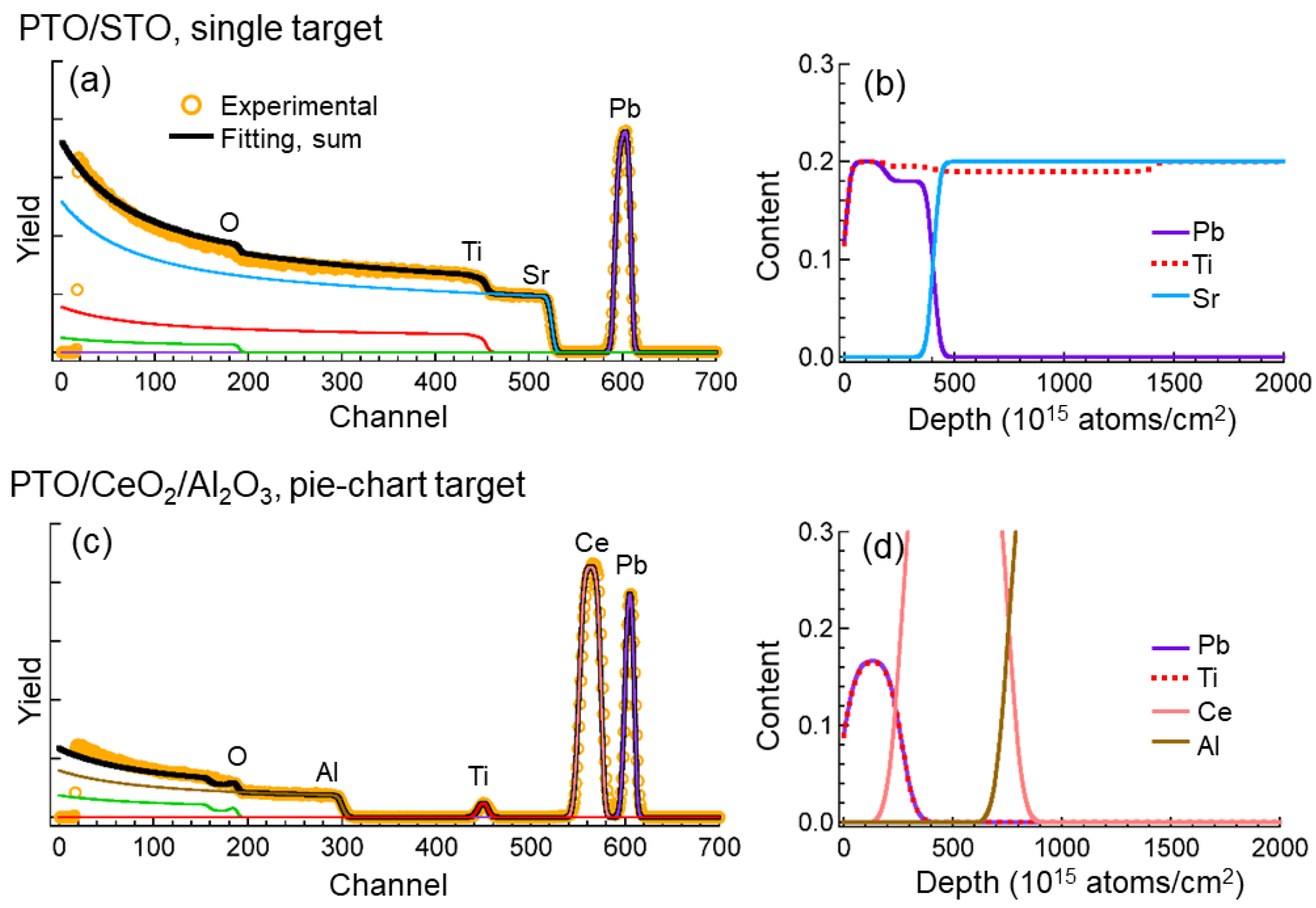
3. Results
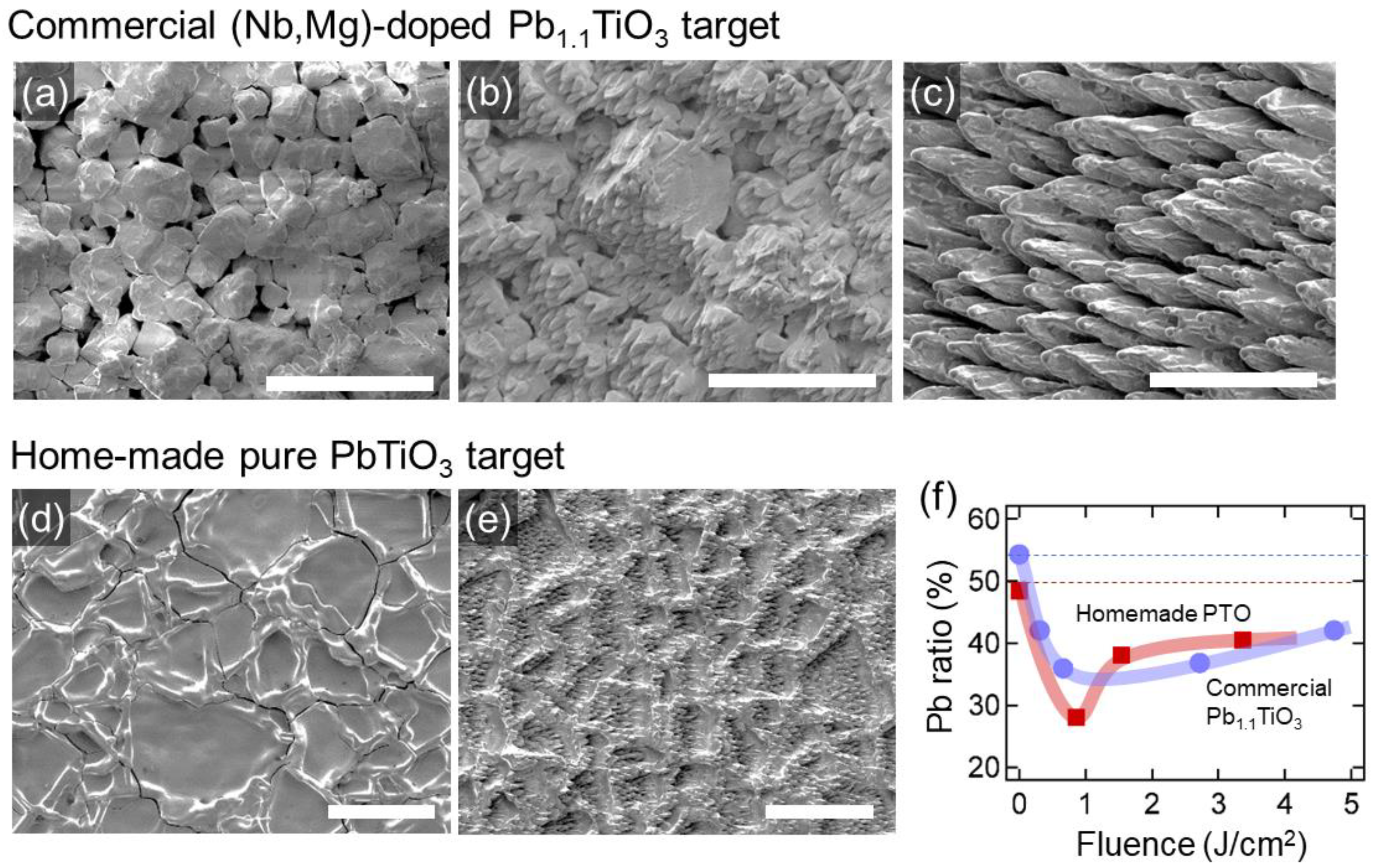
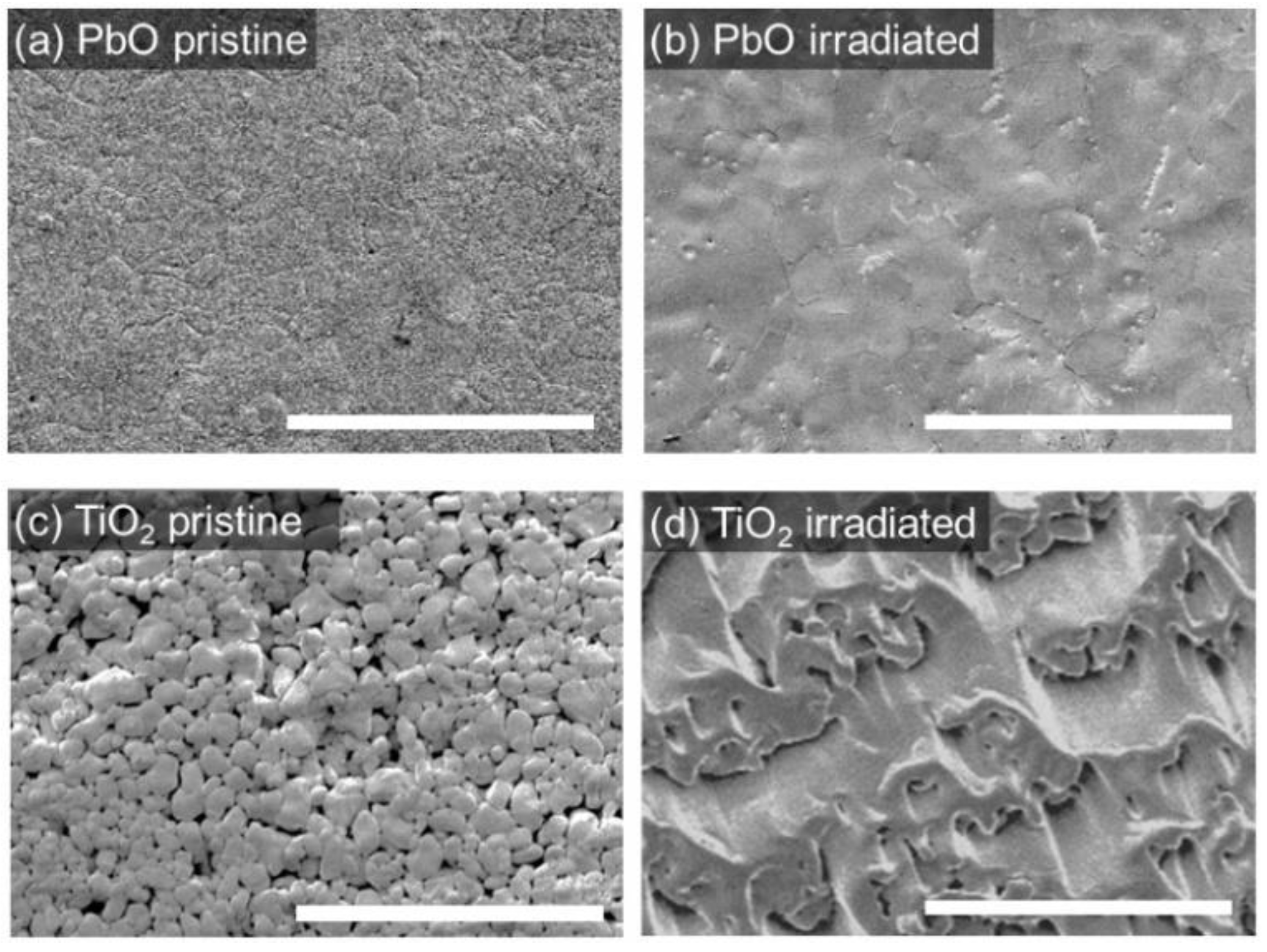
3.1. Morphology and Composition of the Target Surface
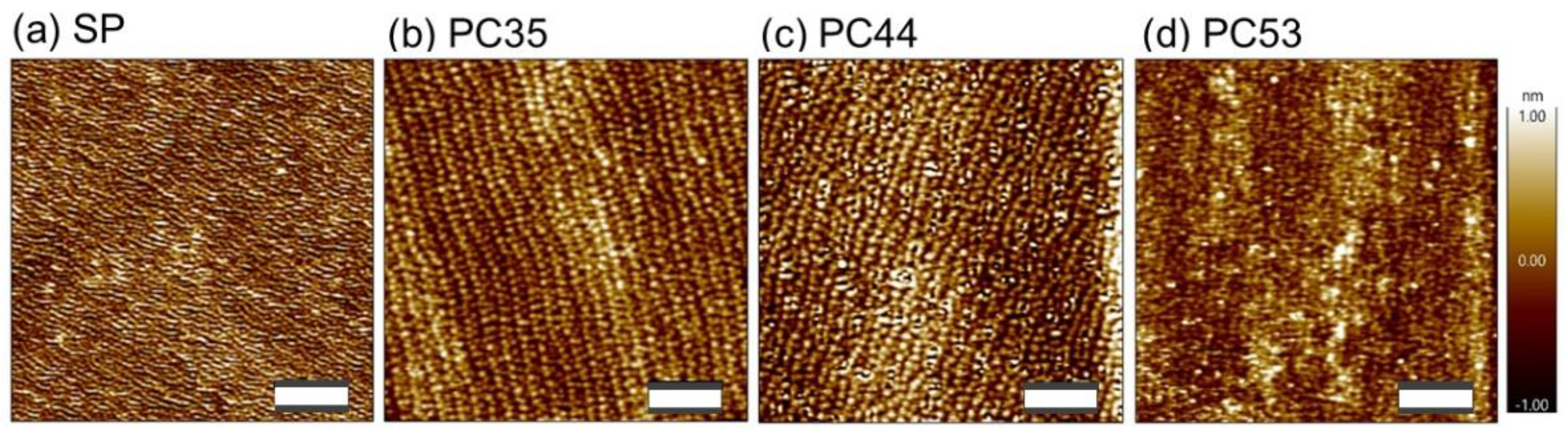
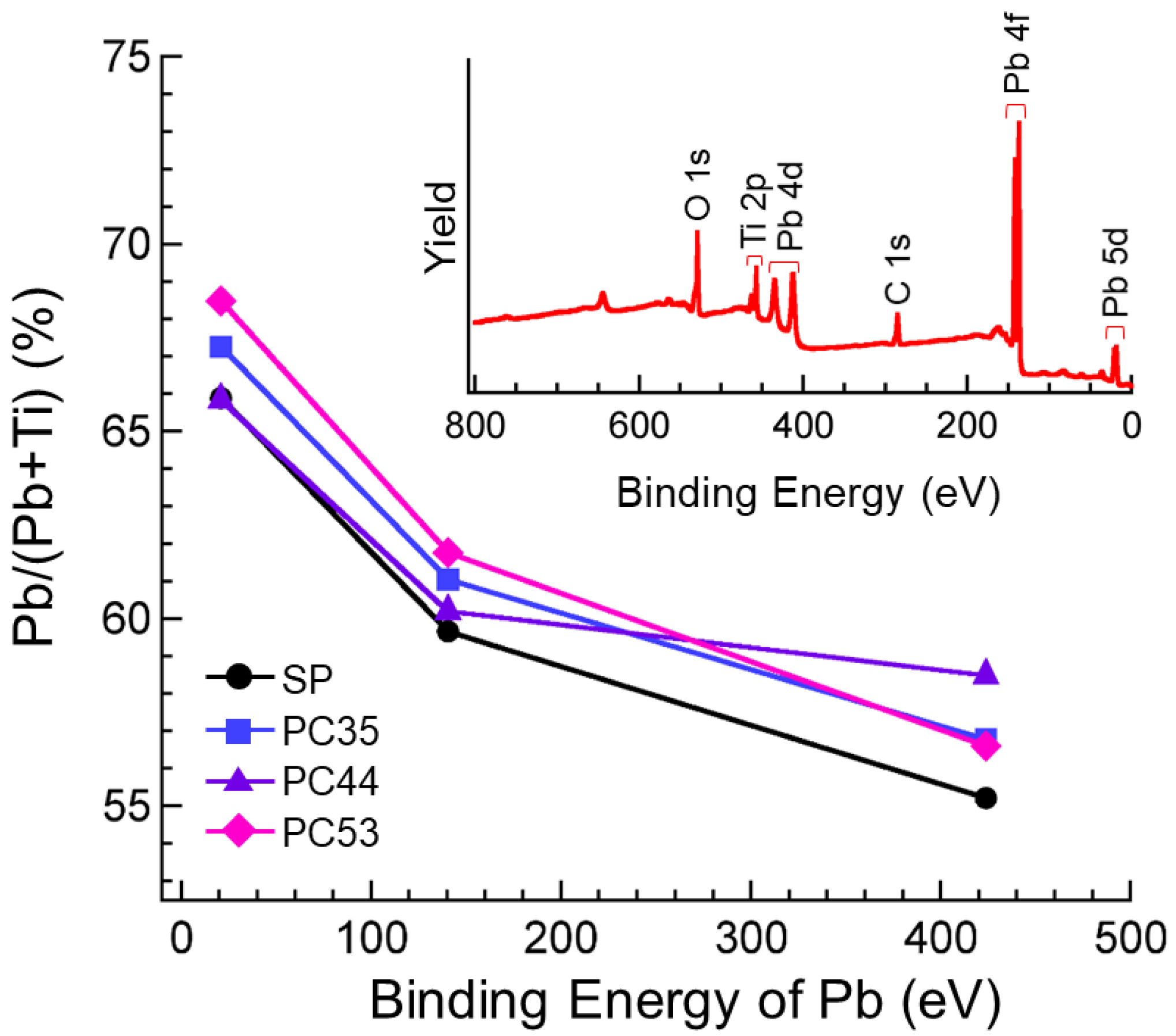
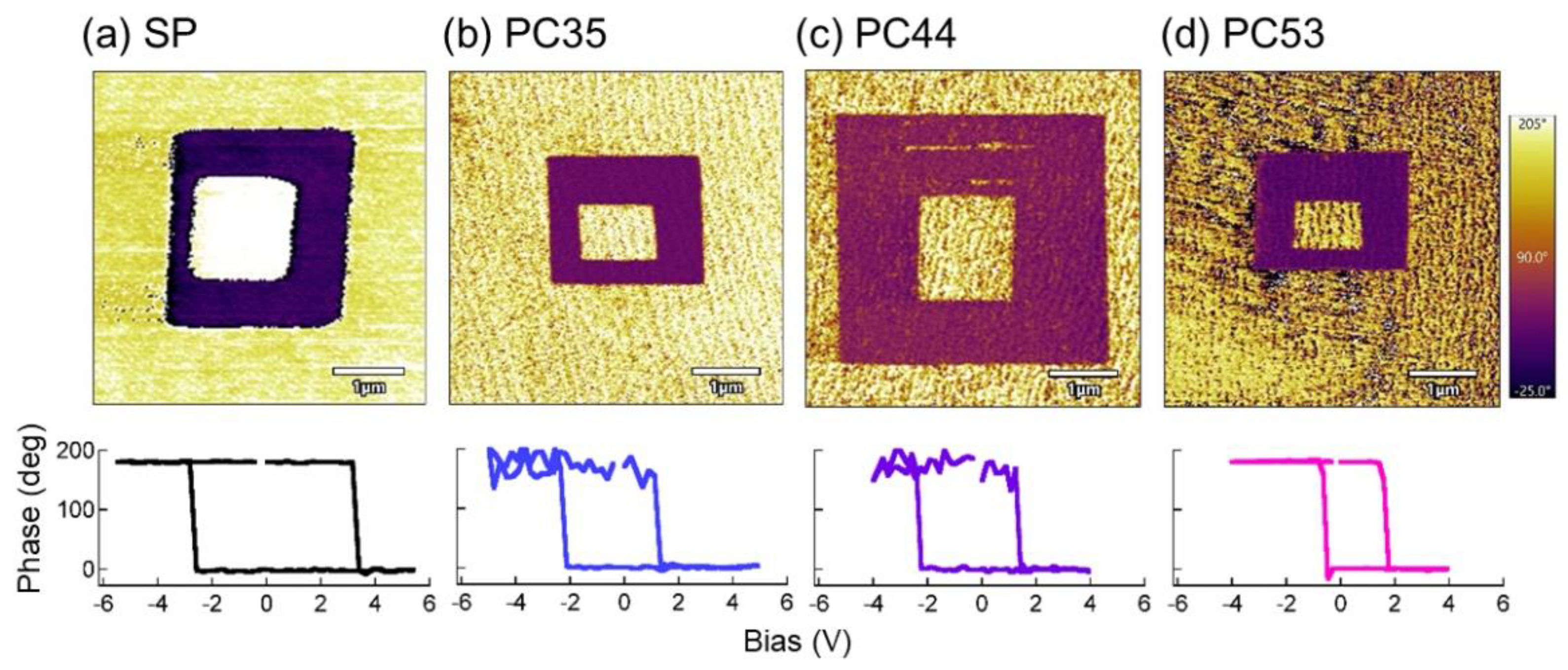
3.2. Properties of PTO Films
4. Discussion
- (i)
- selective ablation at the target (which would drive the film composition to Pb-rich);
- (ii)
- re-evaporation from the substrate (to Pb-poor);
- (iii)
- the CSC mechanism (to stoichiometry).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirane, G.; Hoshino, S.; Suzuki, K. X-ray study of the phase transition in lead titanate. Phys. Rev. 1950, 80, 1105–1106. [Google Scholar] [CrossRef]
- Jaffe, B.; Cook, W.R.; Jaffe, H. Piezoelectric Ceramics; Academic Press: London, UK, 1971. [Google Scholar]
- Ueda, I.; Ikegami, S. Piezoelectric properties of modified PbTiO3 ceramics. Jpn. J. Appl. Phys. 1968, 7, 236–242. [Google Scholar] [CrossRef]
