Application of Nanotechnology in Immunity against Infection
Abstract
1. Introduction
2. Current Situation of Antimicrobial Application
3. Nanotechnology and Anti-Infection Immunity
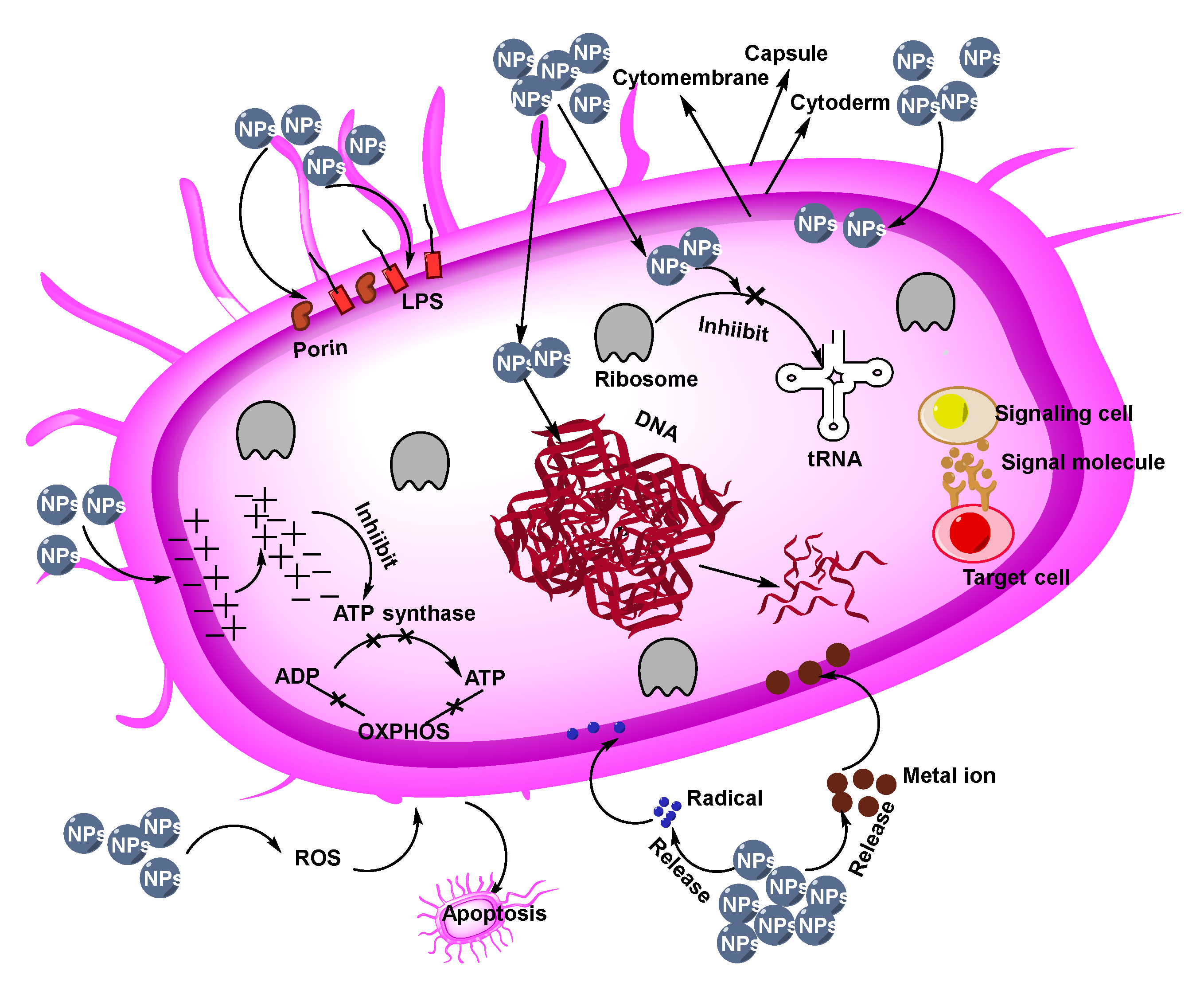
3.1. Antibacterial NPs
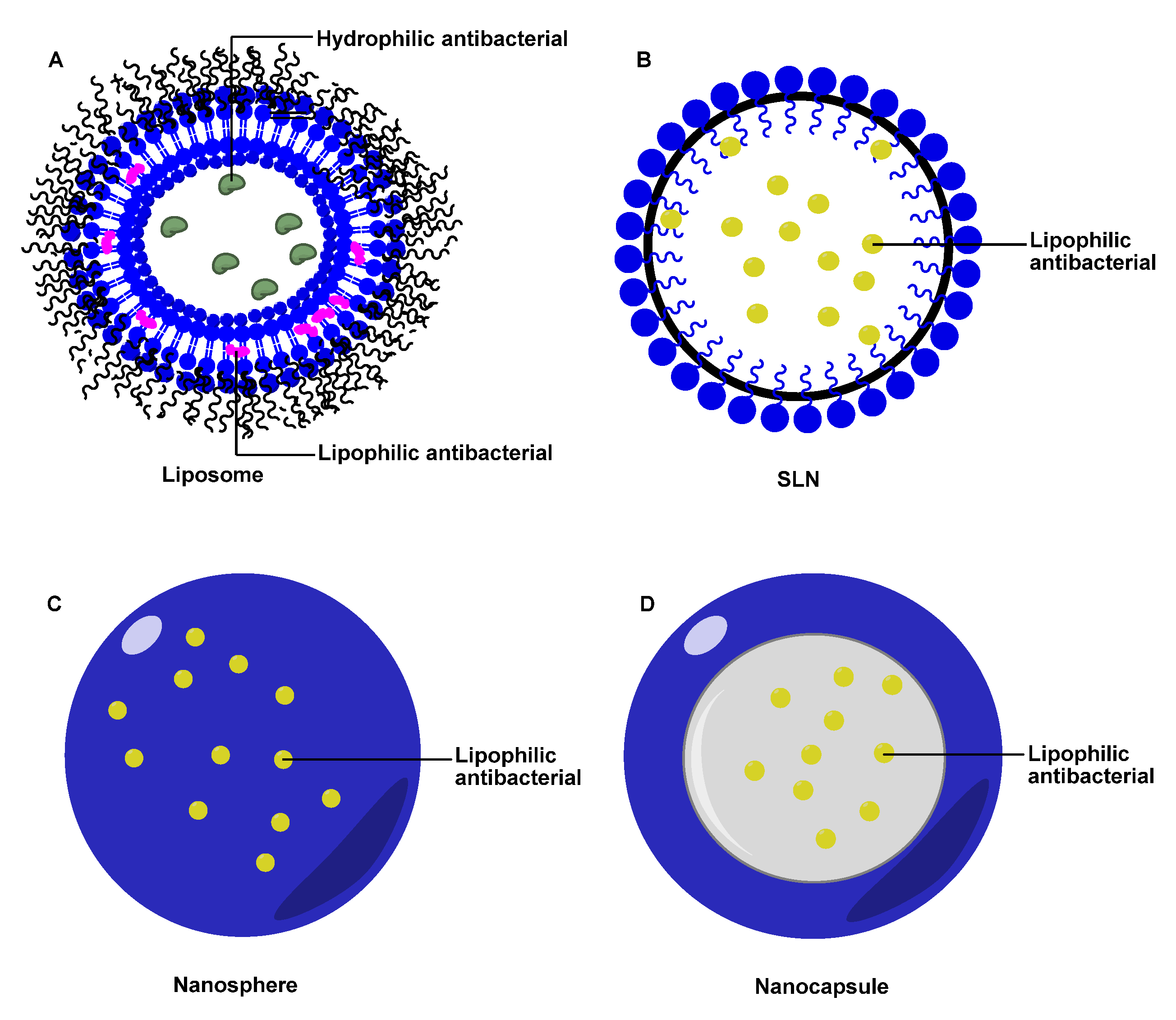
3.2. Antibiotic Nanocarrier
3.2.1. Liposomes
3.2.2. Solid Lipid NPs (SLN)
3.2.3. Polymer NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s Plenty of Room at the Bottom: An Invitation to Enter a New Field of Physics; California Institute of Technology: Los Angeles, CA, USA, 1960. [Google Scholar]
- Drexler, K.E. Engines of Creation: The Coming Era of Nanotechnology; Anchor Books: New York, NY, USA, 1986; pp. 1–20. [Google Scholar]
- Mendes, P.M. Cellular nanotechnology: Making biological interfaces smarter. Chem. Soc. Rev. 2013, 42, 9207–9218. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; CouvreurHillaireau, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4746. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, J.; Xu, S.; Afrin, T.; Xu, W.; Sun, L.; Wang, X. Application of anisotropic silver nanoparticles: Multifunctionalization of wool fabric. J. Colloid Interf. Sci. 2011, 356, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gunawan, P.; Lou, X.W.; Xu, R. Silver nanoparticles deposited layered double hydroxide nanoporous coatings with excellent antimicrobial activities. Adv. Funct. Mater. 2012, 22, 780–787. [Google Scholar] [CrossRef]
- Ma, J.; Xu, R.; Sun, J.; Zhao, D.; Tong, J.; Sun, X. Nanoparticle surface and nanocore properties determine the effect on radiosensitivity of cancer cells upon ionizing radiation treatment. J. Nanosci. Nanotechnol. 2013, 13, 1472–1475. [Google Scholar] [CrossRef]
- Singh, R.; Smitha, M.S.; Surinder, P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef]
- Projan, S.J. Why is big Pharma getting out of antibacterial drug discovery? Curr. Opin. Microbiol. 2003, 6, 427–430. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 2, 86–102. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef]
- Katz, B.; Waites, K. Emerging intracellular bacterial infections. Clin. Lab. Med. 2004, 24, 627–649. [Google Scholar] [CrossRef]
- Briones, E.; Colino, C.I.; Lanao, J.M. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J. Control. Release 2008, 125, 210–227. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W.; Stapleton, P.D.; Paul Luzio, J. New ways to treat bacterial infections. Drug Discov. Today 2002, 7, 1086–1091. [Google Scholar] [CrossRef]
