Synthesis and Characterization of Polyaniline-Based Polymer Nanocomposites as Anti-Corrosion Coatings
Abstract
1. Introduction
2. Experimental Procedure
2.1. Reagents and Materials
2.2. Synthesis of PANI and Polymer Nanocomposites
2.2.1. PANI Synthesis by Rapid Mixing Oxidative Polymerization of Aniline
2.2.2. SiO2, TiO2, and CeO2/PANI Nanocomposites Synthesis by In Situ Chemical Oxidative Polymerization by Rapid Mixing
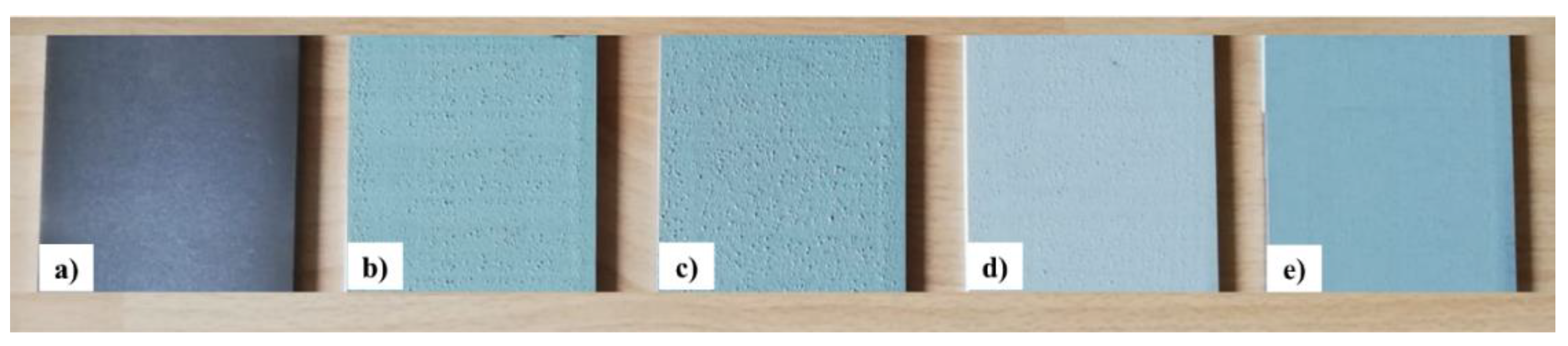
2.2.3. Coating Development
2.3. Characterization of PANI and Polymer Nanocomposites
2.4. Electrochemical Characterization
2.4.1. Tafel Polarization Curves
2.4.2. Electrochemical Impedance Spectroscopy (EIS)
3. Results and Discussion
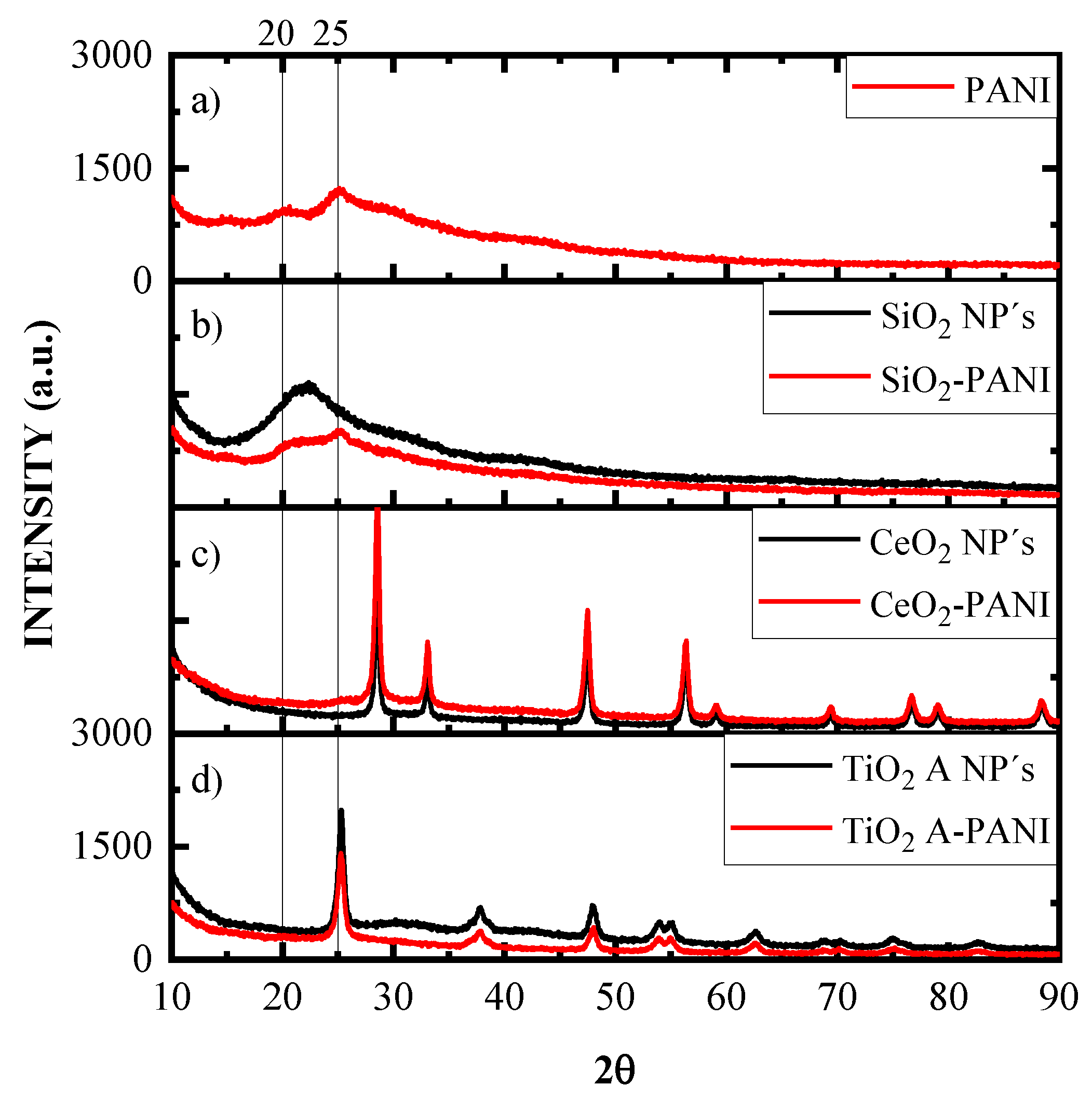
3.1. X-ray Diffraction (XRD) Study
3.2. FTIR Spectroscopic Study
3.3. UV-vis Study
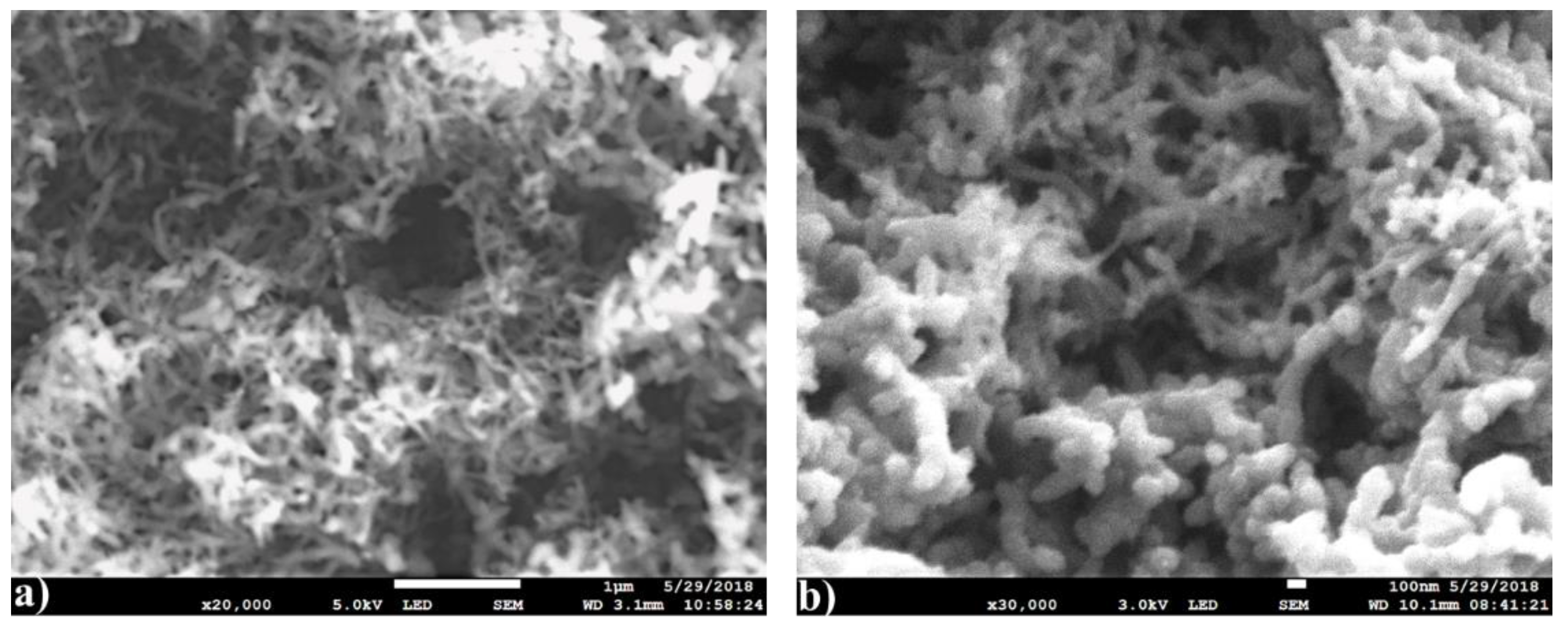
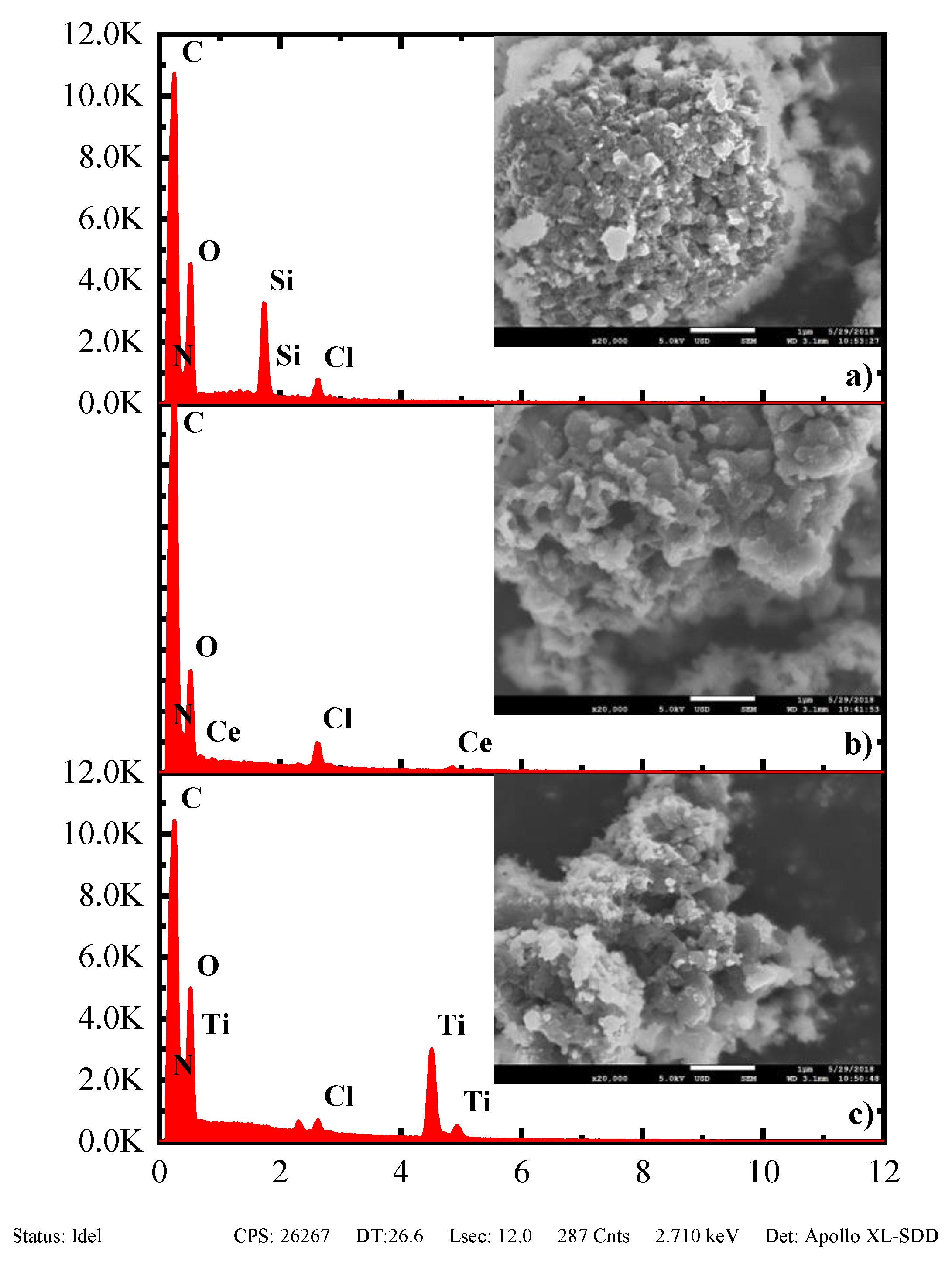
3.4. Morphological Study of PANI and Nanocomposites by SEM
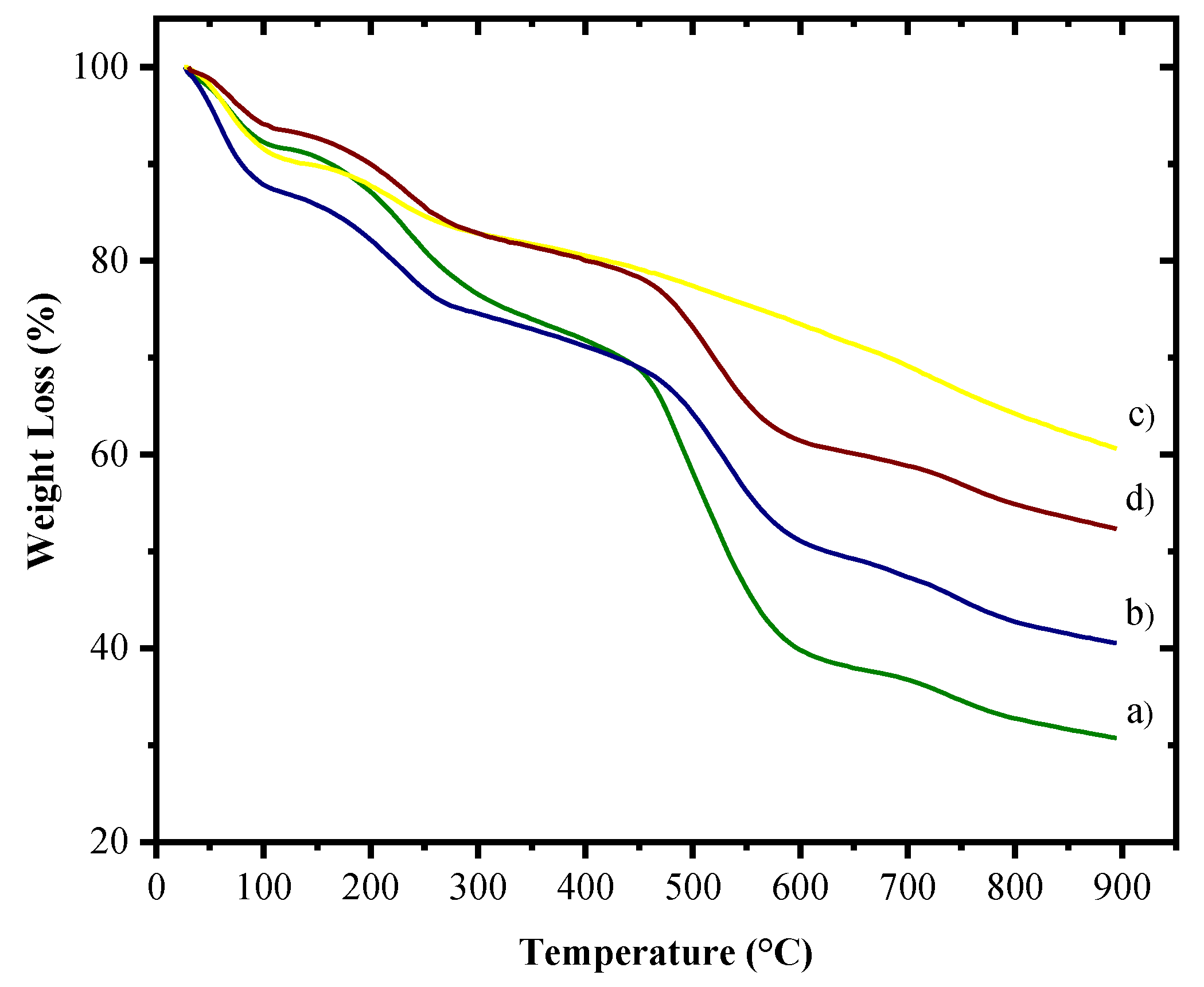
3.5. Thermogravimetric Analysis of Nanocomposites
3.6. Open Circuit Potential (OCP) Study
3.7. Tafel Curves
3.8. Electrochemical Impedance Spectroscopy
Anti-Corrosion Mechanism of Coatings
3.9. Immersion Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| ACS | Analytical grade |
| APS | Ammonium persulfate |
| AR | Alkydalic resin |
| CPE | Constant phase element |
| CPs | Conducting polymers |
| EDS | Energy-dispersive X-ray spectroscopy |
| EIS | Electrochemical impedance spectroscopy |
| ES | Protonated emeraldine |
| FRA | Frequency Response Analyzer System |
| FT-IR | Fourier transform infrared |
| LB | Non-protonated base |
| MO | Metal oxide |
| NP | Nanoparticle |
| NPs | Nanoparticles |
| OCP | Open circuit potential |
| PA | Polyacetylene |
| PANI | Polyaniline |
| PEDOT | Poly(ethylene dioxythiophene) |
| PPy | Polypyrrole |
| PTh | Polythiophene |
| SCE | Saturated calomel electrode |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| UV-vis | Ultraviolet-visible |
| XRD | X-ray diffraction |
| Symbols | |
| Coating capacitance | |
| Corrosion rate | |
| Corrosion potential | |
| Average steel equivalent weight | |
| Corrosion current | |
| Corrosion current of uncoated steel sample | |
| Corrosion current of coated steel sample | |
| Constant that defines the corrosion rate units | |
| Imaginary number | |
