Enhanced Methanol Oxidation Activity of PtRu/C100−xMWCNTsx (x = 0–100 wt.%) by Controlling the Composition of C-MWCNTs Support
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Catalyst Supports on Structure–Composition and Methanol Oxidation Performance of PtRu/C100−xMWCNTsx (x = 0, 40, 100%)
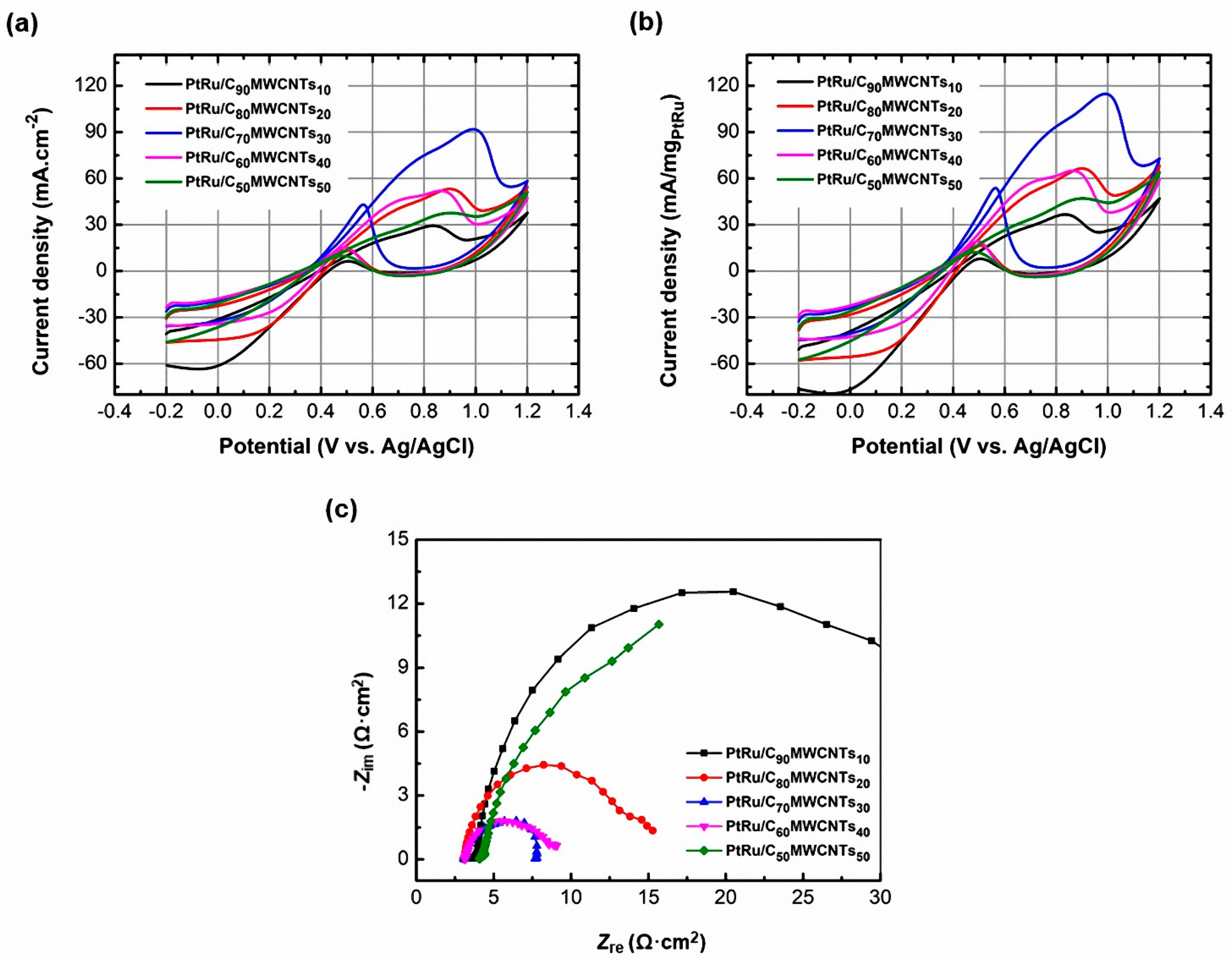
3.2. Effect of MWCNTs Percentage (x = 10–50 wt.%) in PtRu/C100−xMWCNTsx Samples on the Methanol Oxidation Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bokach, D.; de la Fuente, J.L.G.; Tsypkin, M.; Ochal, P.; Endsjø, I.C.; Tunold, R.; Sunde, S.; Seland, F. High-temperature electrochemical characterization of Ru core Pt shell fuel cell catalyst. Fuel Cells 2011, 11, 735–744. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, H.; Du, L.; Yin, G.; Tang, Z.; Liu, S. Three dimensional N-doped graphene/PtRu nanoparticle hybrids as high performance anode for direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 3719–3724. [Google Scholar] [CrossRef]
- Almeida, T.S.; Garbim, C.; Silva, R.G.; De Andrade, A.R. Addition of iron oxide to Pt-based catalyst to enhance the catalytic activity of ethanol electrooxidation. J. Electroanal. Chem. 2017, 796, 49–56. [Google Scholar] [CrossRef]
- Lu, X.; Hu, J.; Foord, J.S.; Wang, Q. Electrochemical deposition of Pt–Ru on diamond electrodes for the electrooxidation of methanol. J. Electroanal. Chem. 2011, 654, 38–43. [Google Scholar] [CrossRef]
- Moura, A.S.; Fajín, J.L.C.; Mandado, M.; Cordeiro, M.N.D.S. Ruthenium-platinum catalysts and direct methanol fuel cells (DMFC): A review of theoretical and experimental breakthroughs. Catalysts 2017, 7, 47. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, R.; Chen, W. Graphene-supported nanoelectrocatalysts for fuel cells: Synthesis, properties, and applications. Chem. Rev. 2014, 114, 5117–5160. [Google Scholar] [CrossRef] [PubMed]