- Carl, K. Ferroelectric properties and fatiguing effects of modified PbTiO3 ceramics. Ferroelectrics 1975, 9, 23–32. [Google Scholar] [CrossRef]
- Norton, D.P. Pulsed laser deposition of complex materials: Progress toward applications. In Pulsed Laser Deposition of Thin Films —Applications-led Growth of Functional Materials; Eason, R., Ed.; A John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 8–9. [Google Scholar]
- Martín, M.J.; Mendiola, J.; Zaldo, C. Pulsed laser deposition of Sm modified PbTiO3 ferroelectric thin films. Ferroelectr. Lett. 1997, 22, 101–105. [Google Scholar] [CrossRef]
- Choi, T.; Lee, J. Structural and dielectric properties of artificial PbZrO3/PbTiO3 superlattices grown by pulsed laser deposition. Thin Solid Films 2005, 475, 283–286. [Google Scholar] [CrossRef]
- Nishino, R.; Kozuka, Y.; Uchida, M.; Kagawa, F.; Kawasaki, M. Electrical conduction on the surface of ferroelectric PbTiO3 thin film inducedby electrolyte gating. Appl. Phys. Lett. 2018, 112, 051602. [Google Scholar] [CrossRef]
- Palkar, V.R.; Purandare, S.C.; Pai, S.P.; Chattopadhyay, S.; Apte, P.R.; Pinto, R.; Multani, M.S. c-axis oriented ferroelectric thin films of PbTiO3 on Si by pulsed laser ablation. Appl. Phys. Lett. 1996, 68, 1582–1584. [Google Scholar] [CrossRef]
- Wu, Y.M.; Lo, J.T. Growth of PbTiO3 thin film on Si(100) with Y2O3 and CeO2 buffer layer. Jpn. J. Appl. Phys. 1998, 37, 4943–4948. [Google Scholar] [CrossRef]
- Seo, J.; Ahn, Y.; Kim, W.H.; Son, J.Y. Local ferroelectric responses of epitaxial PbTiO3 thin films to heated atomic force microscopy. Mater. Lett. 2016, 168, 134–137. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.L.; Tang, Y.L.; Liu, Y.; Zhang, S.R.; Wang, Y.J.; Ma, X.L. Thickness-dependent a1/a2 domain evolution in ferroelectric PbTiO3 films. Acta Mater. 2017, 131, 123–130. [Google Scholar] [CrossRef]