| CPE exponent | |
| Capacitance of coating pore | |
| Double-layer capacitance of the metal surface | |
| Diffusional capacitance | |
| Charge transfer resistance of a coated sample | |
| Charge transfer resistance of an uncoated sample | |
| Diffusional resistance | |
| Resistance of coating pore | |
| Constant value of CPE | |
| Real impedance | |
| Imaginary impedance | |
| Greek Symbols | |
| Anodic slope | |
| Cathodic slope | |
| Protection efficiency | |
| Steel density | |
| Angular frequency | |
References
- Riaz, U.; Nwaoha, C.; Ashraf, S.M. Recent advances in corrosion protective composite coatings based on conducting polymers and natural resource derived polymers. Prog. Org. Coat. 2014, 77, 743–756. [Google Scholar] [CrossRef]
- Wicks, Z.W., Jr. Corrosion Protection by Coatings, Federation of Societies for Coatings Technology; Wiley: Philadelphia, PA, USA, 1987; p. 7. [Google Scholar]
- Tiu, B.D.B.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. Reac. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Eftekhari, A. Nanostructured Conductive Polymers; Wiley: Chichester, UK, 2010. [Google Scholar]
- Muhammad, E.; Mullane, O.; Anthony, P.; Graeme, A. Storing energy in plastics: A review on conducting polymers & their role in electrochemical energy storage. RSC Adv. 2015, 5, 11611–11626. [Google Scholar]
- Snook, G.A.; Bhatt, A.I.; Abdelhamid, M.E.; Best, A.S. Role of H+ in Polypyrrole and Poly(3,4-ethylenedioxythiophene) Formation Using FeCl3∙6H2O in the Room Temperature Ionic Liquid, C4mpyrTFSI. Aust. J. Chem. 2012, 65, 1513–1522. [Google Scholar] [CrossRef]
- Otero, T.F. Biomimetic Conducting Polymers: Synthesis, Materials, Properties, Functions, and Devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar] [CrossRef]
- Camalet, J.L.; Lacroix, J.C.; Aeiyach, S.; Lacaze, P.C. Characterization of polyaniline films electrodeposited on mild steel in aqueous p-toluenesulfonic acid solution. J. Electroanal. Chem. 1998, 445, 117–124. [Google Scholar] [CrossRef]
- De Paoli, M.-A.; Waltman, R.J.; Diaz, A.F.; Bargon, J. Conductive composties form poly(vinyl chloride) and polypyrrole. J. Chem. Soc. Chem. Commun. 1984, 15, 1015–1016. [Google Scholar] [CrossRef]
- Pei, Q.; Bi, X. Electrochemical preparation of electrically conducting polyurethane/polyaniline composite. J. Appl. Polym. Sci. 1989, 38, 1819–1820. [Google Scholar] [CrossRef]
