The Influence of the Roll-Laminating Process on the Bonding Quality of Polymer-Coated Steel Interface
Abstract
1. Introduction
2. Experiment
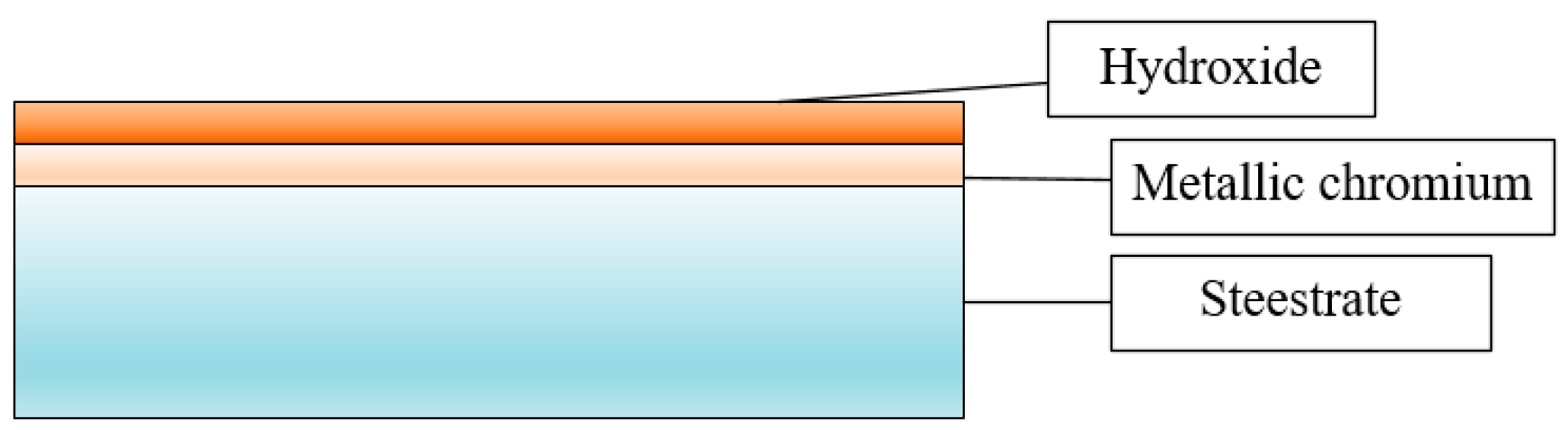
2.1. Materials
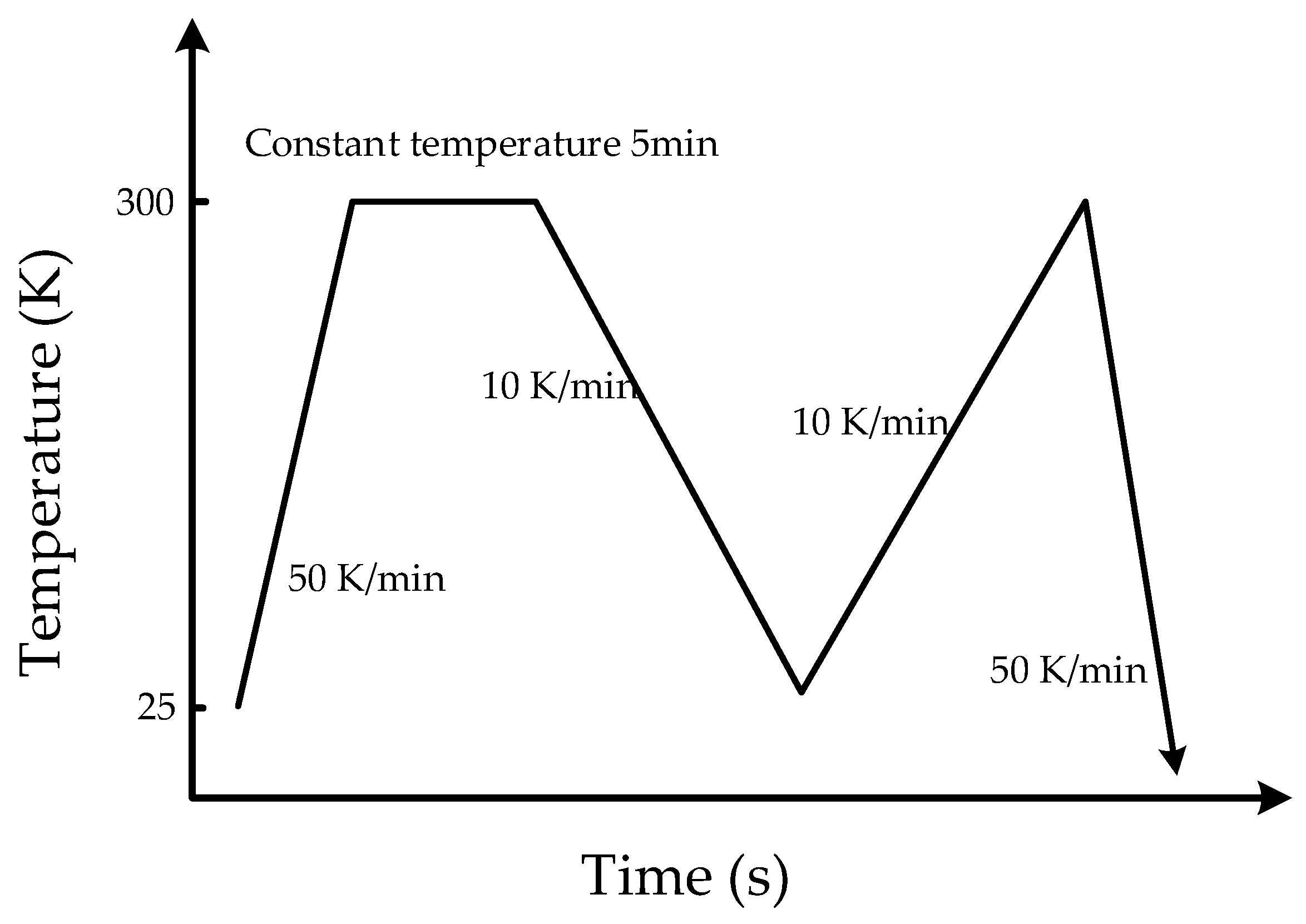
2.2. Laminating Experiment
2.2.1. Laminating Equipment
2.2.2. Experimental Scheme
2.3. Scanning Electron Microscope Experiment (SEM)
2.4. Differential Scanning Calorimetry (DSC)
2.5. Micro-Scratch Tester
3. Results and Discussion
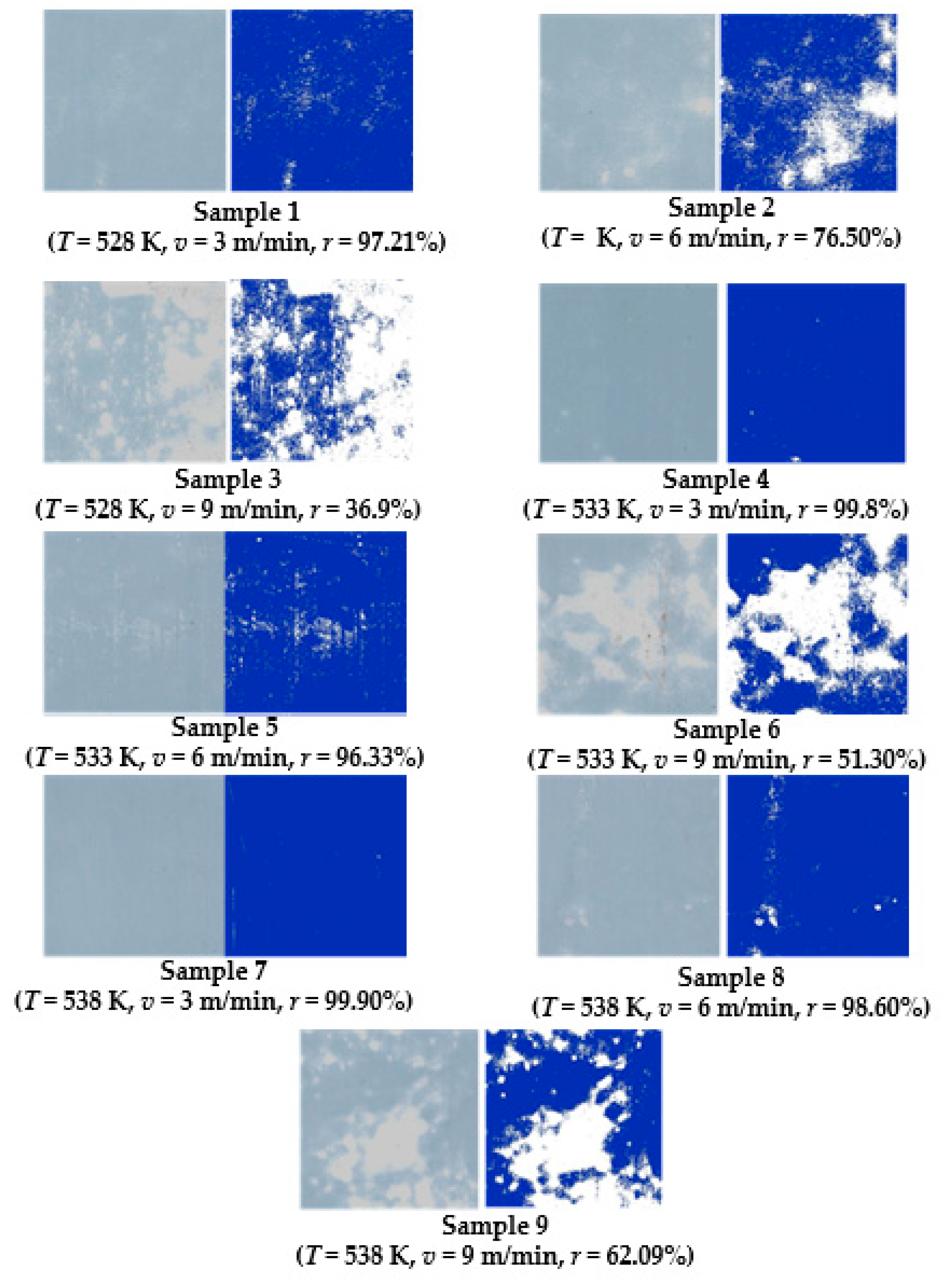
3.1. Characterization of Interface Binding Rate
3.2. Effect of Process Parameters on the Binding Rate
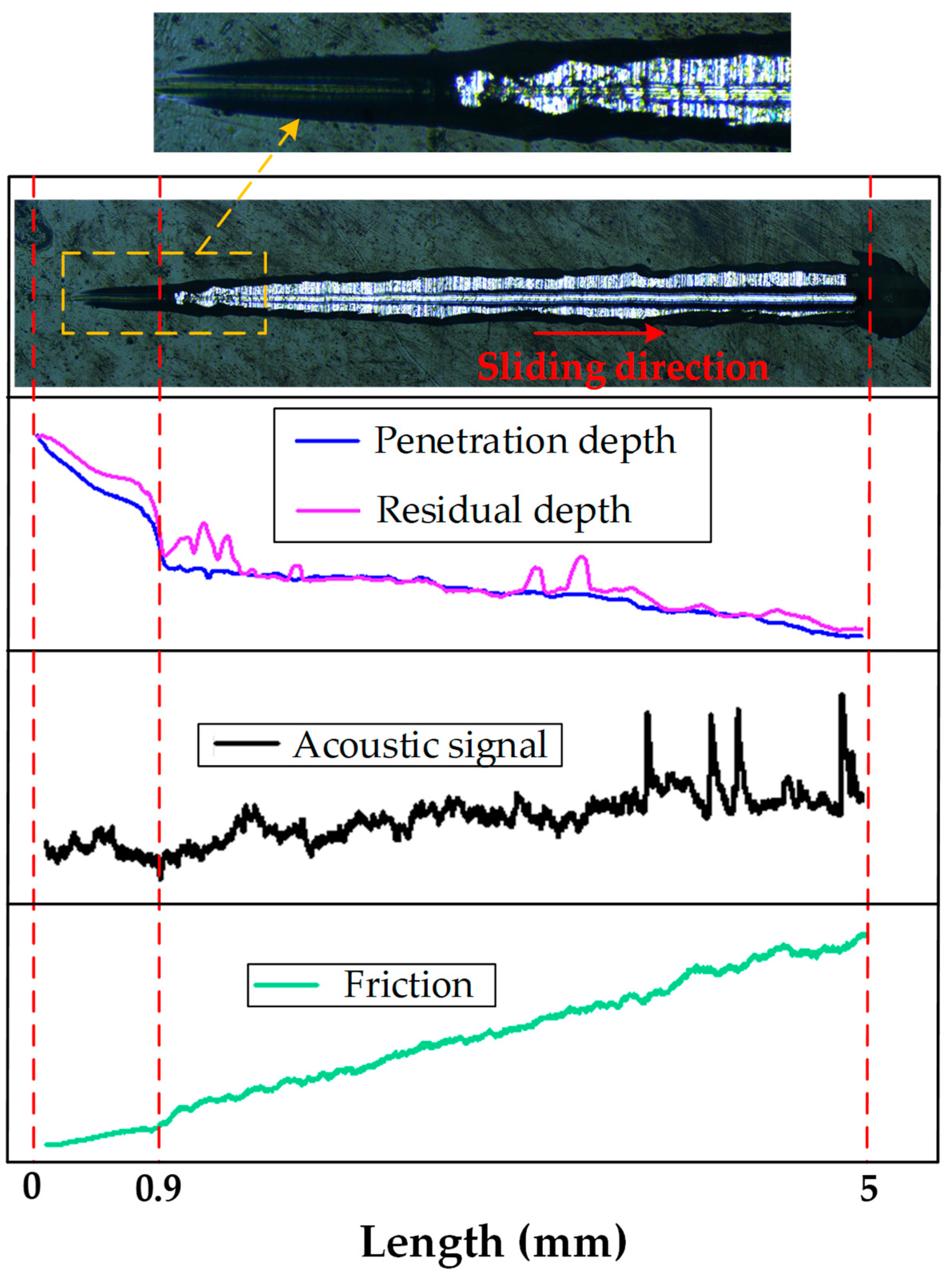
3.3. Interfacial Damage and Failure Mechanisms
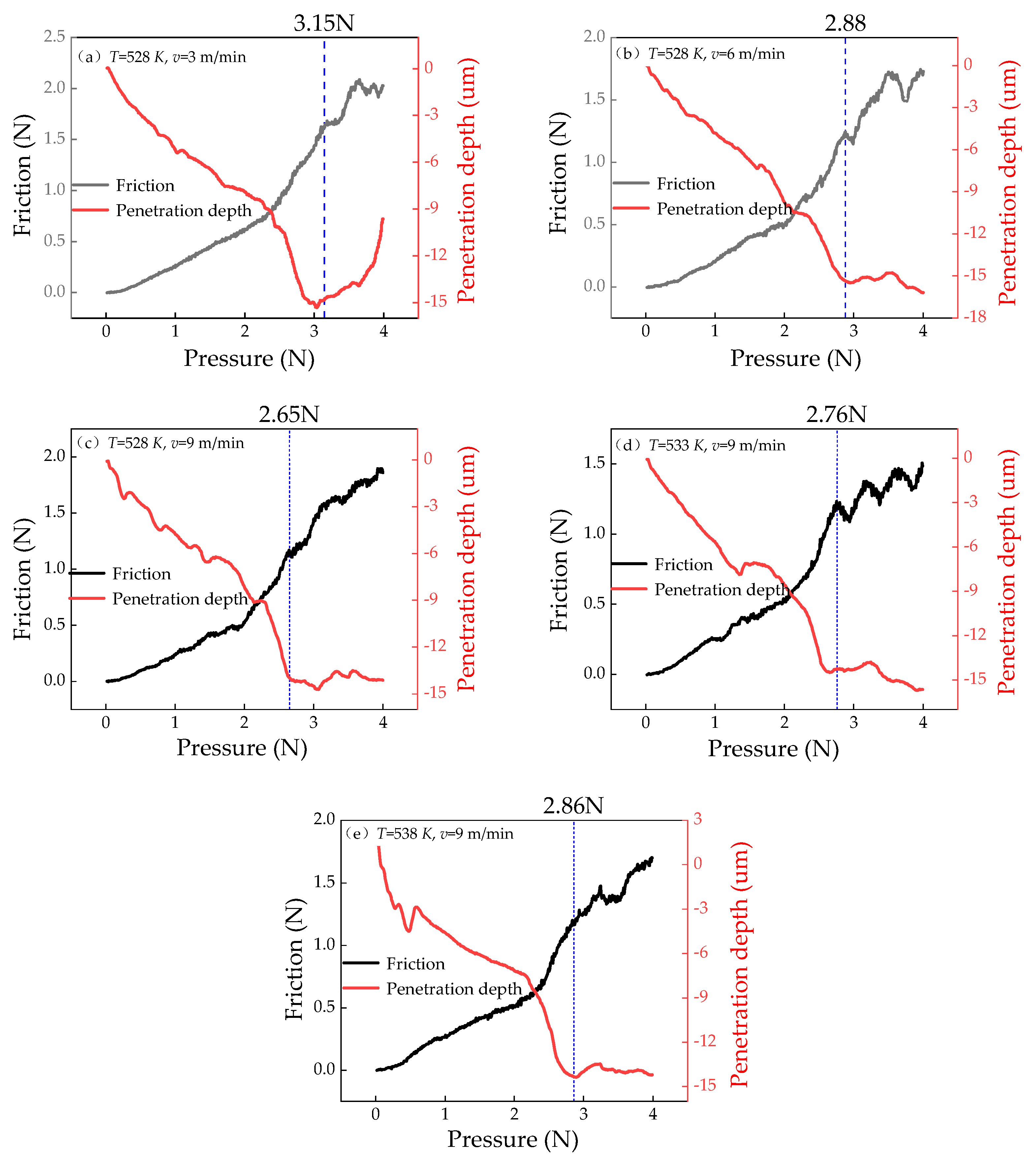
3.4. Effect of Process Parameters on the Bonding Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.D.; Liu, J.Y.; Zhang, B.Y.; Zhang, L.Y. Advances in Theory and Technology for Laminating of Polymer-coated Steel. J. Mech. Eng. 2019, 8, 30–45. [Google Scholar] [CrossRef]
- Yoichiro, Y.; Hiroki, I.; Toyofumi, W. Development of Laminated Tin Free Steel (TFS) ’UNIVERSAL BRITE~®’ Type F for Food Cans. JFE Tech. Rep. 2007, 9, 49–53. [Google Scholar]
- Zumelzu, E.; Cabezas, C.; Delgado, F. Performance and degradation analyses of traditional and ECCS canning tinplates in citric-citrate medium. J. Mater. Process. Technol. 2004, 152, 384–388. [Google Scholar] [CrossRef]