- Lin, J.L.; Wang, Z.J.; Zhao, X.; Liu, W.; Zhang, Z.D. Microstructures and ferroelectric properties of PbTiO3/PbZrO3 superlattices deposited by pulse laser deposition. Ceram. Int. 2018, 44, 20664–20670. [Google Scholar] [CrossRef]
- Shin, H.W.; Son, J.Y. Nonvolatile ferroelectric memory based on PbTiO3 gated single-layer MoS2 field-effect transistor. Electron. Mater. Lett. 2018, 14, 59–63. [Google Scholar] [CrossRef]
- Feng, Y.; Tang, Y.; Ma, D.; Zhu, Y.; Zou, M.; Han, M.; Ma, J.; Ma, X. Thickness-dependent evolution of piezoresponses and stripe 90° domains in (101)-oriented ferroelectric PbTiO3 thin Films. ACS Appl. Mater. Interfaces 2018, 10, 24627–24637. [Google Scholar] [CrossRef]
- Christen, H.M.; Silliman, S.D.; Harshavardhan, K.S. Continuous compositional-spread technique based on pulsed-laser deposition and applied to the growth of epitaxial films. Rev. Sci. Instrum. 2001, 72, 2673–2678. [Google Scholar] [CrossRef][Green Version]
- Koinuma, H.; Takeuchi, I. Combinatorial solid-state chemistry of inorganic materials. Nat. Mater. 2004, 3, 429–438. [Google Scholar] [CrossRef]
- Maruyama, S.; Kubokawa, O.; Nanbu, K.; Fujimoto, K.; Matsumoto, Y. Combinatorial synthesis of epitaxial LiCoO2 thin films on SrTiO3 (001) via on-substrate sintering of Li2CO3 and CoO by pulsed laser deposition. ACS Comb. Sci. 2016, 18, 343–348. [Google Scholar] [CrossRef]
- Sakai, J.; Autret-Lambert, C.; Sauvage, T.; Courtois, B.; Wolfman, J.; Gervais, F. Epitaxial composition-graded perovskite films grown by a dual-beam pulsed laser deposition method. J. Cryst. Growth 2013, 380, 106–110. [Google Scholar] [CrossRef]
- Sakai, J.; Roque, J.M.C.; Vales-Castro, P.; Padilla-Pantoja, J.; Sauthier, G.; Catalan, G.; Santiso, J. Control of lateral composition distribution in graded films of soluble solid systems A1−xBx by partitioned dual-beam pulsed laser deposition. Coatings 2020, 10, 540. [Google Scholar] [CrossRef]