- Park, Y.H.; Park, C.R. Preparation of conducting polyacrylonitrile/polyaniline composite films by electrochemical synthesis and their electroactivity. Synth. Met. 2001, 118, 187–192. [Google Scholar] [CrossRef]
- Gazzoti, W.A., Jr.; Casalbore-Miceli, G.; Mitzakoff, S.; Geri, A.; Gallazi, M.C.; De Paoli, M.-A. Conductive polymer blends as electrochromic materials. Eletrochim. Acta. 1999, 44, 1965–1971. [Google Scholar] [CrossRef]
- Karakisla, M.; Sac, M.; Akbulut ak, U. Conductive polyaniline/poly(methylmethacrylate) films obtained by electropolymerization. J. Appl. Polym. Sci. 1996, 59, 1347–1354. [Google Scholar] [CrossRef]
- Talo, A.; Passiniemi, P.; Forsen, O.; Ylasaari, S. Polyaniline/epoxy coatings with good anti-corrosion properties. Synth. Met. 1997, 85, 1333–1334. [Google Scholar] [CrossRef]
- Talo, A.; Passiniemi, P.; Forsen, O.; Ylasaari, S. Corrosion protective polyaniline epoxy blend coatings on mild steel. Synth. Met. 1999, 102, 1394–1395. [Google Scholar] [CrossRef]
- Wessling, B.; Posdorfer, J. Nanostructures of the dispersed organic metal polyaniline responsible for macroscopic effects in corrosion protection. Synth. Met. 1999, 102, 1400–1401. [Google Scholar] [CrossRef]
- Wang, J. Polyaniline coatings: Anionic membrane nature and bipolar structures for anticorrosion. Synth. Met. 2002, 132, 53–56. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Clark, R.; Yang, S.C. Double-strand polyaniline. Synth. Met. 1997, 84, 67–68. [Google Scholar] [CrossRef]
- Yang, J.P.; Rannou, P.; Planes, J.; Pron, A.; Nechtschein, M. Preparation of low density polyethylene based polyaniline conducting polymer composites with low percolation threshold via extrusion. Synth. Met. 1998, 93, 169–173. [Google Scholar] [CrossRef]
- Ikkala, O.T.; Laakkso, J.; Väkipartab, K.; Ruohonen, H.; Järvinen, H.; Taka, T.; Passiniemi, P.; Osterholm, J.-E.; Cao, Y.; Andreatta, A.; et al. Counter-ion induced processibility of polyaniline: Conducting melt processible polymer blends. Synth. Met. 1995, 69, 97–100. [Google Scholar] [CrossRef]
- Jousseaume, V.; Morsli, M.; Bonnet, A.; Tesson, O.; Lefrant, S. Electrical properties of polyaniline–polystyrene blends above the percolation threshold. J. Appl. Polym. Sci. 1998, 67, 1205–1208. [Google Scholar] [CrossRef]