- Pardo-Figuerez, M.; Lopez-Cordoba, A.; Torres-Giner, S.; Lagaron, J.M. Superhydrophobic bio-coating made by co-continuous electrospinning and electrospraying on polyethylene terephthalate films proposed as easy emptying transparent food packaging. Coatings 2018, 8, 364. [Google Scholar] [CrossRef]
- Mousa, S.; Scheirer, N.; Kim, G.Y. Roll-bonding of metal-polymer-metal sandwich composites reinforced by glass whiskers at the interface. J. Mater. Process. Technol. 2017, 255, 463–469. [Google Scholar] [CrossRef]
- Zumelzu, E.; Rull, F.; Boettcher, A.A. Characterization and micro- and ultra-structural analysis of PET-based Co-rolled composite electrolytic chromium coated steel (ECCS). J. Mater. Process. Tech. 2006, 173, 34–39. [Google Scholar] [CrossRef]
- Zumelzu, E.; Rull, F.; Boettcher, A.A. Deformation and fracture of polymer coated metal sheets: Characterisation and degradation. Surface Eng. 2006, 22, 432–438. [Google Scholar] [CrossRef]
- Fei, S.H. Polymer Coating Effects: Study of Material Properties and Architectural Application Characteristics of Aluminum Template. Coatings 2021, 11, 240. [Google Scholar]