- Hussey, B.W.; Gupta, A. Synthesis of YBa2Cu3O7−d films from separate oxide targets. J. Appl. Phys. 1992, 72, 287–289. [Google Scholar] [CrossRef]
- Lee, Y.E.; Norton, D.P.; Budai, J.D. Enhanced photoluminescence in epitaxial ZnGa2O4: Mn thin-film phosphors using pulsed-laser deposition. Appl. Phys. Lett. 1999, 74, 3155–3157. [Google Scholar] [CrossRef]
- Funakoshi, H.; Fumoto, K.; Okuyama, M.; Hamakawa, Y. Fabrication of lead titanate thin film by laser ablation with alternate deposition of lead oxide and titanium oxide precursors. Jpn. J. Appl. Phys. 1994, 33, 5262–5264. [Google Scholar] [CrossRef]
- Koster, G.; Kropman, B.L.; Rijnders, G.J.H.M.; Blank, D.H.A.; Rogalla, H. Quasi-ideal strontium titanate crystal surfaces through formation of strontium hydroxide. Appl. Phys. Lett. 1998, 73, 2920–2922. [Google Scholar] [CrossRef]
- Doughty, C.; Findikoglu, A.T.; Venkatesan, T. Steady state pulsed laser deposition target scanning for improved plume stability and reduced particle density. Appl. Phys. Lett. 1995, 66, 1276–1278. [Google Scholar] [CrossRef]