- Muthuswamy, N.; de la Fuente, J.L.G.; Tran, D.T.; Walmsley, J.; Tsypkin, M.; Raaen, S.; Sunde, S.; Rønning, M.; Chen, D. Ru@Pt core–shell nanoparticles for methanol fuel cell catalyst: Control and effects of shell composition. Int. J. Hydrogen Energy 2013, 38, 16631–16641. [Google Scholar] [CrossRef]
- Arun, A.; Gowdhamamoorthi, M.; Ponmani, K.; Kiruthika, S.; Muthukumaran, B. Electrochemical characterization of Pt–Ru–Ni/C anode electrocatalyst for methanol electrooxidation in membraneless fuel cells. RSC Adv. 2015, 5, 49643–49650. [Google Scholar] [CrossRef]
- Ávila-García, I.; Ramírez, C.; Hallen López, J.M.; Arce Estrada, E.M. Electrocatalytic activity of nanosized Pt alloys in the methanol oxidation reaction. J. Alloy. Compd. 2010, 495, 462–465. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Paganin, V.A.; Gonzalez, E.R.; Montemora, M.F.; Tacchini, I.; Anson, A.; Salvador, M.A.; Ferreira, P.; Figueiredo, F.M.L.; Ferreira, M.G.S. Characterization and performance evaluation of Pt–Ru electrocatalysts supported on different carbon materials for direct methanol fuel cells. Int. J. Hydrog. Energy 2013, 38, 910–920. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, J.Y.; Chen, W.; Han, M.; Gan, L.M. Physical and electrochemical characterizations of microwave-assisted polyol preparation of carbon-supported PtRu nanoparticles. Langmuir 2004, 20, 181–187. [Google Scholar] [CrossRef]
- Neto, A.O.; Dias, R.R.; Tusi, M.M.; Linardi, M.; Spinacé, E.V. Electro-oxidation of methanol and ethanol using PtRu/C, PtSn/C and PtSnRu/C electrocatalysts prepared by an alcohol-reduction process. J. Power Sources 2017, 166, 87–91. [Google Scholar] [CrossRef]
- Baronia, R.; Goel, J.; Tiwari, S.; Singh, P.; Singh, D.; Singh, S.P.; Singhal, S.K. Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. Int. J. Hydrog. Energy 2017, 42, 10238–10247. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Garcia-Bernabé, A.; Compañ, V. Bimetallic Pt-M electrocatalysts supported on single-wall carbon nanotubes for hydrogen and methanol electrooxidation in fuel cells applications. Int. J. Hydrog. Energy 2018, 43, 872–884. [Google Scholar] [CrossRef]