- Morita, S.I.; Iwashita, H.; Tanaka, A. Effect of Contact Cooling Length between the Laminating Rolls on the Biaxial Orientation Changes of Polyester Film in the Laminating Process. J. Surf. Finish. Soc. Jpn. 2001, 52, 517–521. [Google Scholar] [CrossRef]
- Cho, C.K.; Kim, J.D.; Cho, K.; Park, C.E.; Lee, S.W.; Ree, M. Effects of the lamination temperature on the properties of poly (ethylene terephthalate-co-isophthalate) in polyester-laminated tin-free steel can—I. Characterization of poly (ethylene terephthalate-co-isophthalate). J. Adhes. Sci. Technol. 2000, 14, 1131–1143. [Google Scholar] [CrossRef]
- Cho, C.K.; Kim, J.D.; Cho, K.; Park, C.E.; Lee, S.W.; Ree, M. Effects of the lamination temperature on the properties of poly (ethylene terephthalate-co-isophthalate) in polyester-laminated tin-free steel can—II. Adhesion mechanism of poly (ethylene terephthalate-co-isophthalate) to TFS. J. Adhes. Sci. Technol. 2000, 14, 1145–1157. [Google Scholar] [CrossRef]
- Ruokolainen, R.B.; Sigler, D.R. The Effect of Adhesion and Tensile Properties on the Formability of Laminated Steels. J. Mater. Eng. Perform. 2008, 17, 330–339. [Google Scholar] [CrossRef]
- Braasch, D.A.; Gillis, M.; Pramanik, M.; Ferguson, R.C. Detection of in Situ Early Corrosion on Polymer-Coated Metal Substrates. ACS Appl. Mater. 2019, 11, 37193–37208. [Google Scholar] [CrossRef] [PubMed]