- Roichman, Y.; Titelman, G.I.; Silverstein, M.S.; Siegman, A.; Markis, M. Polyaniline synthesis: Influence of powder morphology on conductivity of solution cast blends with polystyrene. Synth. Met. 1999, 98, 201–209. [Google Scholar] [CrossRef]
- Pron, A.; Nicolau, Y.; Genoud, F.; Nechtschein, M. Flexible highly transparent, and conductive polyaniline-cellulose acetate composite films. J. Appl. Polym. Sci. 1997, 63, 971–977. [Google Scholar] [CrossRef]
- Lenz, D.M.; Delamar, M.; Ferreira, C.A. Methodology for zinc phosphate pigment incorporation into polypyrrole matrix. J. Appl. Electrochem. 2005, 35, 1051–1057. [Google Scholar] [CrossRef]
- Brusic, V.; Angelopoulos, M.; Graham, T. Use of polyaniline and its derivatives in corrosion protection of copper and silver. J. Electrochem. Soc. 1997, 144, 436–442. [Google Scholar] [CrossRef]
- Tallman, D.E.; Spinks, G.; Dominis, A.; Wallace, G.G.; Tallman, D.E. Electroactive conducting polymers for corrosion control. J. Solid State Electrochem. 2002, 6, 73–84. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D.; Stejskal, J. Organic coatings containing polyaniline and inorganic pigments as corrosion inhibitors. Prog. Org. Coat. 2008, 62, 105–116. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Yang, L.; Han, E. Characterization of protective performance of epoxy reinforced with nanometer-sized TiO2 and SiO2. Prog Org Coat. 2008, 62, 359. [Google Scholar] [CrossRef]
- Caldas, C.M.; Calheiros, L.F.; Soares, B.G. Silica-polyaniline hybrid materials prepared by inverse emulsion polymerization for epoxy-based anticorrosive coating. J. Appl. Polym. Sci. 2017, 134, 45505–45514. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Siju, C.R.; Debajyoti Mahanta Satish, P.; Giridhar, M. Conducting polyaniline-nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Galliano, P.; Damborenea, J.J.D.; Pascual, M.J.; Duran, A. Sol-Gel Coatings on 316L Steel for Clinical Applications. J. Sol Gel Sci. Technol. 1998, 13, 723–727. [Google Scholar] [CrossRef]
- Vasconcelos, D.C.L.; Carvalho, J.A.N.; Mantel, M.; Vasconcelos, W.L. Corrosion resistance of stainless steel coated with sol-gel silica. J. Non-Cryst. Solids 2000, 273, 135–139. [Google Scholar] [CrossRef]
- Nazeri, A.; Trzaskoma-Paulette, P.P.; Bauer, D. Synthesis and Properties of Cerium and Titanium Oxide Thin Coatings for Corrosion Protection of 304 Stainless Steel. J. Sol Gel Sci. Technol. 1997, 10, 317–331. [Google Scholar] [CrossRef]
