Evaluation of Passivation Process for Stainless Steel Hypotubes Used in Coronary Angioplasty Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. The Hypotubes’ Characteristics
Sampling Description
2.2. The Passivation Method
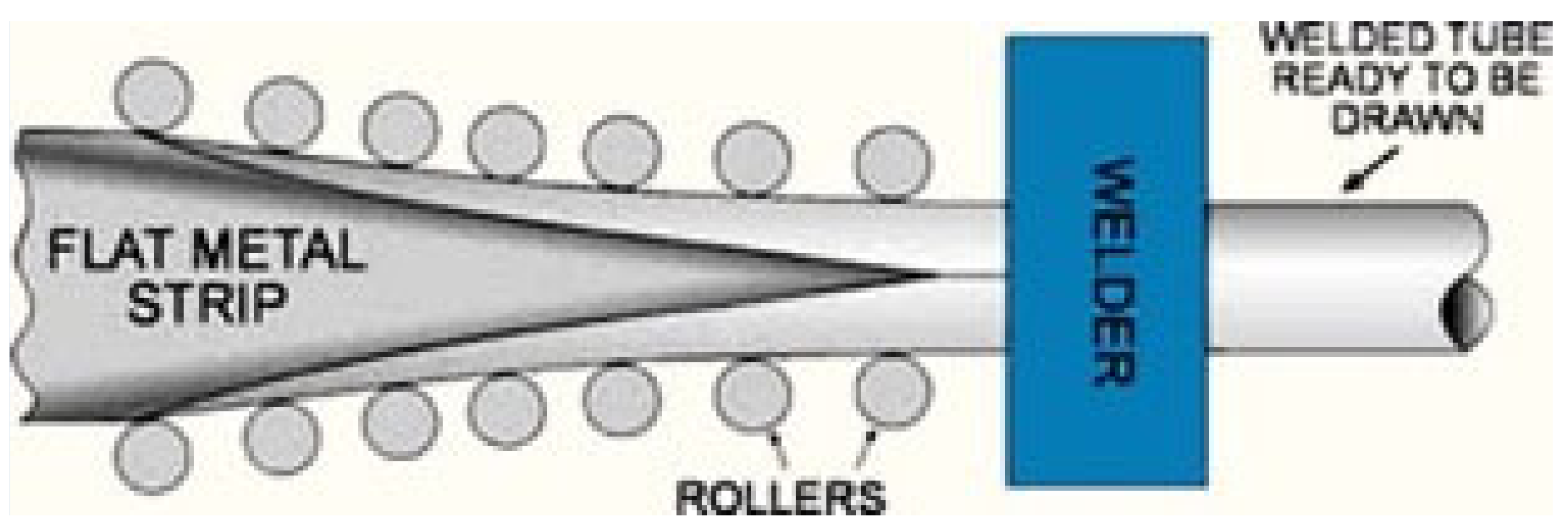
2.2.1. The Micro-Pilot
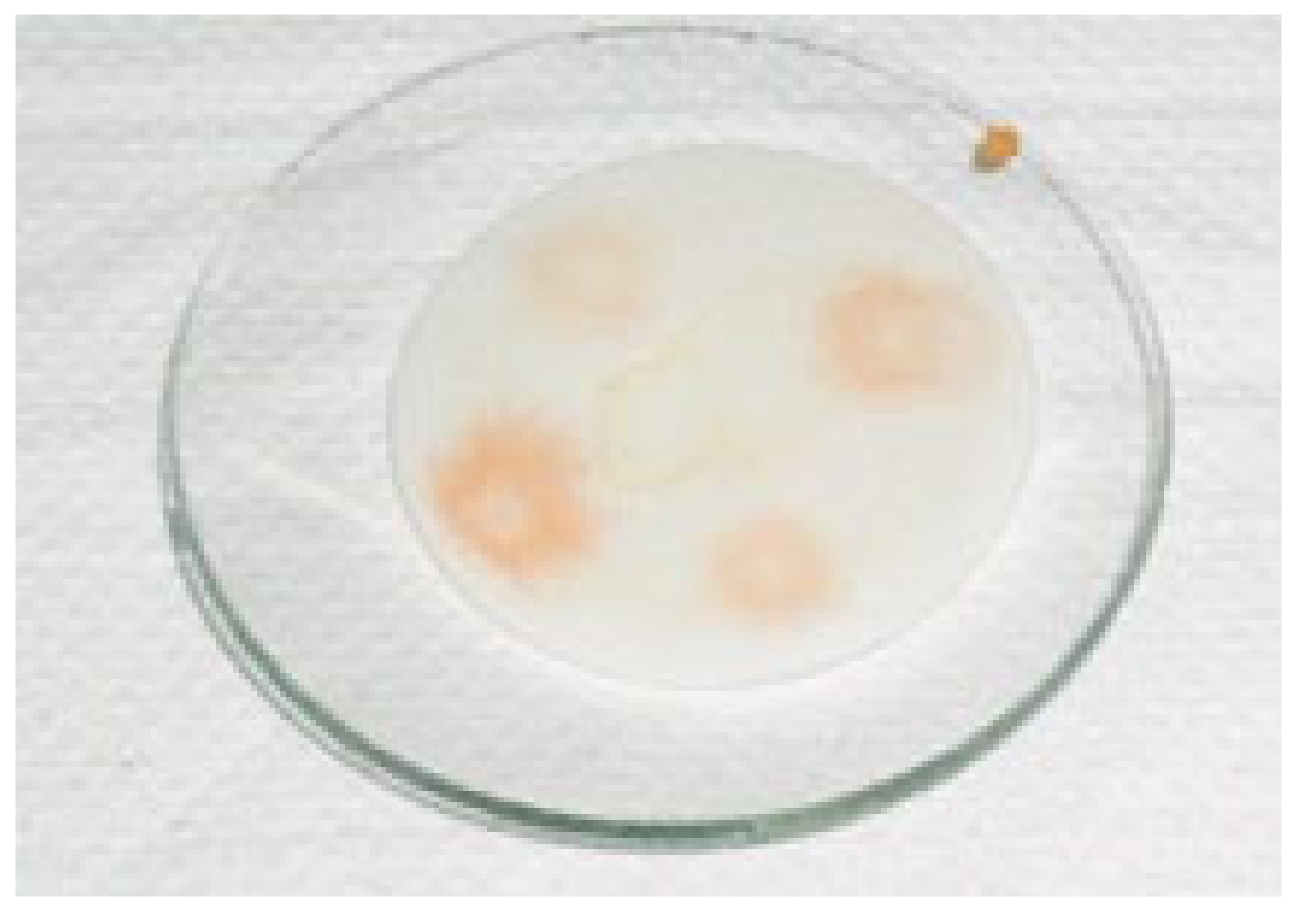
2.2.2. The Orthophenanthroline Hydrochloride Test
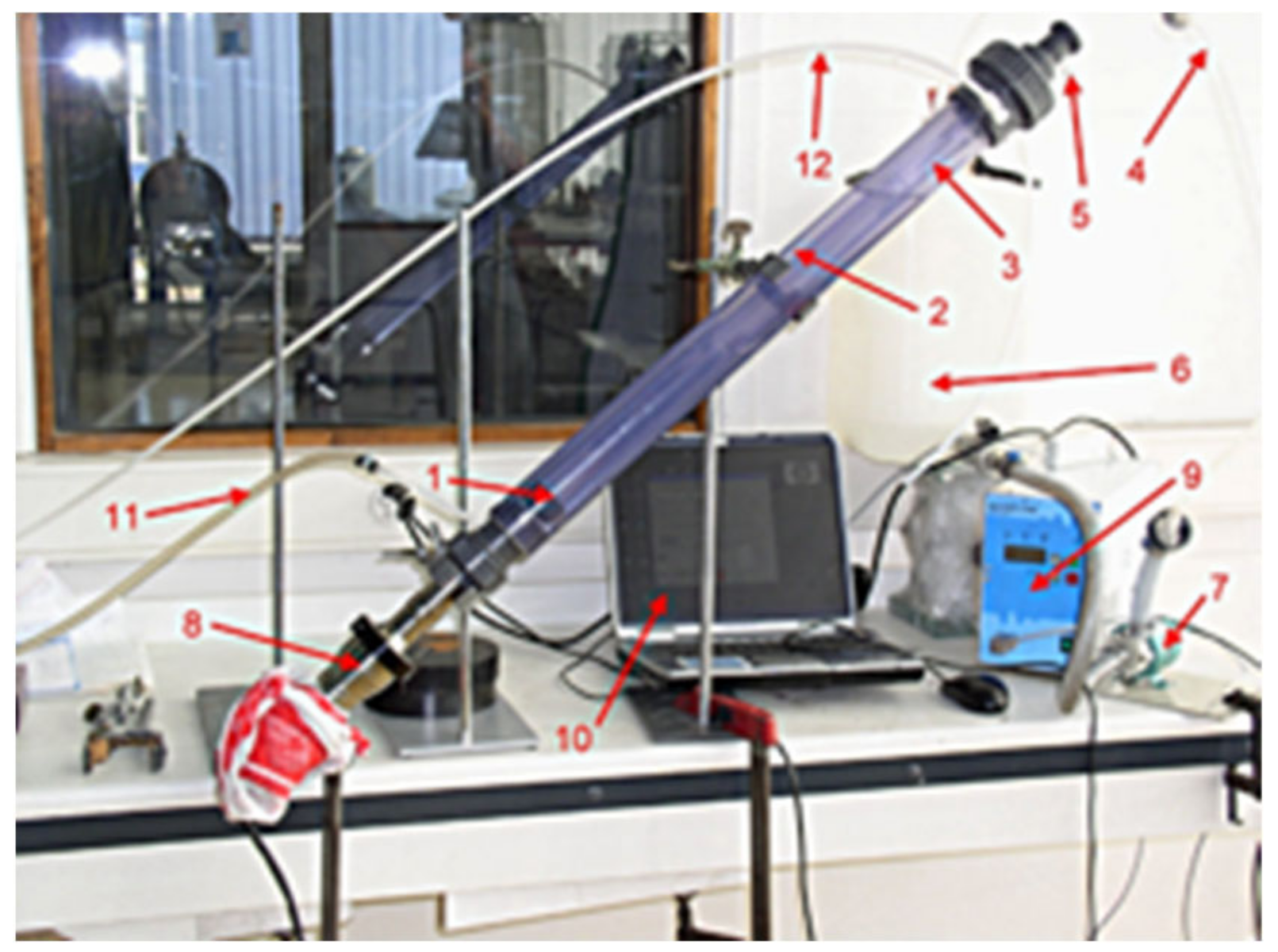
2.2.3. The under Pressure Passivation