- Wang, Z.N.; Huo, S.H.; Zhou, P.X. Rich-grain-boundary PtRuNi with network structure as efficient catalysts for methanol oxidation reaction. Int. J. Electrochem. Sci. 2019, 14, 10576–10581. [Google Scholar] [CrossRef]
- Wang, Z.B.; Zuo, P.J.; Yin, G.P. Investigations of compositions and performance of PtRuMo/C ternary catalysts for methanol electrooxidation. Fuel Cells 2009, 9, 106–113. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-based catalysts on various carbon supports and conducting polymers for direct methanol fuel cell applications: A review. Nanoscale Res. Lett. 2018, 13, 1–25. [Google Scholar] [CrossRef]
- Rambabu, G.; Bhat, D.S.; Figueiredo, F.M. Carbon nanocomposite membrane electrolytes for direct methanol fuel cells—A concise review. Nanomaterials 2019, 9, 1292. [Google Scholar] [CrossRef] [PubMed]
- Yaldagard, M.; Jahanshahi, M.; Seghatoleslami, N. Carbonaceous nanostructured support materials for low temperature fuel cell electrocatalysts—A review. World J. Nano Sci. Eng. 2013, 3, 121–153. [Google Scholar] [CrossRef]
- Maass, S.; Finsterwalder, F.; Frank, G.; Hartmann, R.; Merten, C. Carbon support oxidation in PEM fuel cell cathodes. J. Power Sources 2008, 176, 444–451. [Google Scholar] [CrossRef]
- Kannan, R.; Pillai, V.K. Applications of carbon nanotubes in polymer electrolyte membrane fuel cells. J. Indian Inst. Sci. 2009, 89, 425–436. [Google Scholar]
- Cui, Z.; Liu, C.; Liao, J.; Xing, W. Highly active PtRu catalysts supported on carbon nanotubes prepared by modified impregnation method for methanol electro-oxidation. Electrochim. Acta 2008, 53, 7807–7811. [Google Scholar] [CrossRef]
- Fard, H.F.; Khodaverdi, M.; Pourfayaz, F.; Ahmadi, M.H. Application of N-doped carbon nanotube-supported Pt–Ru as electrocatalyst layer in passive direct methanol fuel cell. Int. J. Hydrog. Energy 2020, 45, 25307–25316. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yang, S.Y.; Li, S.M.; Tien, H.W.; Hsiao, S.T.; Liao, W.H.; Liu, C.H.; Chang, K.H.; Ma, C.C.M.; Hu, C.C. Three-dimensionally porous graphene–carbon nanotube composite-supported PtRu catalysts with an ultrahigh electrocatalytic activity for methanol oxidation. Electrochim. Acta 2013, 87, 261–269. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Grüner, G. Percolation in transparent and conducting carbon nanotube networks. Nano Lett. 2004, 4, 2513–2517. [Google Scholar] [CrossRef]
- Do, J.W.; Chang, N.N.; Estrada, D.; Lian, F.; Cha, H.; Duan, X.J.; Haasch, R.T.; Pop, E.; Girolami, G.S.; Lyding, J.W. Solution-mediated selective nanosoldering of carbon nanotube junctions for improved device performance. ACS Nano 2015, 9, 4806–4813. [Google Scholar] [CrossRef]
- Alegre, C.; Calvillo, L.; Moliner, R.; González-Expósito, J.A.; Guillén-Villafuerte, O.; Huerta, M.M.; Pastor, E.; Lázaro, M.J. Pt and PtRu electrocatalysts supported on carbon xerogels for direct methanol fuel cells. J. Power Sources 2011, 196, 4226–4235. [Google Scholar] [CrossRef]
- Kang, S.; Lim, S.; Peck, D.H.; Kim, S.K.; Jung, D.H.; Hong, S.H.; Jung, H.G.; Shul, Y. Stability and durability of PtRu catalysts supported on carbon nanofibers for direct methanol fuel cells. Int. J. Hydrog. Energy 2012, 37, 4685–4693. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Alaje, T.O.; Scott, K.K. Highly stable Pt–Ru nanoparticles supported on three-dimensional cubic ordered mesoporous carbon (Pt–Ru/CMK-8) as promising electrocatalysts for methanol oxidation. J. Phys. Chem. C 2012, 116, 2630–2638. [Google Scholar] [CrossRef]
- Lee, S.H.; Kakati, N.; Jee, S.H.; Maiti, J.; Yoon, Y.S. Hydrothermal synthesis of PtRu nanoparticles supported on graphene sheets for methanol oxidation in direct methanol fuel cell. Mater. Lett. 2011, 65, 3281–3284. [Google Scholar] [CrossRef]