- Lukachova, L.V.; Shkerin, E.A.; Puganova, E.A.; Karyakina, E.E.; Kiseleva, S.G.; Orlov, A.V.; Karpacheva, G.P.; Karyakin, A.A. Electroactivity of chemically synthesized polyaniline in neutral and alkaline aqueous solutions: Role of self-doping and external doping. J. Electroanal. Chem. 2003, 544, 59–63. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A Polymer with Many Interesting Intrinsic Redox States. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Ciric-Marjanovic, G. Recent Advances in Polyaniline Research: Polymerization Mechanisms, Structural Aspects, Properties and Applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Huang, J.; Kaner, B. Nanofiber Formation in the Chemical Polymerization of Aniline: A Mechanistic Study. Angew. Chem. Int. Ed. 2004, 43, 5817–5821. [Google Scholar] [CrossRef]
- Khatoon, H.; Ahmed, S. A review on conducting polymer reinforced polyurethane composites. Ind. Eng. Chem. Res. 2017, 53, 1–22. [Google Scholar] [CrossRef]
- Pawar, S.G.; Patil, S.L.; Chougule, M.A.; Mane, A.T.; Jundale, D.M.; Patil, V.B. Synthesis and Characterization of Polyaniline: TiO2 Nanocomposites. Int. J. Polymer. Mater. 2010, 59, 777–785. [Google Scholar] [CrossRef]
- Mostaferi, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. 2012, 22, 273–280. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, X. Formation of Polyaniline Nanofibers: A Morphological Study. J. Phys. Chem. B 2008, 112, 1157–1162. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Preparation of polyaniline–TiO2 composite and its comparative corrosion protection performance with polyaniline. Synth. Met. 2007, 157, 205–213. [Google Scholar] [CrossRef]
- Anilkumar, P.; Jayakannan, M. New Renewable Resource Amphiphilic Molecular Design for Size-Controlled and Highly Ordered Polyaniline Nanofibers. Langmuir 2006, 22, 5952–5957. [Google Scholar] [CrossRef]
- Kumar, E.; Selvarajan, P.; Muthuraj, D. Preparation and characterization of polyaniline/cerium dioxide (CeO2) nanocomposite via in situ polymerization. J. Mater. Sci. 2012, 47, 7148–7156. [Google Scholar] [CrossRef]
- Najafi, V.; Ahmadi, E.; Ziaee, F.; Omidian, H.; Sedaghat, H. Polyaniline-Modified TiO2, a Highly Effective Photo-catalyst for Solid-Phase Photocatalytic Degradation of PVC. J. Polym. Environ. 2019, 27, 784–793. [Google Scholar] [CrossRef]