- First bullet; At 10 bars, the cleaning time was 180 s, with a flow rate of 38.17 µL/sec.
- At 50 bars, the cleaning time was 60 s, with a flow rate of 92.59 µL/sec.
2.3. EDX Analysis
2.4. The Carbon (C) and Sulfur (S) Analysis
2.5. Evaluation of Intergranular Corrosion (the Strauss Test)
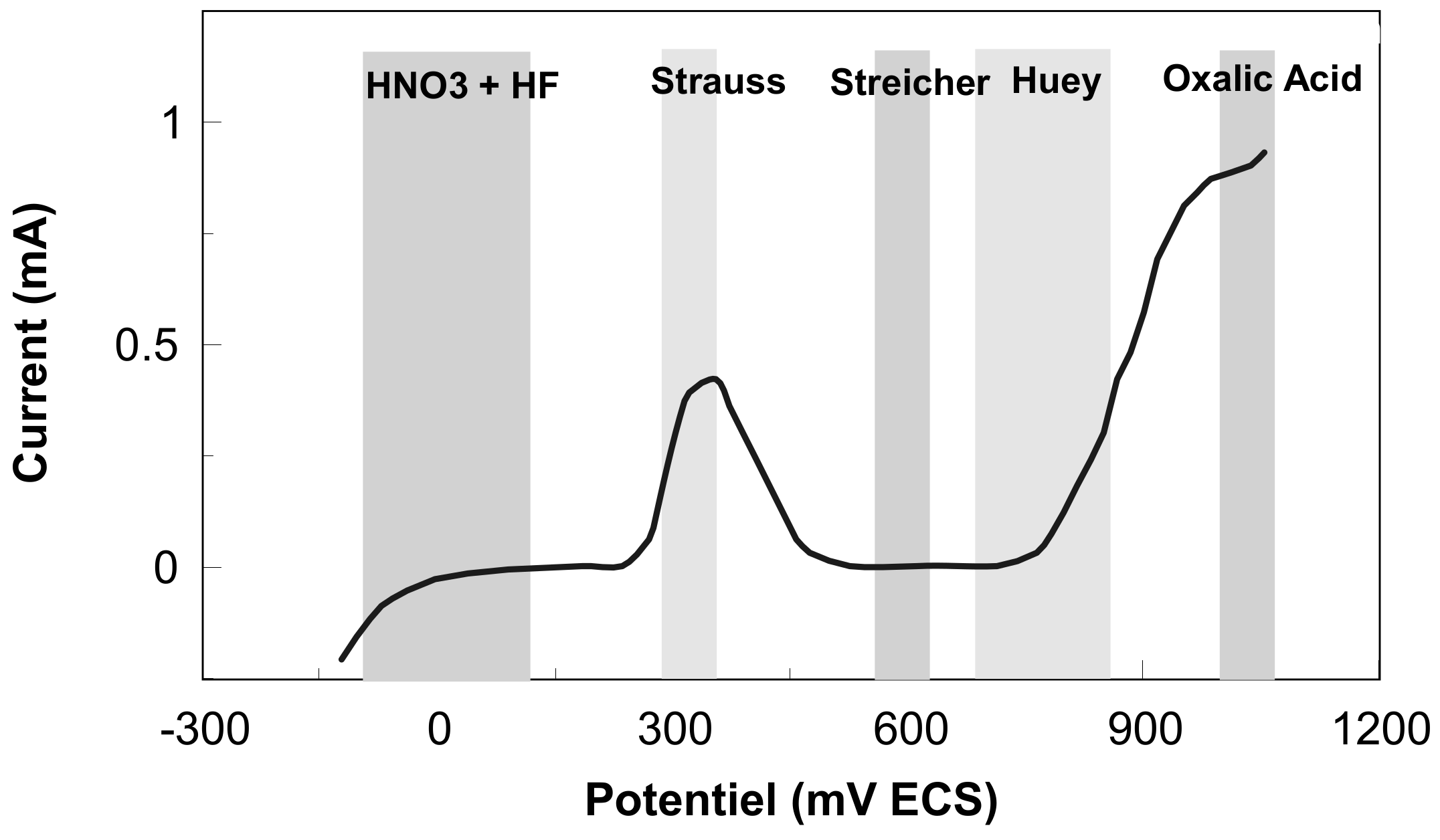
2.6. Electrochemical Evaluation
2.6.1. External Surface
2.6.2. Internal Surface
3. Results and Discussion
3.1. Chemical Composition Determination by EDX
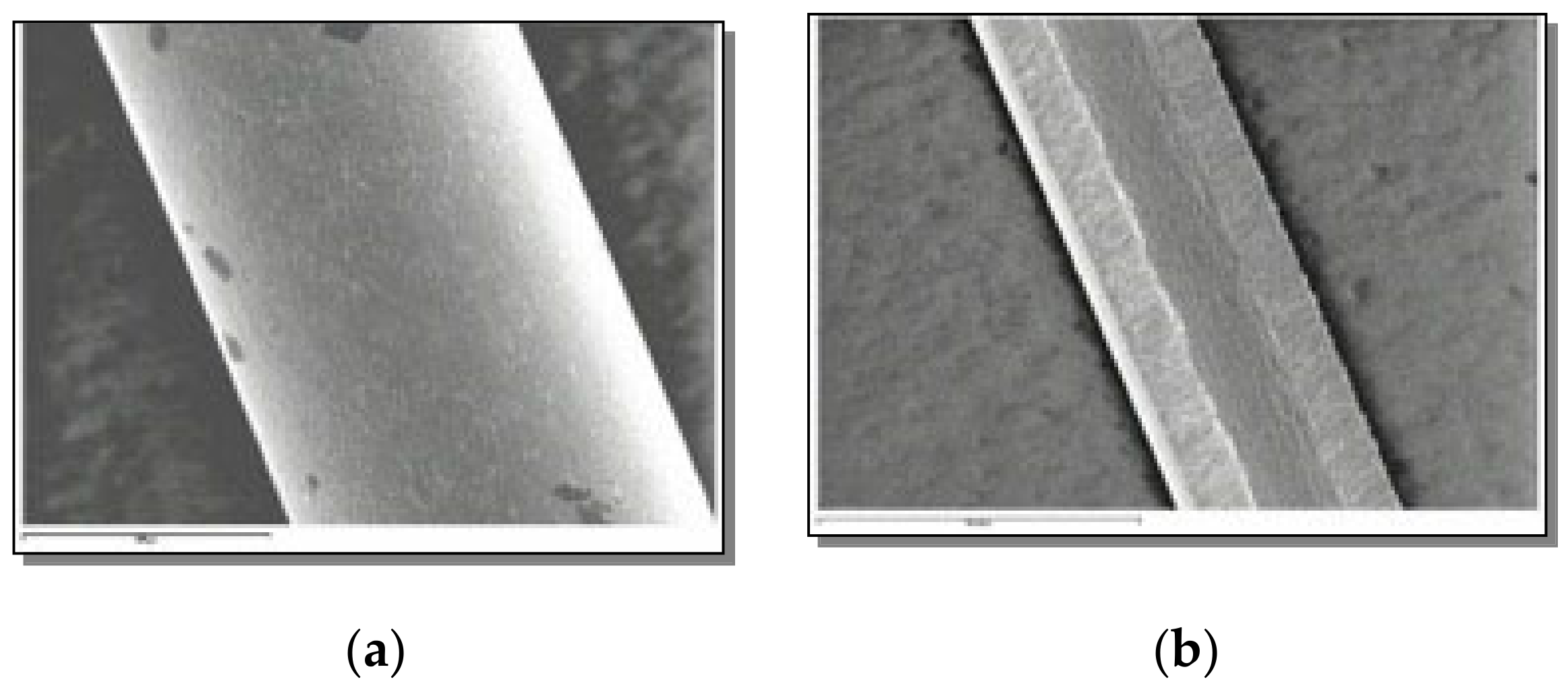