- Reddy, G.V.; ORaghavendra, P.; Ankamwar, B.; Chandana, P.S.; Kumar, S.S.; Sarma, L.S. Ultrafine Pt–Ru bimetallic nanoparticles anchored on reduced graphene oxide sheets as highly active electrocatalysts for methanol oxidation. Mater. Chem. Front. 2017, 1, 757–766. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Xue, H.; Wang, Z.; Hu, H. Methanol electrocatalytic oxidation on highly dispersed platinum–ruthenium/graphene catalysts prepared in supercritical carbon dioxide–methanol solution. RSC Adv. 2012, 2, 9651–9659. [Google Scholar] [CrossRef]
- Cheng, Y. Highly effective and CO-tolerant PtRu electrocatalysts supported on poly(ethyleneimine) functionalized carbon nanotubes for direct methanol fuel cells. Electrochim. Acta 2013, 99, 124–132. [Google Scholar] [CrossRef]
- Yang, Z.; Berber, M.R.; Nakashima, N. Design of polymer-coated multi-walled carbon nanotube/carbon black-based fuel cell catalysts with high durability and performance under non-humidified condition. Electrochim. Acta 2015, 170, 1–8. [Google Scholar] [CrossRef][Green Version]
- Hsu, N.Y.; Chien, C.C.; Jeng, K.T. Characterization and enhancement of carbon nanotube-supported PtRu electrocatalyst for direct methanol fuel cell applications. Appl. Catal. B 2008, 84, 196–203. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Y.; Yin, G.; Lin, Y. Carbon nanotubes decorated with Pt nanoparticles via electrostatic self-assembly: A highly active oxygen reduction electrocatalyst. J. Mater. Chem. 2010, 20, 2826–2830. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, L.; Ren, J.; Hong, B. Electrodeposition of Pt–Ru and Pt–Ru–Ni nanoclusters on multi-walled carbon nanotubes for direct methanol fuel cell. Int. J. Hydrogen Energy 2014, 39, 4544–4557. [Google Scholar] [CrossRef]
- Giordano, N.; Passalacqua, E.; Pino, L.; Arico, A.S.; Antonucci, V.; Vivaldi, M.; Kinoshita, K. Analysis of platinum particle size and oxygen reduction in phosphoric acid. Electrochim. Acta 1991, 36, 1979–1984. [Google Scholar] [CrossRef]
- Antolini, E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A review. Mater. Chem. Phys. 2003, 78, 563–573. [Google Scholar] [CrossRef]