- Babazadeh, M.; Zalloi, F.; Olad, A. Fabrication of Conductive Polyaniline Nanocomposites Based on Silica Nanoparticles via In-Situ Chemical Oxidative Polymerization Technique. Synth. React. Inorg. Met. Org. Nano Metal Chem. 2015, 45, 86–91. [Google Scholar] [CrossRef]
- Bian, C.; Yu, Y.; Xue, G. Synthesis of Conducting Polyaniline/TiO2 Composite Nanofibres by One-step In Situ Polymerization Method. Appl. Polym. Sci. 2006, 104, 21–26. [Google Scholar] [CrossRef]
- Luvita, N.R.D.; Kusumawati, D.H.; Putri, N.P.; Supardi, Z.A. Synthesis of PANi-SiO2 Nanocomposite with In-Situ Polymerization Method: Nanoparticle Silica (NPS) Amorphous and Crystalline Phase. J. Phys. Conf. Ser. 2018, 997, 012052. [Google Scholar]
- Babitha, K.K.; Sreedevi, A.; Priyanka, K.P.; Sabu, B.; Varghese, T. Structural characterization and optical studies of CeO2 nanoparticles synthesized by chemical precipitation. IJPAP 2015, 53, 596–603. [Google Scholar]
- Liu, F.; He, Y.; Huh, J. Study on the synthesis and characterization of PANi/nano-CeO2 composites. In Solid State Phenomena; Trans Tech Publications Ltd: Stafa-Zurich, Switzerland, 2007; pp. 287–290. [Google Scholar]
- Li, X.; Dai, N.; Wang, G.; Song, X. Water-Dispersible Conducting Polyaniline/Nano-SiO2 Composites without Any Stabilizer. J. Appl. Polym. Sci. 2008, 107, 403–408. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Z.; Wang, J. Thermal stability of several polyaniline/rare earth oxide composites: Polyaniline/CeO2 composites. J. Therm. Anal. Calorim. 2011, 107, 1199–1203. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Bian, C.; He, J.; Xu, N.; Xue, G. Surface modification of TiO2 nanoparticles by polyaniline. Appl. Surf. Sci. 2003, 217, 16–22. [Google Scholar] [CrossRef]
- Mareci, D.; Cailean, A.; Ciurescu, G.; Sutiman, D. Electrochemical Determination of the Corrosion Resistance of NiCr Dental Casting Alloys. Open Corros. J. 2010, 3, 45–53. [Google Scholar] [CrossRef]
- Alam, A.; Sherif, S.M.; Al-Zahrani, S.M. Fabrication of various epoxy coatings for offshore applications and evaluating their mechanical properties and corrosion behavior. Int. J. Electrochem. Sci. 2013, 8, 3121–3131. [Google Scholar]
- Balaji, J.; Sethuraman, M.G. Chitosan-doped-hybrid/TiO2 nanocomposite based sol-gel coating forthe corrosion resistance of aluminum metal in 3.5% NaCl medium. Int. J. Biol. Macromol. 2017, 104, 1730–1739. [Google Scholar]