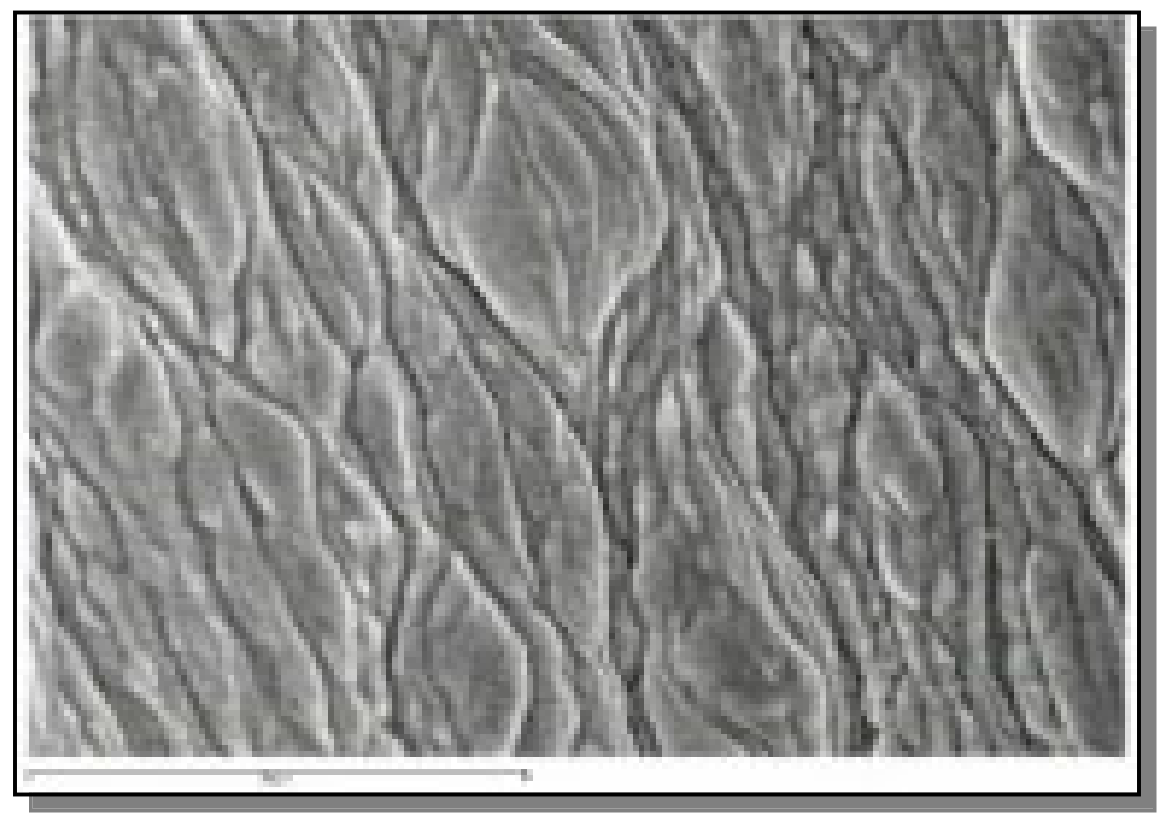
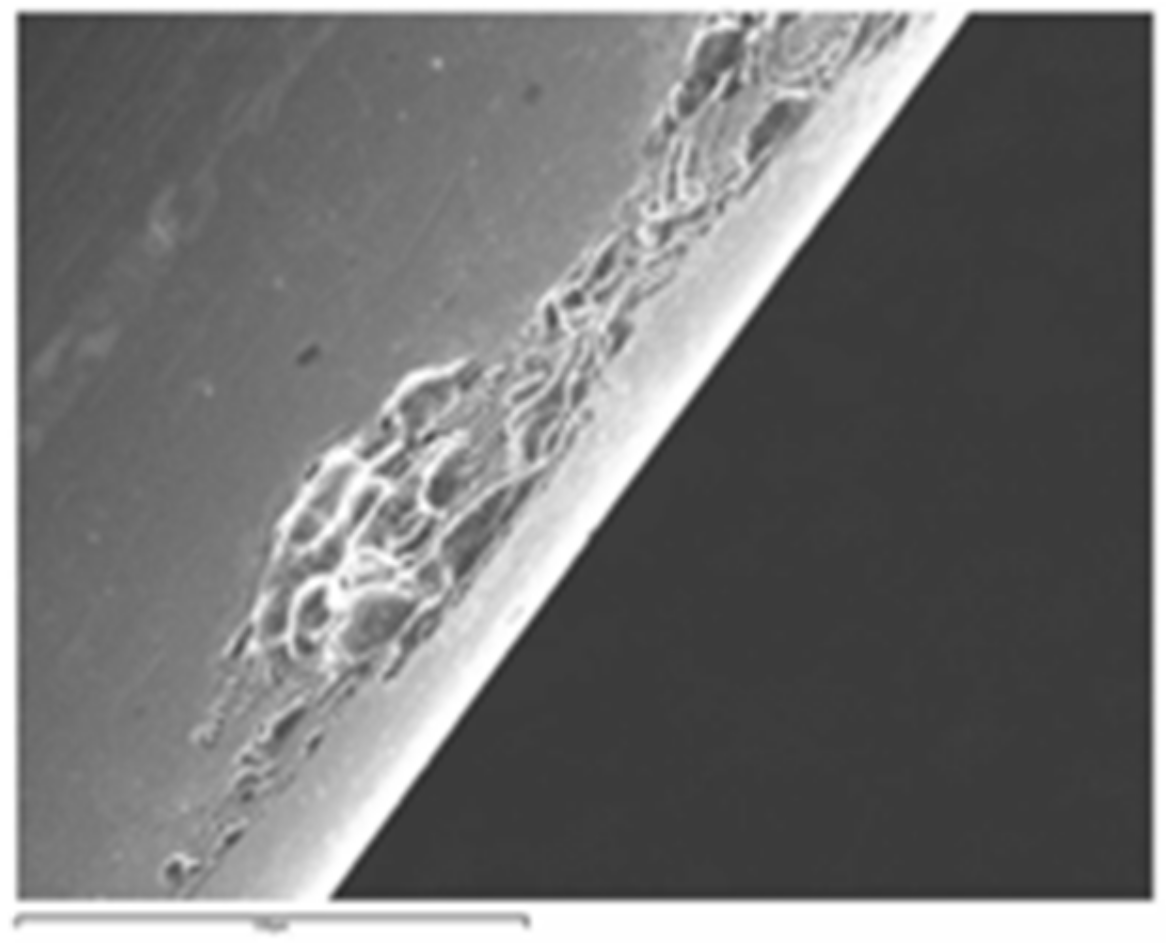
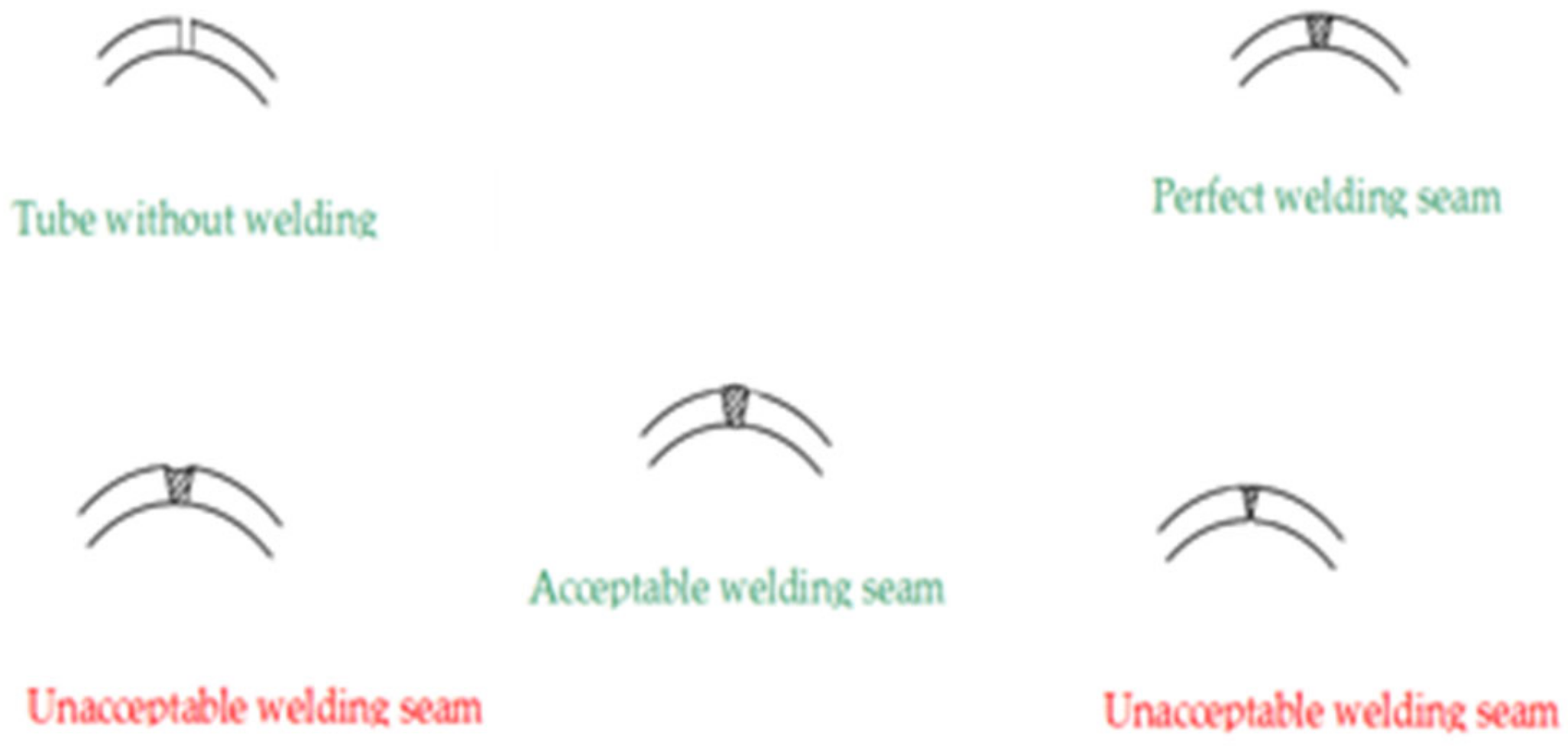
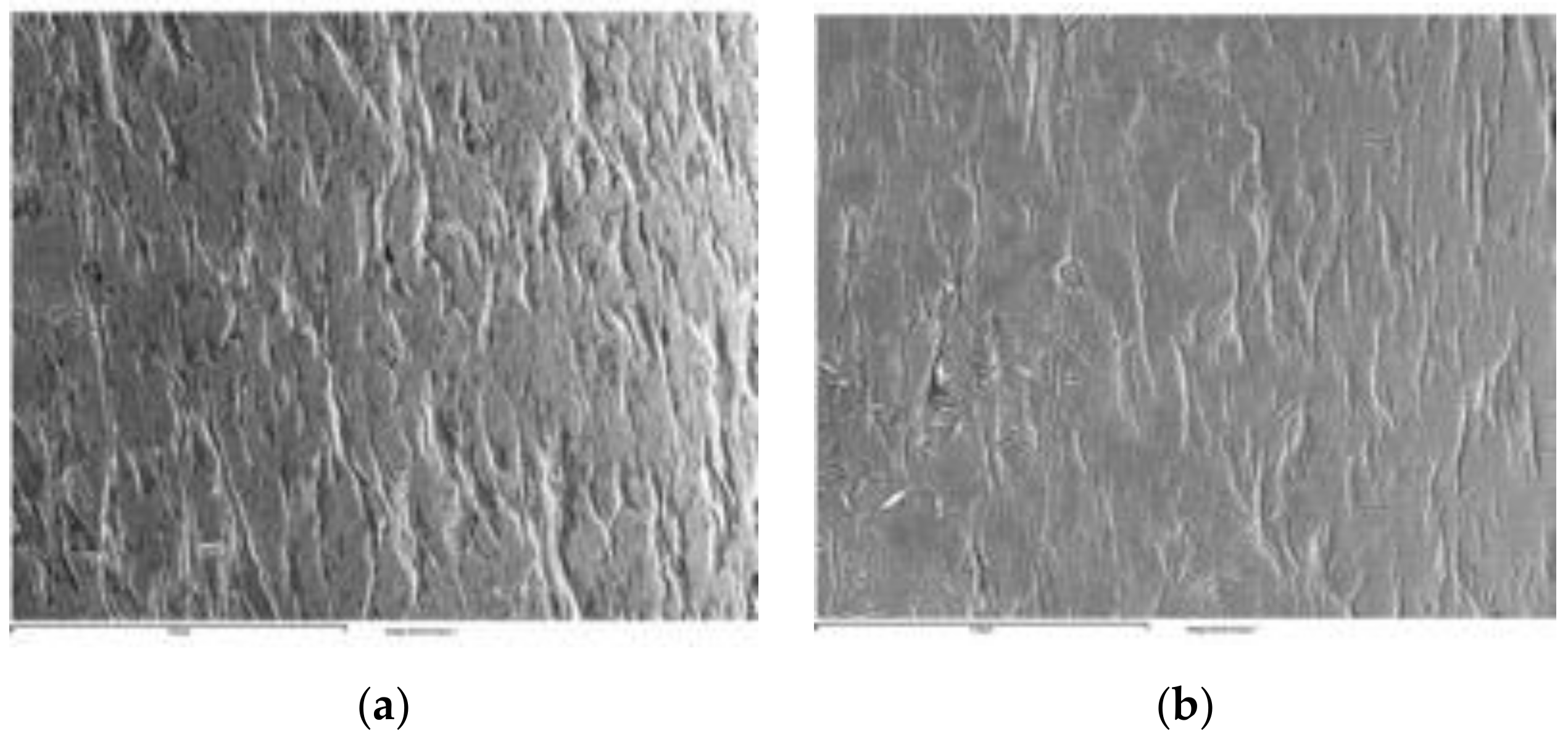
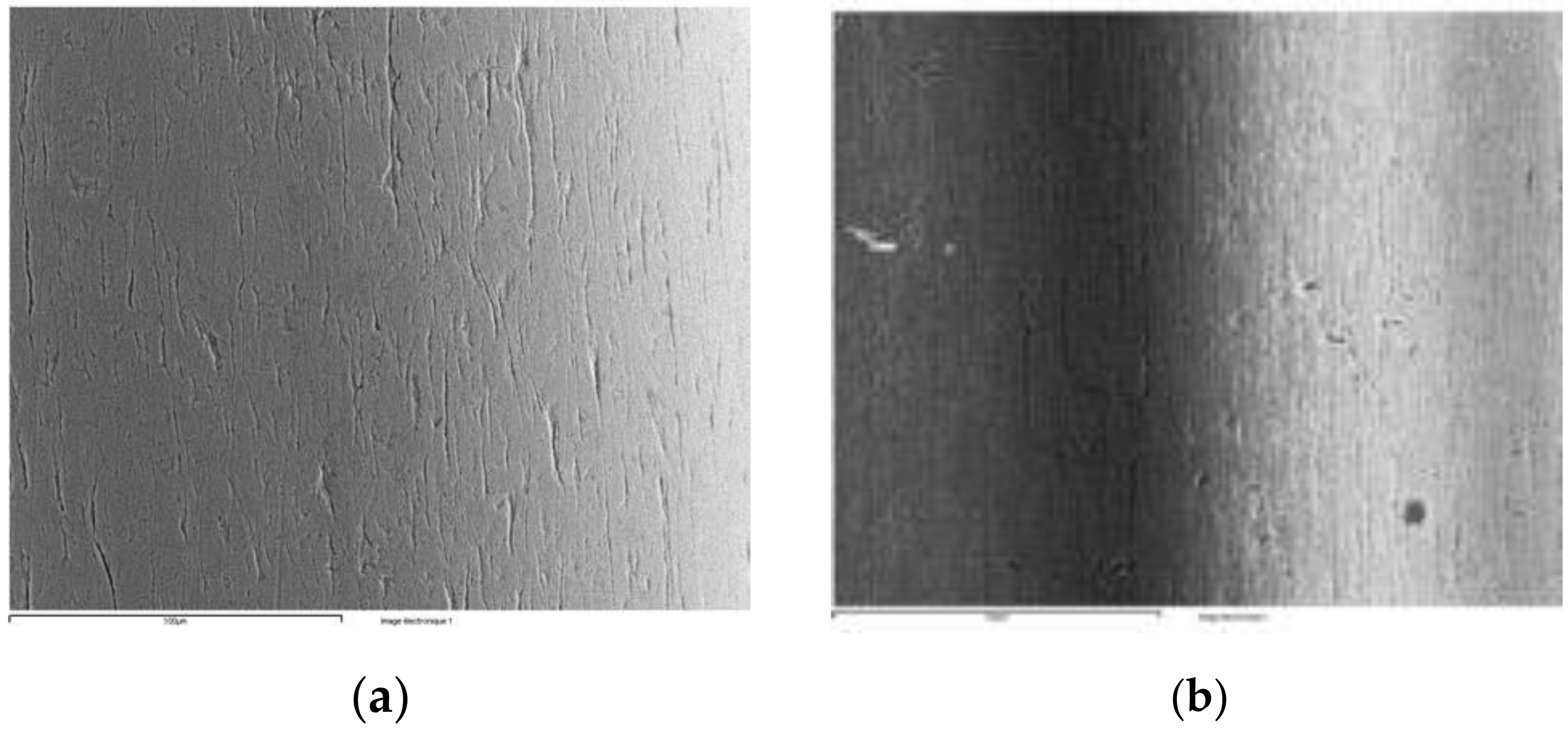
3.2. Microstructure Observation by SEM
3.3. Detection of Susceptibility to Intergranular Corrosion (the Strauss Test)
3.4. Corrosion Evaluation by Electrochemical Methods
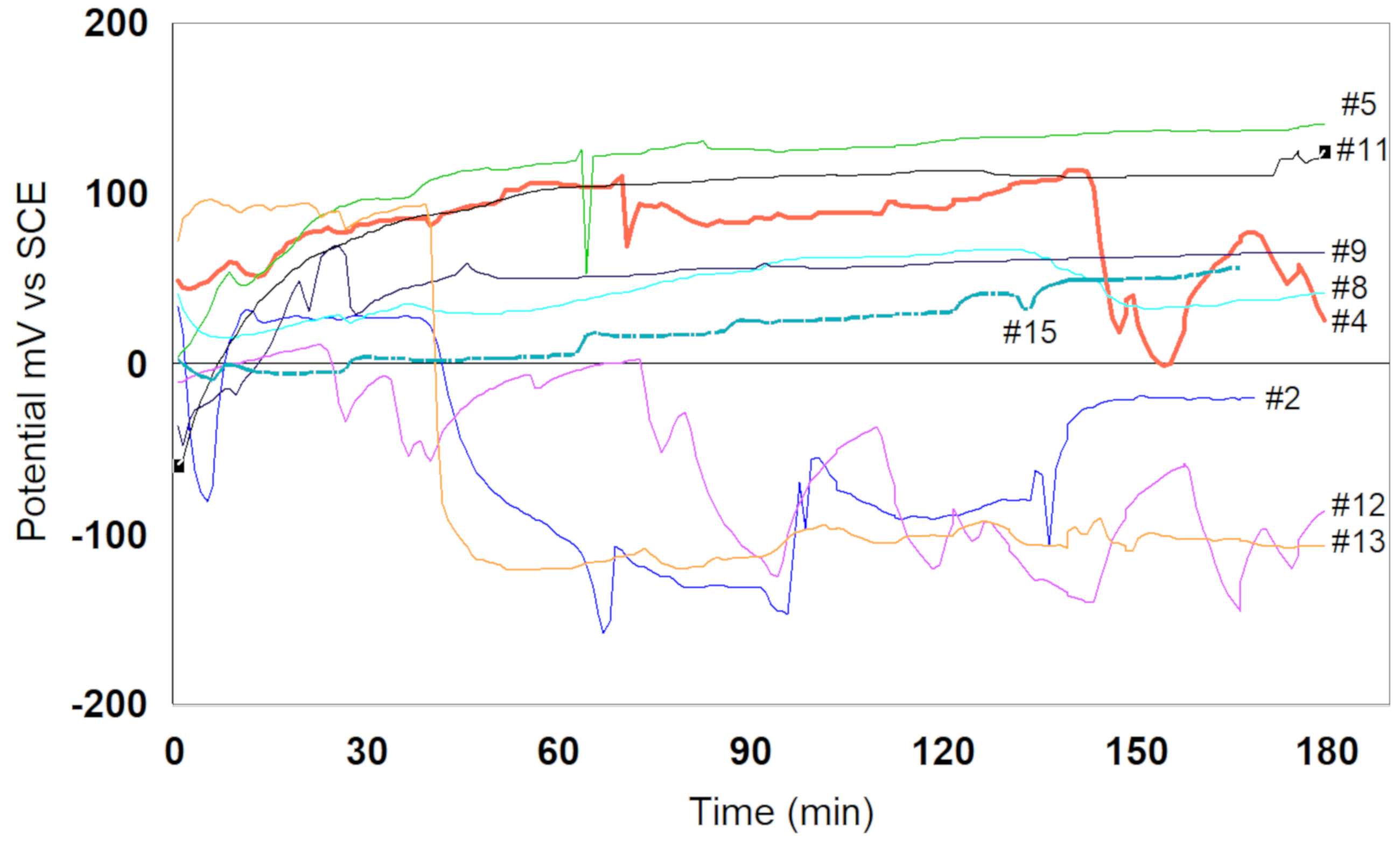
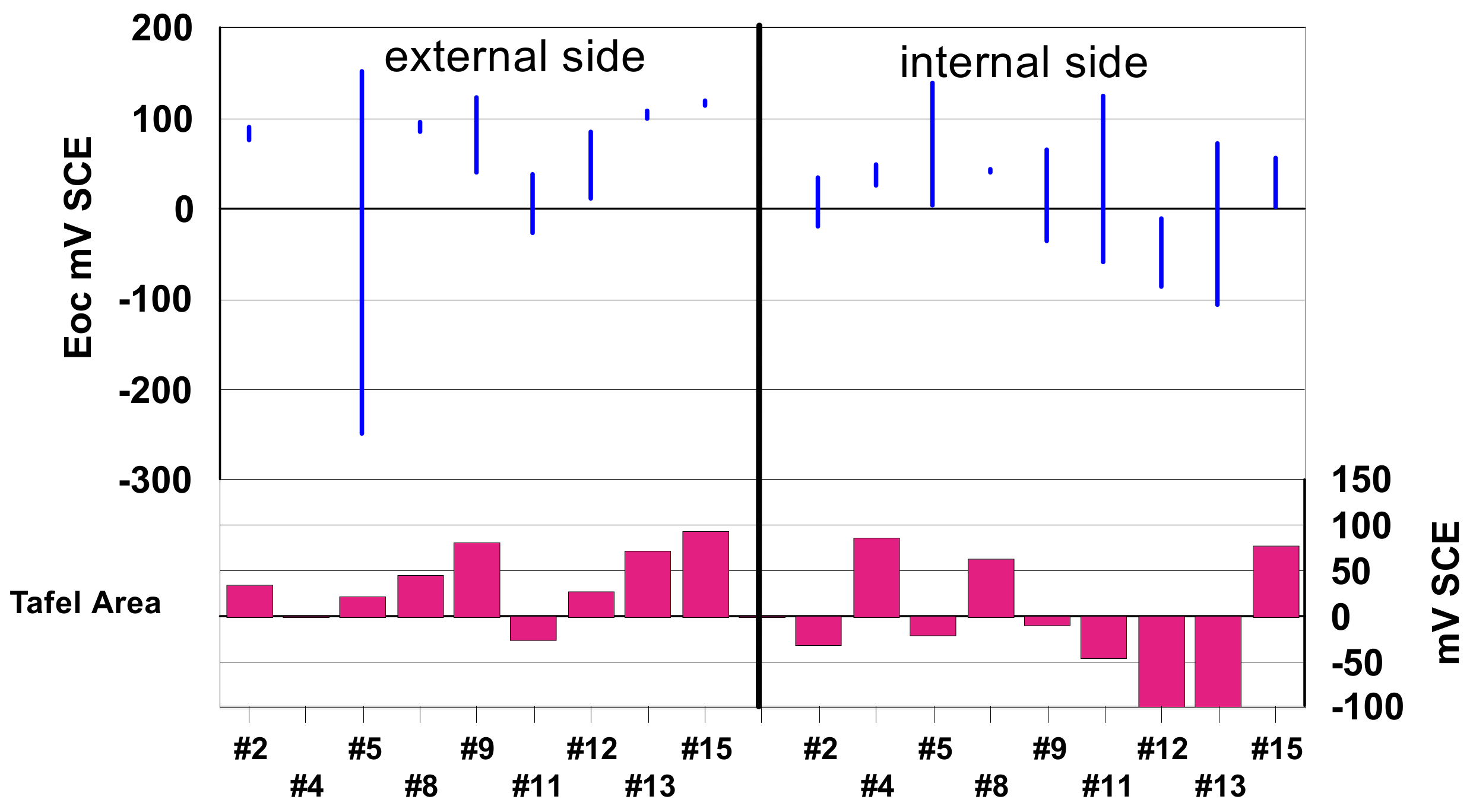
3.4.1. The Open Circuit Potential