- Allaker, R.P.; Guogang, R. Potential impact of nanotechnology on the control of infectious disease. T Roy. Soc. Trop. Med. H 2008, 102, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver NPs as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold NPs functionalized with cationic and anionic side chains. Bioconjugate Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Durmus, N.; Taylor, E.; Kummer, K.; Webster, T.J. Enhanced efficacy of superparamagnetic iron oxide NPs against antibiotic-resistant biofilms in the presence of metabolites. Adv Mater. 2013, 25, 5706–5713. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO NPs toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver NPs depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microb. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, S.; Prasad, S.; Gambhir, I.S. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig. J. Nanomater. Bios. 2008, 3, 115–122. [Google Scholar]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold NPs on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Lok, C.; Ho, C.; Chen, R.; He, Q.; Yu, W.; Sun, H.; Tam, P.K.; Chiu, J.; Che, C. Proteomic analysis of the mode of antibacterial action of silver NPs. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 213–225. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.O.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver NPs. Nanomed. Nanotechnol. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and antibacterial activity of Fe3O4@Ag NPs. Nanotechnology 2007, 18, 604–611. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver NPs and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Leid, J.G.; Ditto, A.J.; Knapp, A.; Shah, P.N.; Wright, B.D.; Blust, R.; Christensen, L.; Clemons, C.B.; Wilber, J.P.; Young, G.W.; et al. In vitro antimicrobial studies of silver carbene complexes: Activity of free and nanoparticle carbene formulations against clinical isolates of pathogenic bacteria. J. Antimicrob. Chemoth. 2011, 67, 138–148. [Google Scholar] [CrossRef]
- Schaller, M.; Laude, J.; Bodewaldt, H.; Hammb, G.; Kortinga, H.C. Toxicity and antimicrobial activity of a hydrocolloid dressing containing silver particles in an ex vivo model of cutaneous infection. Skin Pharmacol. Phys. 2004, 17, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Heggers, J.P.; Richard, J.W.; Spencer, B.A.; McCoy, L.F.; Carino, E.; Washington, J.W.; Edgar, P.L.; Rosenblatt, J.; Goodheart, R. Acticoat versus silverlon: The truth. Burn Care Rehabil. 2002, 23, S115. [Google Scholar] [CrossRef]
- Ip, M.; Lui, S.L.; Poon, V.K.; Lung, I.; Burd, A. Antimicrobial activities of silver dressings: An in vitro comparison. J. Med. Microbiol. 2006, 55, 59–63. [Google Scholar] [CrossRef]
- Jun, J.; Yuan-Yuan, D.; Shao-hai, W.; Zhang, S.F.; Wang, Z.Y. Preparation and characterization of antibacterial silver-containing nanofifibers for wound dressing applications. J. US-China Med. Sci. 2007, 4, 45–52. [Google Scholar]