- Zumelzu, E.; Wehrhahn, M.J.; Rull, F.; Pesenti, H.; Munoz, O.; Ugarte, R. Evaluation of salmon adhesion on PET-metal interface by ATR, FT-IR, and raman spectroscopy. J. Spectrosc. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Iwashita, H.; Morita, S.I.; Tanaka, A. Development of Amorphous Layer of Biaxially Oriented Polyester Film on Laminated Steel in the Laminating Process. Tetsu-to-Hagane 2001, 87, 175–182. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Zhang, Q.; Geng, L.; Zhang, B.; Zhang, L. Study on evolution law of lateral thickness difference of polymer-coated steel in laminating process. Mater. Today Proc. 2020, 33, 2059–2065. [Google Scholar] [CrossRef]
- Terauchi, F.; Iwashita, H.; Tanaka, A.; Morita, S. Effect of Annealing Temperature on Adhesion of Polyester Film to Electrolytically Chromium Coated Steel. Tetsu-to-Hagane 1999, 85, 236–240. [Google Scholar] [CrossRef]
- Matsubayashi, H. Metal Can using Polyester Film Laminated ECCS (Electrolytic chromium/chromium oxide-coated steel). Zairyo Kankyo 2002, 51, 299–304. [Google Scholar] [CrossRef][Green Version]
- Ranjbar, N.; Talebian, S.; Mehrali, M.; Kuenzel, C.; Metselaar, H.S.C.; Jumaat, M.Z. Mechanisms of interfacial bond in steel and polypropylene fiber reinforced geopolymer composites. Compos. Sci. Technol. 2016, 122, 73–81. [Google Scholar] [CrossRef]