3.4.2. The Polarization Curves in the Tafel Domain
- First bullet; Surfaces with a positive open circuit potential in the Tafel domain after scanning, where Ecorr is always positive.
- Surfaces with a positive open circuit potential (at the end of the test) and a negative after scanning Ecorr in the Tafel domain.
- Surfaces with a negative open circuit potential and a negative after scanning Ecorr in the Tafel domain.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Streicher, M.A. Austenitic and Ferritic Stainless Steels. In Uhlig’s Corrosion Handbook, 3rd ed.; Winston Revie, R., Ed.; Revised by Grubb, J.F.; Wiley: Hoboken, NJ, USA, 2011; pp. 657–694. [Google Scholar]
- Fondation Suisse de Cardiologie, Angioplastie Coronaire. Available online: https://www.swissheart.ch/fr/maladies-cardiaques-avc/traitements/traitements/angioplastie-coronaire.html (accessed on 12 December 2020).
- Ali, R.; Greenbaum, A.B.; Kugelmass, A.D. A Review of Available Angioplasty Guiding Catheters, Wires and Balloons–Making the Right Choice. Interv. Cardiol. Rev. 2012, 7, 100–103. [Google Scholar] [CrossRef]
- Lin, Z.; Hsiao, H.-M.; Mackiewicz, D.; Anukhin, B.; Pike, K. Anisotropic Behavior of Radiopaque NiTiPt Hypotube for Biomedical Applications. Adv. Eng. Mater. 2009, 11, B189–B193. [Google Scholar] [CrossRef]
- Diagnostic and Interventional Cardiology, The Basics of Guide Wire Technology. Available online: https://www.dicardiology.com/article/basics-guide-wire-technology (accessed on 12 December 2020).