- Tian, Y.; Cao, H.; Qiao, Y.; Meng, F.; Liu, X. Antibacterial activity and cytocompatibility of titanium oxide coating modified by iron ion implantation. Acta Biomater. 2014, 10, 4505–4517. [Google Scholar] [CrossRef]
- Cao, H.; Meng, F.; Liu, X. Antimicrobial activity of tantalum oxide coatings decorated with Ag nanoparticles. J. Vac. Sci. Technol. A 2016, 34, 04C102. [Google Scholar] [CrossRef]
- Drake, P.L.; Hazelwood, K.J. Exposure-related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver NPs: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Taglietti, A.; Yuri, A.D.F.; Amato, E.; Cucca, L.; Dacarro, G.; Grisoli, P.; Necchi, V.; Pallavicini, P.; Pasotti, L.; Patrini, M. Antibacterial activity of glutathione-coated silver nanoparticles against gram positive and gram negative bacteria. Langmuir 2012, 28, 8140−8148. [Google Scholar] [CrossRef]
- Brobbey, K.J.; Haapanen, J.; Mäkelä, J.M.; Gunell, M.; Eerola, E.; Rosqvist, E.; Peltonen, J.; Saarinen, J.J.; Tuominen, M.; Toivakka, M. Effect of plasma coating on antibacterial activity of silver nanoparticles. Thin Solid Films 2019, 672, 75−82. [Google Scholar] [CrossRef]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based NPs and their toxicity assessment. Wires Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharmaceut. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Santos-Magalhães, N.S.; Mosqueira, V.C. Nanotechnology applied to the treatment of malaria. Adv. Drug Deliver Rev. 2010, 62, 560–575. [Google Scholar] [CrossRef]
- Mansour, H.M.; Rhee, Y.S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 12, 299–319. [Google Scholar] [CrossRef]
- Sosnik, A.; Carcaboso, A.M.; Glisoni, R.J.; Moretton, M.A.; Chiappetta, D.A. New old challenges in tuberculosis: Potentially effective nanotechnologies in drug delivery. Adv. Drug Deliver Rev. 2010, 62, 547–559. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.M.; Huang, C.M. Development of NPs for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Abeylath, S.C.; Turos, E. Drug delivery approaches to overcome bacterial resistance to β-lactam antibiotics. Expert Opin. Drug Deliv. 2008, 5, 931–949. [Google Scholar] [CrossRef]
- Lasic, D.D. Stealth liposomes. Ann. Biomed. Eng. 1991, 19, 594. [Google Scholar]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Schumacher, I.; Margalit, R. Liposome-encapsulted ampicillin: Physicochemical and antibacterial properties. J. Pharm. Sci. US 1997, 86, 635–641. [Google Scholar] [CrossRef]
- Magallanes, M.; Dijkstra, J.; Fierer, J. Liposome-incorporated ciproflfloxacin in treatment of murine salmonellosis. Antimicrob. Agents Chemother. 1993, 37, 2293–2297. [Google Scholar] [CrossRef]