- Antolini, E.; Giorgi, L.; Cardellini, F.; Passalacqua, E. Physical and morphological characteristics and electrochemical behaviour in PEM fuel cells of PtRu/C catalysts. J. Solid State Chem. 2001, 5, 131–140. [Google Scholar] [CrossRef]
- Arico, A.S.; Antonucci, P.L.; Modica, E.; Baglio, V.; Kim, H.; Antonucci, V. Effect of Pt–Ru alloy composition on high-temperature methanol electro-oxidation. Electrochim. Acta 2002, 47, 3723–3732. [Google Scholar] [CrossRef]
- Takasu, Y.; Itaya, H.; Iwazaki, T.; Miyoshi, R.; Ohnuma, T.; Sugimoto, W.; Murakami, Y. Size effects of ultrafine Pt–Ru particles on the electrocatalytic oxidation of methano. Chem. Commun. 2001, 4, 341–342. [Google Scholar] [CrossRef]
- Liu, Z.; Ling, X.Y.; Su, X.; Lee, J.Y. Carbon-supported Pt and PtRu nanoparticles as catalysts for a direct methanol fuel cell. J. Phys. Chem. B 2004, 108, 8234–8240. [Google Scholar] [CrossRef]
- Manoharan, R.; Goodenough, J.B. Methanol oxidation in acid on ordered NiTi. J. Mater. Chem. 1992, 2, 875–887. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Wickman, B.; Fanta, A.B.; Burrows, A.; Hellman, A.; Wagner, J.B.; Iandolo, B. Iron oxide films prepared by rapid thermal processing for solar energy conversion. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; Rajkumar, M.; Chen, S.M.; Hu, C.C.; Boopathi, K.M.; Chu, C.W. High electrocatalytic performance of platinum and manganese dioxide nanoparticle decorated reduced graphene oxide sheets for methanol electro-oxidation. RSC Adv. 2014, 4, 41387–41397. [Google Scholar] [CrossRef]
- Dang, Q.L.; Nguyen, T.M.; Truong, N.V.; Le, P.H.; Long, N.V. Investigation of carbon supported Ru–Pt nanoparticles for high–performance electrocatalytic oxidation of methanol. Int. J. Electrochem. Sci. 2017, 12, 10187–10198. [Google Scholar] [CrossRef]
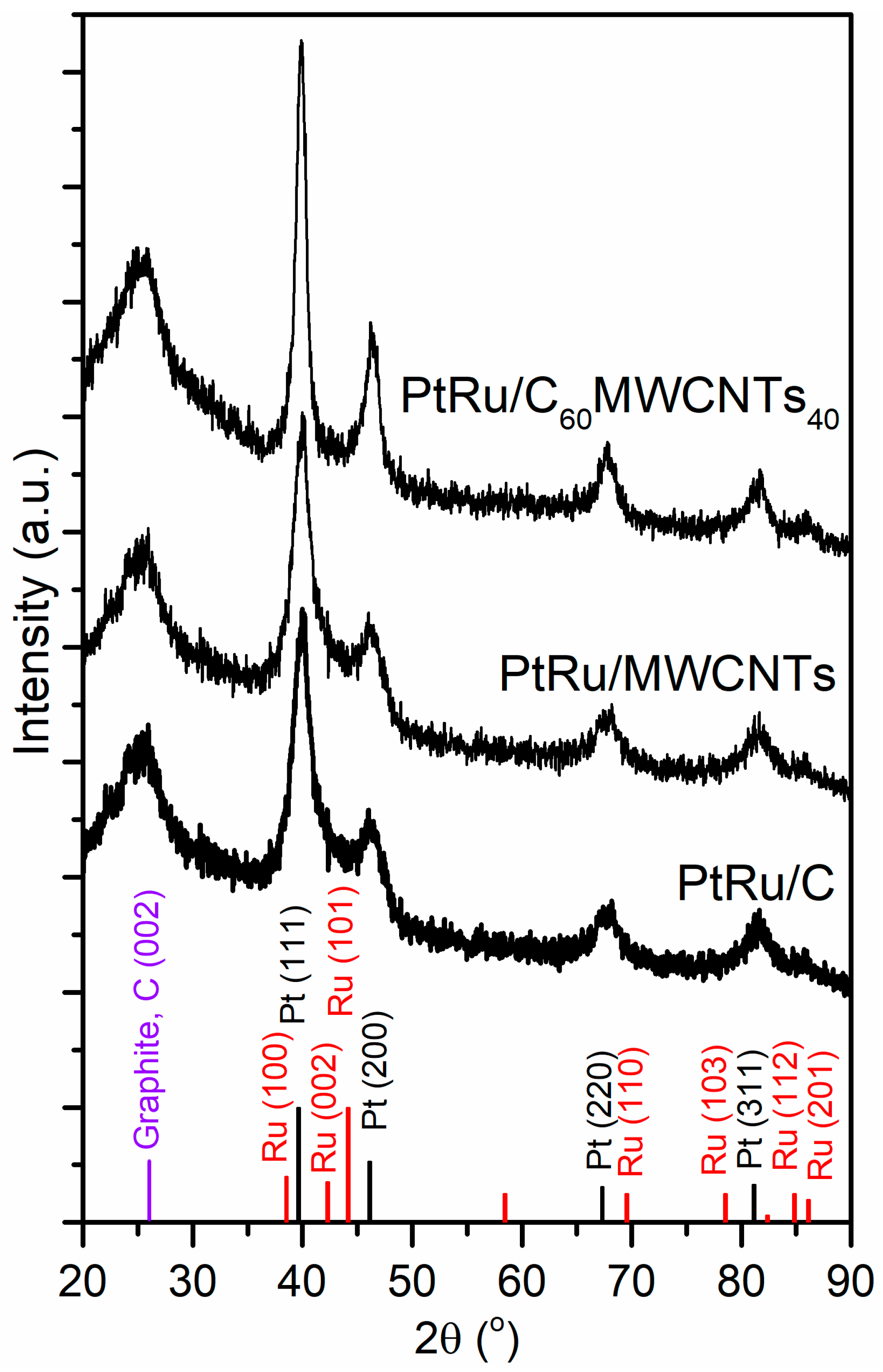

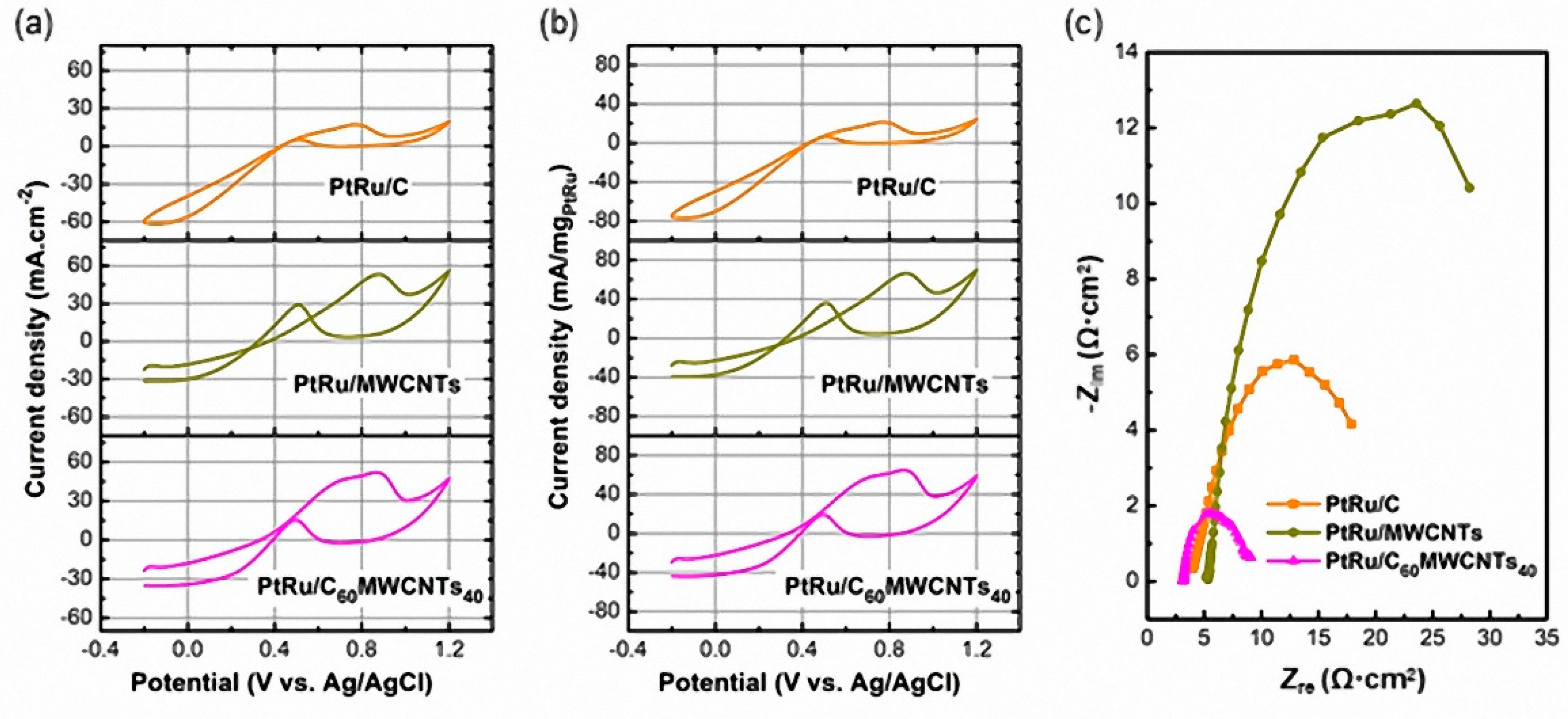
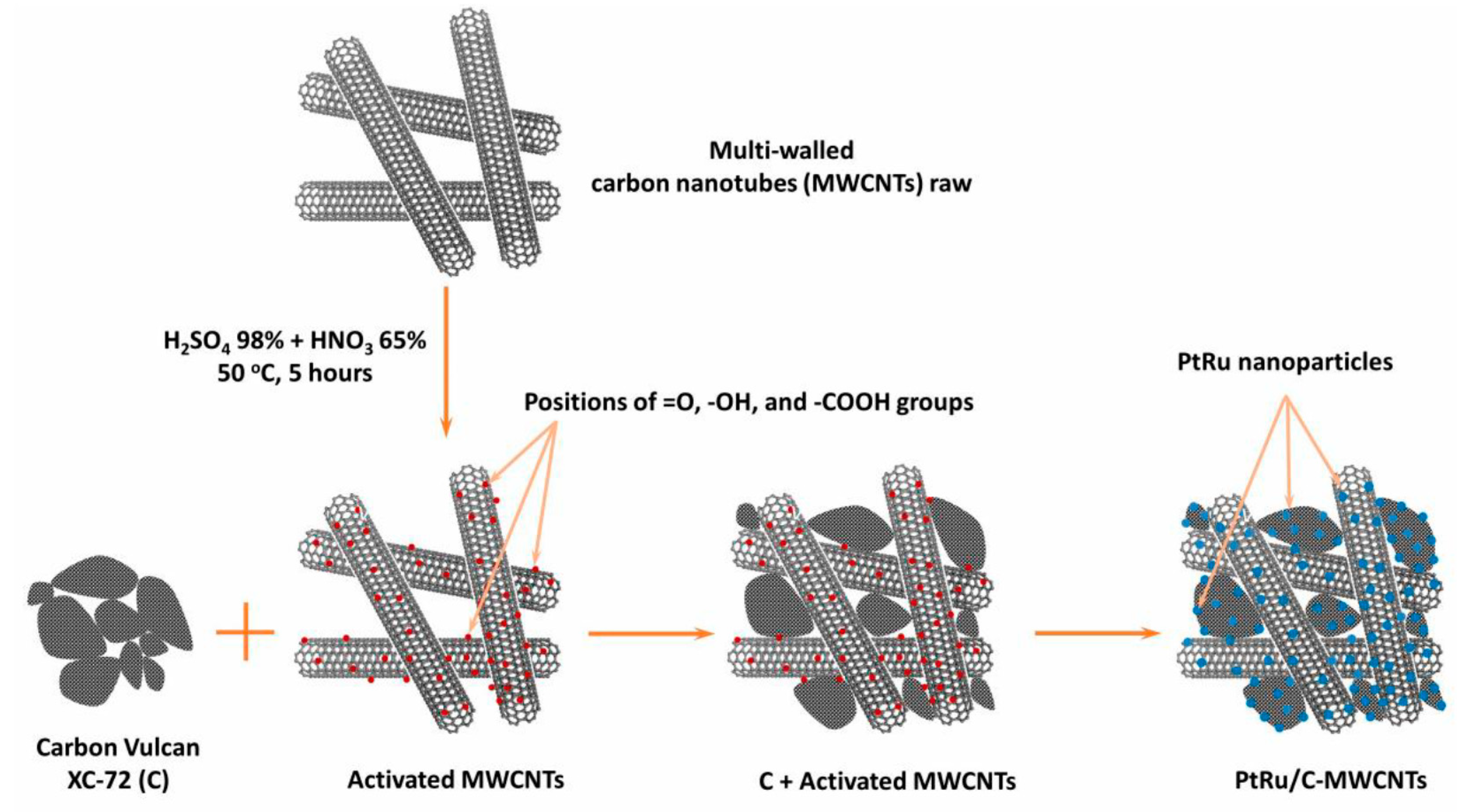
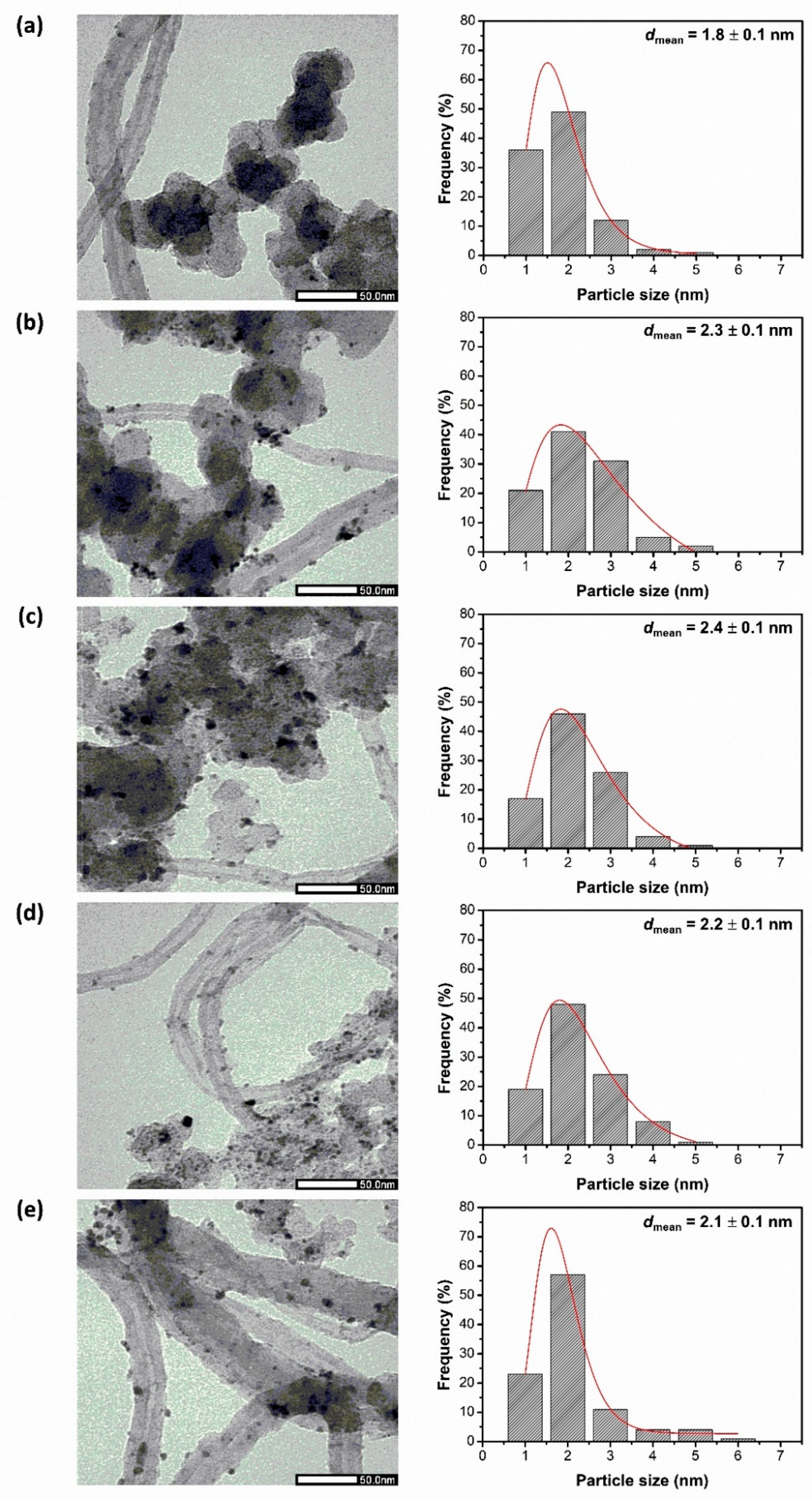
| Sample | C-MWCNTs Support | Pt and Ru Metal | ||||||
|---|---|---|---|---|---|---|---|---|