- Dam, B.; Rector, J.; Chang, M.F.; Kars, S.; de Groot, D.G.; Griessen, R. Laser ablation threshold of YBa2Cu3O6+x. Appl. Phys. Lett. 1994, 65, 1581–1583. [Google Scholar] [CrossRef]
- Hase, T.; Hirata, K.; Amanuma, K.; Hosokawa, N.; Miyasaka, Y. Preparation of Pb(Zr, Ti)O3 thin films by multitarget sputtering. Jpn. J. Appl. Phys. 1994, 33, 5244–5248. [Google Scholar] [CrossRef]


| Sample Name | PbO:TiO2 Areal Ratio | Number of Pulses Irradiated on PbO:TiO2 per 1 Cycle | PTO Growth Rate (10−3 nm/pulse) | PTO out-of-Plane Lattice Spacing (Å) | PTO 002 Rocking Curve FWHM (deg) |
|---|---|---|---|---|---|
| SP | - | - | 28 | 4.158 | 0.080 |
| PC35 | 3:5 | 12:30 | 15 | 4.146 | 0.081 |
| PC44 | 4:4 | 18:24 | 14 | 4.146 | 0.087 |
| PC53 | 5:3 | 24:18 | 8.6 | 4.141 | 0.090 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, J.; Roque, J.M.C.; Vales-Castro, P.; Padilla-Pantoja, J.; Sauthier, G.; Santiso, J. Pulsed Laser Deposition of Epitaxial Non-Doped PbTiO3 Thin Films from PbO–TiO2 Mosaic Targets. Coatings 2021, 11, 662. https://doi.org/10.3390/coatings11060662
Sakai J, Roque JMC, Vales-Castro P, Padilla-Pantoja J, Sauthier G, Santiso J. Pulsed Laser Deposition of Epitaxial Non-Doped PbTiO3 Thin Films from PbO–TiO2 Mosaic Targets. Coatings. 2021; 11(6):662. https://doi.org/10.3390/coatings11060662
Chicago/Turabian StyleSakai, Joe, José Manuel Caicedo Roque, Pablo Vales-Castro, Jessica Padilla-Pantoja, Guillaume Sauthier, and José Santiso. 2021. "Pulsed Laser Deposition of Epitaxial Non-Doped PbTiO3 Thin Films from PbO–TiO2 Mosaic Targets" Coatings 11, no. 6: 662. https://doi.org/10.3390/coatings11060662
APA StyleSakai, J., Roque, J. M. C., Vales-Castro, P., Padilla-Pantoja, J., Sauthier, G., & Santiso, J. (2021). Pulsed Laser Deposition of Epitaxial Non-Doped PbTiO3 Thin Films from PbO–TiO2 Mosaic Targets. Coatings, 11(6), 662. https://doi.org/10.3390/coatings11060662