- Grgur, B.N.; Gvozdenović, M.M.; Mišković-Stanković, V.B.; Kačarević-Popović, Z. Corrosion behavior and thermal stability of electrodeposited PANI/epoxy coating system on mild steel in sodium chloride solution. Prog. Org. Coat. 2016, 56, 214–219. [Google Scholar] [CrossRef]
- Mansfeld, F. Recording and Analysis of AC Impedance Data for Corrosion Studies. J. Appl. Sci. Eng. 1981, 37, 301–307. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthukrishnan, S.; Venkatachari, G.; Trivedi, D.C. Corrosion protection of steel by polyaniline (PANI) pigmented paint coating. Prog. Org. Coat. 2005, 53, 297–301. [Google Scholar] [CrossRef]
- Sun, Q.; Schork, F.J.; Deng, Y. Water-based polymer/clay nanocomposite suspension for improving water and moisture barrier in coating. Compos. Sci. Technol. 2007, 67, 1823–1829. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Sonawane, N.; Siju, C.R. Epoxy powder coatings containing polyaniline for enhanced corrosion protection. Prog. Org. Coat. 2009, 64, 383–386. [Google Scholar] [CrossRef]
- Wicks, Z.W.; Jone, F.N.; Pappas, S.P. Organic Coatings: Science and Technology; Wiley: New Jersey, NJ, USA, 2007. [Google Scholar]
- Sasikumar, Y.; Kumar, A.M.; Gasem, Z.M.; Ebenso, E.E. Hybrid nanocomposite from aniline and CeO2 nanoparticles: Surface protective performance on mild steel in acidic environment. Appl. Surf. Sci. 2015, 330, 207–215. [Google Scholar] [CrossRef]







| Samples | Tafel Parameters | |||||
|---|---|---|---|---|---|---|
(V) | (μA cm−2) | (V dec−1) | (V dec−1) | (mpy) | (%) | |
| 1018 STEEL | −0.698 | 2.418 | 0.515 | 0.060 | 1.106 | - |
| PANI/RA | −0.522 | 0.079 | 0.225 | 0.188 | 0.036 | 97.10 |
| SiO2-PANI/RA | −0.479 | 0.073 | 0.385 | 0.124 | 0.033 | 97.35 |
| CeO2-PANI/RA | −0.637 | 0.147 | 0.194 | 0.119 | 0.067 | 94.28 |
| TiO2A-PANI/RA | −0.362 | 0.018 | 0.195 | 0.192 | 0.008 | 98.63 |
| Samples | EIS Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1018 STEEL | 49.5 | 1.34 × 10−3 | 0.80 | 70.54 | 1.72 × 10−3 | 0.81 | 1143 | - | - | - | |
| PANI/RA | 157 | 6.71 × 10−9 | 0.79 | 3400 | 6.63 × 10−6 | 0.80 | 1.78 × 104 | 4.74 × 10−6 | 0.60 | 1.54 × 105 | 93.58 |
| SiO2-PANI/RA | 203 | 9.67 × 10−7 | 0.44 | 5028 | 2.36 × 10−7 | 0.85 | 4.556 × 104 | 4.56 × 10−5 | 0.40 | 1.11 × 105 | 97.49 |