- Lindner, M.; Rodler, N.; Jesdinszki, M.; Schmid, M.; Sängerlaub, S. Surface energy of corona treated PP, PE and PET films, its alteration as function of storage time and the effect of various corona dosages on their bond strength after lamination. J. Appl. Polym. Sci. 2018, 135, 45842. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Guo, Y.; Zhang, Z. Surface modification of a biomedical polyethylene terephthalate (PET) by air plasma. Appl. Surface Sci. 2009, 255, 4446–4451. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, Z.J. Plasma techniques for the fabrication of polymer electrolyte membranes for fuel cells. J. Membr. Sci. 2014, 456, 85–106. [Google Scholar] [CrossRef]
- Komai, M.; Taniguchi, A.; Shimizu, N.; Shimizu, K.; Tanaka, A. Effects of composition and microstructure of hydrated chromium oxide on adhesive property of PET/I film laminated TFS. Tetsu-to-Hagane 2009, 83, 377–382. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Iwasa, H.; Yamashita, M. The Mechanism of Wet Adhesion of the PET-laminated Steel Sheet. Tetsu-to-Hagane 2003, 89, 142–148. [Google Scholar] [CrossRef][Green Version]
- Zumelzu, E.; Angulo, C.; Cabezas, C.; Ugarte, R. Characterisation of nanometric chromium coatings in metal–polymer composites. Surface Eng. 2013, 29, 620–626. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Zhang, B.; Yu, M. The Bonding Mechanism of the Micro-Interface of Polymer Coated Steel. Polymers 2020, 12, 3052. [Google Scholar] [CrossRef] [PubMed]