- Iñigo-García, L.A.; Iñigo-Almansa, L.; Gil-Jiménez, T.; Siles-Rubio, J.R.; Ramírez-Moreno, A.; Pons, J.; Sanz Vazquez, O.; Munoz Bellido, J.F.; Urbaneja Salas, A.; Ruiz Mateas, F. Coronary Angioplasty Guidewires: Differential Characteristics and Technology. J. Cardiol. Curr. Res. 2017, 8, 00278. [Google Scholar] [CrossRef]
- Cunat, P.J. Soudabilité et métallurgie des soudures. In Les Aciers Inoxydables; Lacombe, P., Baroux, B., Beranger, G., Colombier, L., Hochmann, J., Eds.; Les Editions de Physique: Les Ulis, France, 1990; pp. 798–855. [Google Scholar]
- Siddhagiri Metals & Tubes, Welded Tubes. Available online: http://www.siddhagirimetals.com/welded-tube-supplier-astm-b516-b704-b515-b730-b626-a789-a269-b467-b468-b674-b338-a519.html (accessed on 12 December 2020).
- VitaNeedle Stainless Steel, Welded and Drawn Tubing. Available online: https://www.vitaneedle.com/welded-and-drawn-tubing (accessed on 12 December 2020).
- Høl, P.J.; Gjerdet, N.R.; Jonung, T. Corrosion and metal release from overlapping arterial stents under mechanical and electrochemical stress-An experimental study. Mech. Behav. Biomed. Mater. 2019, 93, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, L.; Chen, Y.; Dai, D.; Su, Y.; Shao, L. Corrosion Behavior and in Vitro Cytotoxicity of Ni-Ti and Stainless Steel Arch Wires Exposed to Lysozyme, Ovalbumin, and Bovine Serum Albumin. ACS Omega 2020, 5, 18995–19003. [Google Scholar] [CrossRef]
- Gotman, I. Characteristics of metals used in implants. J. Endourol. 1997, 11, 38338–38339. [Google Scholar] [CrossRef] [PubMed]
- ASTM A262-15, Standard Practices for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steels, ASTM International. 2015. Available online: www.astm.org (accessed on 2 May 2020).
- Streicher, M.A. Intergranular. In Corrosion Tests and Standards: Application and Interpretation, 2nd ed.; Baboian, R., Ed.; ASTM International: West Conshohocken, PA, USA, 2004; pp. 244–266. [Google Scholar]
- Henthorne, M. Intergranular corrosion in Iron and Nickel Base Alloys. In Localized Corrosion-Cause of Metal Failure; Henthorne, M., Ed.; ASTM Technical Publication: Philadelphia, PA, USA, 1972; pp. 66–119. [Google Scholar]
- Reclaru, L.; Lüthy, H.; Ziegenhagen, R.; Eschler, P.-Y.; Blatter, A. Anisotropy of nickel release and corrosion in austenitic stainless steels. Acta Biomater. 2008, 4, 680–685. [Google Scholar] [CrossRef]
- Reclaru, L.; Ziegenhagen, R.; Eschler, P.-Y.; Blatter, A.; Lüthy, H. Effects of Crystallographic Orientation of Stainless Steels on Pitting and Crevice Corrosion and Nickel Release. In New Research on Biomaterials; Bloomington, D.R., Ed.; Nova Science Publisher Inc.: New York, NY, USA, 2007; pp. 237–279. [Google Scholar]
- Reclaru, L.; Ardelean, L.C. Corrosion Susceptibility and Allergy Potential of Austenitic Stainless Steels. Materials 2020, 13, 4187. [Google Scholar] [CrossRef]
- ASM Handbook. Corrosion of Stainless Steel Weldments, Corrosion: Fundamentals, Testing, and Protection; ASM International: Russell Township, OH, USA, 2003; Volume 13A, pp. 301–316. [Google Scholar]
- Wagner, C.; Traud, W. Über die Deutung von Korrosionsvorgängen durch Überlagerung von elektrochemischen Teilvorgängen und über die Potentialbildung an Mischelektroden. Z. Electrochem. 1938, 44, 391–402. [Google Scholar] [CrossRef]
- Haruyama, S. Electrochemical methods in passivity study. Corros. Sci. 1990, 31, 29–38. [Google Scholar] [CrossRef]
- Olefjord, I.; Elfstrom, B.-O. The Composition of the Surface during Passivation of Stainless Steels. Corrosion 1982, 38, 46–52. [Google Scholar] [CrossRef]
- Müller, W.J. Die Bedeckungstheorie der Passivität der Metalle und ihre experimentelle Begründung. J. Phys. Chem. 1934, 38, 132. [Google Scholar] [CrossRef]
- Fleischmann, M.; Pattison, J.; Thirsk, H.R. Electrocrystallization of thin films of thallous chloride on thallium amalgam. Trans. Faraday Soc. 1965, 61, 1256–1269. [Google Scholar] [CrossRef]
- Lukac, C.; Lumsden, J.B.; Smialowska, S.; Staehle, R.W. Effects of Temperature on the Kinetics of Passive Film Growth on Iron. J. Electrochem. Soc. 1975, 122, 1571–1580. [Google Scholar] [CrossRef]
- Bockris, J.O.; Genshaw, M.A.; Brusic, V.; Wroblowa, H. The mechanism of the passivation of iron in neutral solutions: An ellipsometric and coulometric investigation. Electrochim. Acta 1971, 16, 1859–1894. [Google Scholar]
- Foroulis, Z.A.; Uhlig, H.H. Effect of Cold-Work on Corrosion of Iron and Steel in Hydrochloric Acid. J. Electrochem. Soc. 1964, 111, 522–528. [Google Scholar] [CrossRef]
- Kabanov, B.N.; Leikis, Z.I. Untersuchung der Passivität von Metallen durch Messung der Elektrodenimpedanz bei hohen Frequenzen. Z. Elektrochem. 1958, 62, 660–663. [Google Scholar]
- Kolotyrkin, Y.M. Electrochemical behaviour and anodic passivity mechanism of certain metals in electrolyte solutions. Z. Elektrochem. 1958, 62, 664–669. [Google Scholar]
- Frankenthal, R.P. On the Passivity of Iron-Chromium Alloys: II. The Activation Potential. J. Electrochem. Soc. 1969, 116, 580–585. [Google Scholar] [CrossRef]
- Lara Banda, M.; Gaona Tiburcio, C.; Zambrano-Robledo, P.; Cabral, J.A.; Estupinán, F.; Baltazar-Zamora, M.A.; Croche, R.; Vera, E.; Almeraya-Calderón, F. Corrosion Behaviour of 304 Austenitic, 15-5PH and 17-4PH Passive Stainless Steels in Acid Solutions. Int. J. Electrochem. Sci. 2018, 13, 10314–10324. [Google Scholar] [CrossRef]
- Hegelmann, E.; Hengst, P.; Hollmann, P.; Thronicke, P.; Buchwalder, J.A.; Schimpf, C.; Hunger, R.; Zenker, R. Improvement of Wear and Corrosion Resistance of Austenitic Stainless Steel by Combined Treatment Using Electron Beam Cladding and Subsequent Gas Nitrocarburizing. Adv. Eng. Mater. 2019, 21, 1900365. [Google Scholar] [CrossRef]
- Gietzelt, T.; Toth, V.; Huell, A. Challenges of Diffusion Bonding of Different Classes of Stainless Steels. Adv. Eng. Mater. 2017, 23, 1700367. [Google Scholar] [CrossRef]