| Carbon Vucal XC-72 Mass (mg) | MWCNTs Mass (mg) | Mass Ratio of C and C-MWCNTs (wt.%) | Mass Ratio of MWCNTs and C-MWCNTs (wt.%) | Mass Ratio of C-MWCNTs and Sample (wt.%) | Pt Mass (mg) | Ru Mass (mg) | Mass Ratio of Pt + Ru and Sample (wt.%) | |
| PtRu/C | 80 | 0 | 100 | 0 | 80 | 13 | 7 | 20 |
| PtRu/MWCNTs | 0 | 80 | 0 | 100 | 80 | 13 | 7 | 20 |
| PtRu/C90MWCNTs10 | 72 | 8 | 90 | 10 | 80 | 13 | 7 | 20 |
| PtRu/C80MWCNTs20 | 64 | 16 | 80 | 20 | 80 | 13 | 7 | 20 |
| PtRu/C70MWCNTs30 | 56 | 24 | 70 | 30 | 80 | 13 | 7 | 20 |
| PtRu/C60MWCNTs40 | 48 | 32 | 60 | 40 | 80 | 13 | 7 | 20 |
| PtRu/C50MWCNTs50 | 40 | 40 | 50 | 50 | 80 | 13 | 7 | 20 |
| Sample | Jf | Jr | Jf/Jr | Ret | ||
|---|---|---|---|---|---|---|
| (mA·cm−2) | (mA/mgPtRu) | (mA·cm−2) | (mA/mgPtRu) | – | (Ω·cm2) | |
| PtRu/C | 17.3 | 21.6 | 4.8 | 6.0 | 3.6 | 7.39 |
| PtRu/MWCNTs | 53.6 | 67.0 | 28.8 | 36.0 | 1.9 | 11.19 |
| PtRu/C60MWCNTs40 | 52.3 | 65.4 | 15.5 | 19.4 | 3.4 | 6.37 |
| Sample | Jf | Jr | Jf/Jr | Ret | ||
|---|---|---|---|---|---|---|
| (mA/cm2) | (mA/mgPtRu) | (mA/cm2) | (mA/mgPtRu) | – | (Ω·cm2) | |
| PtRu/C90MWCNTs10 | 29.8 | 37.3 | 6.7 | 8.4 | 4.4 | 29.2 |
| PtRu/C80MWCNTs20 | 52.8 | 66.0 | 13.5 | 16.9 | 4.0 | 6.16 |
| PtRu/C70MWCNTs30 | 92.6 | 115.8 | 42.9 | 53.6 | 2.2 | 5.31 |
| PtRu/C60MWCNTs40 | 52.3 | 65.4 | 15.5 | 19.4 | 3.4 | 6.37 |
| PtRu/C50MWCNTs50 | 38.6 | 48.3 | 9.7 | 12.1 | 3.9 | 8.87 |
| Sample | Support Material | Measurement Condition | Evaluate Performance | Reference | |
|---|---|---|---|---|---|
| Current Density (Jf) | Current Density Ratio (Jf/JfC) | ||||
| PtRu/E-Tek PtRu/CX | Vulcan XC-72R Carbon xerogels | 2 M CH3OH + 0.5 M H2SO4, 0.02 V·s−1 | 0.29 mA·cm−2 0.36 mA·cm−2 | – 1.44 | [27] |
| PtRu/C PtRu 70%/CNF | Carbon Carbon nanofiber | 1 M CH3OH + 0.5 M H2SO4, 2 mV·s−1 | 340 mA·cm−2 390 mA·cm−2 | – 1.15 | [28] |
| PtRu/XC-72 PtRu/CMK-8-II | Vulcan XC-72R Mesoporous Carbon | 1 M CH3OH + 0.5 M H2SO4, 50 mV·s−1 | 27 mA·cm−2 60 mA·cm−2 | – 2.22 | [29] |