- Manasoglu, G.; Elen, R.; Kanik, M.; Ulcay, Y. An Investigation on the Thermal and Solar Properties of Graphene-Coated Polyester Fabrics. Coatings 2021, 11, 125. [Google Scholar] [CrossRef]
- Ding, L.; Xie, L.; Cao, J.; Bai, Y. Crystallization behavior of poly (ethylene terephthalate-co-neopentyl terephthalate-co-ethylene isophthalate-co-neopentyl isophthalate) copolyester and its application in laminated tin-free steel. J. Appl. Polym. Sci. 2015, 42308. [Google Scholar] [CrossRef]
- Holmberg, K.; Laukkanen, A.; Ronkainen, H.; Wallin, K.; Varjus, S. A model for stresses, crack generation and fracture toughness calculation in scratched TiN-coated steel surfaces. Wear 2003, 254, 278–291. [Google Scholar] [CrossRef]






| Sample Number | Temperature (K) | Laminating Speed(m/min) |
|---|---|---|
| 1 | 528 | 3 |
| 2 | 528 | 6 |
| 3 | 528 | 9 |
| 4 | 533 | 3 |
| 5 | 533 | 6 |
| 6 | 533 | 9 |
| 7 | 538 | 3 |
| 8 | 538 | 6 |
| 9 | 538 | 9 |
| Sample Number | Temperature (K) | Laminating Speed (m/min) | Binding Rate | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Average Value (%) | Standard Deviation (%) | |||
| 1 | 528 | 3 | 98.91 | 99.58 | 99.14 | 99.21 | 0.28 |
| 2 | 528 | 6 | 73.2 | 84.36 | 71.94 | 76.5 | 5.58 |