| CeO2-PANI/RA | 194 | 4.75 × 10−7 | 0.71 | 3700 | 6.40 × 10−7 | 0.67 | 3.804 × 104 | 2.11 × 10−6 | 0.78 | 5.68 × 104 | 96.99 |
| TiO2A-PANI/RA | 168 | 1.06 × 10−8 | 0.77 | 6140 | 6.40 × 10−7 | 0.44 | 6.882 × 104 | 8.83 × 10−6 | 0.48 | 3.84 × 105 | 98.34 |
| Samples | Tafel Parameters | Immersion Time (h) | |||
|---|---|---|---|---|---|
| 3 | 8 | 24 | 72 | ||
| PANI/RA | (μA cm−2) | 0.079 | 0.112 | 0.070 | 0.075 |
| (V) | −0.522 | −0.596 | −0.679 | −0.680 | |
| (V dec−1) | 0.225 | 0.174 | 0.108 | 0.081 | |
| (V dec−1) | 0.188 | 0.197 | 0.205 | 0.149 | |
| (%) | 97.10 | 95.73 | 97.48 | 97.27 | |
| SiO2-PANI/RA | (μA cm−2) | 0.073 | 0.123 | 0.096 | 0.069 |
| (V) | −0.479 | −0.650 | −0.726 | −0.699 | |
| (V dec−1) | 0.385 | 0.172 | 0.117 | 0.096 | |
| (V dec−1) | 0.124 | 0.189 | 0.224 | 0.170 | |
| (%) | 97.35 | 95.28 | 96.40 | 97.52 | |
| CeO2-PANI/RA | (μA cm−2) | 0.147 | 0.163 | 0.135 | 0.227 |
| r (V) | −0.637 | −0.683 | −0.737 | −0.750 | |
| (V dec−1) | 0.194 | 0.162 | 0.110 | 0.071 | |
| (V dec−1) | 0.119 | 0.122 | 0.167 | 0.162 | |
| (%) | 94.28 | 93.62 | 94.78 | 90.96 | |
| TiO2A-PANI/RA | (μA cm−2) | 0.018 | 0.139 | 0.074 | 0.059 |
| (V) | −0.362 | −0.498 | −0.616 | −0.662 | |
| (V dec−1) | 0.195 | 0.264 | 0.149 | 0.119 | |
| (V dec−1) | 0.192 | 0.192 | 0.232 | 0.221 | |
| (%) | 99.63 | 94.61 | 97.31 | 97.93 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel-Olivares, F.R.; Arce-Estrada, E.M.; Cabrera-Sierra, R. Synthesis and Characterization of Polyaniline-Based Polymer Nanocomposites as Anti-Corrosion Coatings. Coatings 2021, 11, 653. https://doi.org/10.3390/coatings11060653
Rangel-Olivares FR, Arce-Estrada EM, Cabrera-Sierra R. Synthesis and Characterization of Polyaniline-Based Polymer Nanocomposites as Anti-Corrosion Coatings. Coatings. 2021; 11(6):653. https://doi.org/10.3390/coatings11060653
Chicago/Turabian StyleRangel-Olivares, Francisco R., Elsa M. Arce-Estrada, and Román Cabrera-Sierra. 2021. "Synthesis and Characterization of Polyaniline-Based Polymer Nanocomposites as Anti-Corrosion Coatings" Coatings 11, no. 6: 653. https://doi.org/10.3390/coatings11060653
APA StyleRangel-Olivares, F. R., Arce-Estrada, E. M., & Cabrera-Sierra, R. (2021). Synthesis and Characterization of Polyaniline-Based Polymer Nanocomposites as Anti-Corrosion Coatings. Coatings, 11(6), 653. https://doi.org/10.3390/coatings11060653