| PtRu/C PtRu/CNTs | Vulcan XC-72R Carbon nanotube | 1 M CH3OH + 0.5 M H2SO4, 20 mV·s−1 | 22.5 mA·cm−2 33.5 mA·cm−2 | – 1.49 | [22] |
| PtRu/CB PtRu/CNT PtRu/N-CNTs | Carbon Carbon nanotube Carbon nanotube doping N | 1 M CH3OH + 0.5 M H2SO4, 50 mV s−1 | 27.5 mA·cm−2 44.1 mA·cm−2 82.7 mA·cm−2 | – 1.60 3.00 | [23] |
| PtRu/C PtRu/CNTs PtRu/GS PtRu/CTNs-GS | Carbon Carbon nanotubes Graphene sheet Carbon nanotubes + Graphene sheet | 1 M CH3OH + 0.5 M H2SO4, 20 mV·s−1 | 42.7 mA·mg−1 56.0 mA·mg−1 78.7 mA·mg−1 136.7 mA·mg−1 | – 1.31 1.84 3.20 | [24] |
| PtRu/C PtRu/RGO | Carbon Reduced graphene oxide (RGO) | 0.5 M H2SO4 + 1 M CH3OH | 430 mA·mg−1 570 mA·mg−1 | – 1.33 | [31] |
| PtRu/C PtRu/FGSs | Carbon Functionalized graphene sheets | 1 M CH3OH + 0.5 M H2SO4, 50 mV·s−1 | 8.21 mA·cm−2 14.05 mA·cm−2 | – 1.71 | [32] |
| PtRu/C PtRu/MWCNTs PtRu/C70MWCNTs30 | Carbon Vulcan XC-72 Multi-walled carbon nanotubes C + MWCNTs | 1 M CH3OH + 0.5 M H2SO4, 50 mV·s−1 | 17.3 mA·cm−2 53.6 mA·cm−2 92.6 mA·cm−2 | – 3.10 5.35 | Our results |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, D.L.; Le, P.H. Enhanced Methanol Oxidation Activity of PtRu/C100−xMWCNTsx (x = 0–100 wt.%) by Controlling the Composition of C-MWCNTs Support. Coatings 2021, 11, 571. https://doi.org/10.3390/coatings11050571
Quan DL, Le PH. Enhanced Methanol Oxidation Activity of PtRu/C100−xMWCNTsx (x = 0–100 wt.%) by Controlling the Composition of C-MWCNTs Support. Coatings. 2021; 11(5):571. https://doi.org/10.3390/coatings11050571
Chicago/Turabian StyleQuan, Dang Long, and Phuoc Huu Le. 2021. "Enhanced Methanol Oxidation Activity of PtRu/C100−xMWCNTsx (x = 0–100 wt.%) by Controlling the Composition of C-MWCNTs Support" Coatings 11, no. 5: 571. https://doi.org/10.3390/coatings11050571
APA StyleQuan, D. L., & Le, P. H. (2021). Enhanced Methanol Oxidation Activity of PtRu/C100−xMWCNTsx (x = 0–100 wt.%) by Controlling the Composition of C-MWCNTs Support. Coatings, 11(5), 571. https://doi.org/10.3390/coatings11050571