| 3 | 528 | 9 | 37.4 | 33.11 | 40.19 | 36.9 | 2.91 |
| 4 | 533 | 3 | 99.85 | 99.89 | 99.66 | 99.8 | 0.1 |
| 5 | 533 | 6 | 94.25 | 97.63 | 97.11 | 96.33 | 1.49 |
| 6 | 533 | 9 | 56.33 | 48.33 | 49.24 | 51.3 | 3.57 |
| 7 | 538 | 3 | 99.89 | 99.93 | 99.88 | 99.9 | 0.02 |
| 8 | 538 | 6 | 99.3 | 99.67 | 96.83 | 98.6 | 1.26 |
| 9 | 538 | 9 | 59.31 | 66.43 | 60.53 | 62.09 | 3.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, Q.; Zhang, B.; Yu, M. The Influence of the Roll-Laminating Process on the Bonding Quality of Polymer-Coated Steel Interface. Coatings 2021, 11, 472. https://doi.org/10.3390/coatings11040472
Liu J, Zhang Q, Zhang B, Yu M. The Influence of the Roll-Laminating Process on the Bonding Quality of Polymer-Coated Steel Interface. Coatings. 2021; 11(4):472. https://doi.org/10.3390/coatings11040472
Chicago/Turabian StyleLiu, Jiyang, Qingdong Zhang, Boyang Zhang, and Mingyang Yu. 2021. "The Influence of the Roll-Laminating Process on the Bonding Quality of Polymer-Coated Steel Interface" Coatings 11, no. 4: 472. https://doi.org/10.3390/coatings11040472
APA StyleLiu, J., Zhang, Q., Zhang, B., & Yu, M. (2021). The Influence of the Roll-Laminating Process on the Bonding Quality of Polymer-Coated Steel Interface. Coatings, 11(4), 472. https://doi.org/10.3390/coatings11040472