- Srinivasan, N.; Kain, V.; Birbilis, N.; Sunil Kumar, B.; Gandhi, M.N.; Sivaprasad, P.V.; Chai, G.; Lodh, A.; Ahmedabadi, P.M.; Samajdar, I. Plastic deformation and corrosion in austenitic stainless steel: A novel approach through microtexture and infrared spectroscopy. Corros. Sci. 2016, 111, 404–413. [Google Scholar] [CrossRef]
- Tranchida, G.; Clesi, M.; Di Franco, F.; Di Quarto, F.; Santamaria, M. Electronic properties and corrosion resistance of passive films on austenitic and duplex stainless steels. Electrochim. Acta 2018, 273, 412–423. [Google Scholar] [CrossRef]
- Jargelius-Pettersson, R.F.A.; Pound, B.G. Examination of the Role of Molybdenum in Passivation of Stainless Steels Using AC Impedance Spectroscopy. J. Electrochem. Soc. 1998, 145, 1462–1469. [Google Scholar] [CrossRef]
- Kruger, J. Passivity. In Uhlig’s Corrosion Handbook, 3rd ed.; Winston Revie, R., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 151–157. [Google Scholar]
- Bohni, H. Localized Corrosion of Passive Metals. In Uhlig’s Corrosion Handbook, 3rd ed.; Winston Revie, R., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 157–171. [Google Scholar]
- Lizlovs, E.A.; Bond, A.P. Anodic Polarization Behavior of High-Purity 13 and 18% Cr Stainless Steels Bond. J. Electrochem. Soc. 1975, 122, 719–723. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Revie, R.W. Corrosion and Corrosion Control, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1985. [Google Scholar]
- Crow, W.B.; Myers, J.R.; Marvin, B.D. Anodic polarization behaviour of Ni-Al alloys in sulfuric acid solutions. Corrosion 1971, 27, 459–465. [Google Scholar] [CrossRef]
- Okamoto, G. Passive film of 18-8 stainless steel structure and its function. Corros. Sci. 1973, 13, 471–489. [Google Scholar] [CrossRef]
- Tomashov, N.D.; Chernova, G.P.; Ruscol, Y.S.; Ayuyan, G.A. The passivation of alloys on titanium bases. Electrochim. Acta 1974, 19, 159–172. [Google Scholar] [CrossRef]










| DIN | AISI | C | Si | Mn | P | S | Cr | Mo | Ni | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.4306 | 304 L | <0.030 | <1.50 | <1.50 | <0.035 | <0.020 | 17.0–20.0 | -- | 8.0–12.0 | N 0.10–0.20 |
| Sampling | #1 | #2(ref) | #3 | #4 | #5 | #6 | #7 | #8(ref) | #9 | #10 | #11 | #12 | #13(ref) | #14 | #15 | #16 | #17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Passivation process | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |||
| EDX analysis | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| Carbon (C) and sulphur (S) analysis | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| Metallography (welding) | x | x | x | x | x | x | x | ||||||||||
| Strauss Test (ASTM A262-15) | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| Optical and SEM observation | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| Passivation polarization test | x | x | x | x | x | x | x | x | x |
| Procedure | Medium | Time (min) |
|---|---|---|
| Pickling | HNO3 + H3PO4 + CH3COOH | 60 |
| Rinsing | Deionized H2O | 15 |
| Passivation | 20% HNO3 | 20 |
| Rinsing | Deionized H2O | 15 |
| Neutralisation | Sol. 2% ETA | 10 |
| Rinsing | Deionized H2O | 10 |
| Drying | N2 of N55 purity | - |
| Spectre | Si | Cr | Mn | Fe | Ni | %C | %S |
|---|---|---|---|---|---|---|---|
| Internal surface | 0.74 | 18.87 | 1.53 | 65.93 | 8.42 | 0.046 | 0.004 |
| External surface | 0.76 | 18.71 | 1.61 | 66.58 | 9.04 |
| Code | Loss [mg] |
|---|---|
| #1 | 0.44 |
| #2(ref) | 0.04 |
| #3 | 0.065 |
| #4 | 0.235 |
| #6 | 0.045 |
| #7 | 0.035 |
| #8(ref) | 0.025 |
| #10 | 0.02 |
| #13(ref) | 0.02 |
| #14 | 0.02 |
| #15 | 0.035 |
| #16 | 0.165 |
| #17 | 0.09 |
| Code | External Surface | Internal Surface | ||
|---|---|---|---|---|
| Start potential | Final potential | Start potential | Final potential | |
| (mV vs. SCE) | (mV vs. SCE) | (mV vs. SCE) | (mV vs. SCE) | |
| #2(ref) | 77 | 90 | 34 | −20 |
| #4 | - | - | 49 | 26 |
| #5 | −249 | 152 | 4 | 140 |
| #8(ref) | 86 | 96 | 41 | 41 |
| #9 | 41 | 123 | −36 | 65 |
| #11 | −26 | 38 | −59 | 125 |
| #12 | 12 | 85 | −11 | −86 |
| #13(ref) | 109 | 99 | 72 | −106 |
| #15 | 115 | 119 | 3 | 56 |
| Code | E(i=o) mV | Icorr nA | ba mV/dec | bc mV/dec | °(Ei = 0 + 150 mV) µC | Code | E(i=o) mV | Icorr nA | ba mV/dec | bc mV/dec | °(Ei = 0 + 150 mV) µC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #2E | 24 | 6.4 | 246 | 69 | 0.99 | #2I | −4 | 24.44 | 249 | 83 | 3.88 |
| #4E | - | - | - | - | #4I | 81 | 0.48 | 124 | 54 | 0.78 | |
| #5E | 29 | 17.5 | 168 | 60 | 5.56 | #5I | 82 | 0.11 | 162 | 43 | 8.74 |
| #8E | 70 | 7.58 | 201 | 57 | 1.19 | #8I | 20 | 2.86 | 220 | 55 | 5.71 |
| #9E | 82 | 0.77 | 200 | 72 | 4.14 | #9I | −12 | 2.85 | 259 | 50 | 0.91 |
| #11E | −30 | 1.71 | 187 | 34 | 4.69 | #11I | 30 | 1.79 | 174 | 40 | 4.99 |
| #12E | 26 | 6.81 | 89 | 52 | 19.72 | #12I | −100 | 3.04 | 86 | 72 | 5.76 |
| #13E | 60 | 6.15 | 136 | 75 | 0.75 | #13I | −100 | 70.2 | 166 | 148 | 2.68 |
| #15E | 78 | 0.18 | 99 | 43 | 0.66 | #15I | 70 | 1.15 | 218 | 20 | 1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reclaru, L.; Ardelean, L.C. Evaluation of Passivation Process for Stainless Steel Hypotubes Used in Coronary Angioplasty Technique. Coatings 2021, 11, 448. https://doi.org/10.3390/coatings11040448
Reclaru L, Ardelean LC. Evaluation of Passivation Process for Stainless Steel Hypotubes Used in Coronary Angioplasty Technique. Coatings. 2021; 11(4):448. https://doi.org/10.3390/coatings11040448
Chicago/Turabian StyleReclaru, Lucien, and Lavinia Cosmina Ardelean. 2021. "Evaluation of Passivation Process for Stainless Steel Hypotubes Used in Coronary Angioplasty Technique" Coatings 11, no. 4: 448. https://doi.org/10.3390/coatings11040448
APA StyleReclaru, L., & Ardelean, L. C. (2021). Evaluation of Passivation Process for Stainless Steel Hypotubes Used in Coronary Angioplasty Technique. Coatings, 11(4), 448. https://doi.org/10.3390/coatings11040448