- Onyeji, C.O.; Nightingale, C.H.; Marangos, M.N. Enhanced killing of methicillin-resistant Staphylococcus aureus in human macrophages by liposome-entrapped vancomycin and teicoplanin. Infection 1994, 22, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Gangadhanun, P.R.; Ashtekar, D.A.; Ghori, N.; Goldstein, J.A.; Debs, R.J.; Gangadharam, D.N.; Gangadharam, P.R. Chemotherapentic potential of free and liposome encapsulated streptomycin against experimental Mycobacterium avium complex infections in beige mice. J. Antimicrob. Chemother. 1991, 28, 425–435. [Google Scholar]
- Omri, A.; Suntres, Z.E.; Shek, P.N. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem. Pharmacol. 2002, 64, 1407–1413. [Google Scholar] [CrossRef]
- Schiffelers, R.; Storm, G.; Bakker-Woudenberg, I. Liposome-encapsulated aminoglycosides in pre-clinical and clinical studies. J. Antimicrob. Chemother. 2001, 48, 333–344. [Google Scholar] [CrossRef]
- Kim, H.J.; Jones, M.N. The delivery of benzyl penicillin to Staphylococcus aureus. J. Liposome Res. 2004, 14, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Müller, R.H. Cosmetic applications for solid lipid NPs (SLN). Int. J. Pharmaceut. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid NPs (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Pandey, R.; Khuller, G.K. Solid lipid particle-based inhalable sustained drug delivery system against experimenta tuberculosis. Tuberculosis 2005, 85, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Gasco, M.R.; Chetoni, P.; Burgalassi, S.; Saettone, M.F. Solid lipid NPs (SLN) as ocular delivery system for tobramycin. Int. J. Pharmaceut. 2002, 238, 241–245. [Google Scholar] [CrossRef]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid lipid NPs (SLN) as carriers for the topical delivery of econazole nitrate: In-vitro characterization, ex-vivo and in-vivo studies. J. Pharm. Pharmacol. 2007, 59, 1057–1064. [Google Scholar] [CrossRef]
- Jain, D.; Banerjee, R. Comparison of ciproflfloxacin hydrochloride-loaded protein, lipid, and chitosan NPs for drug delivery. J. Biomed. Mater. Res. B 2008, 86B, 105–112. [Google Scholar] [CrossRef]
- Fattal, E.; Youssef, M.; Couvreur, P.; Andremont, A. Treatment of experimental salmonellosis in mice with ampicillin-bound NPs. Antimicrob. Agents Chemother. 1989, 33, 1540–1543. [Google Scholar] [CrossRef]
- Turos, E.; Reddy, G.S.K.; Greenhalgh, K.; Ramaraju, P.; Abeylath, S.C.; Jang, S.; Dickeyc, S.; Lim, D.V. Penicillin-bound polyacrylate NPs: Restoring the activity of β- lactam antibiotics against MRSA. Bioorg. Med. Chem. Lett. 2007, 17, 3468–3472. [Google Scholar] [CrossRef] [PubMed]
- Turos, E.; Shim, J.Y.; Wang, Y.; Greenhalgh, K.; Reddy, G.S.; Dickey, S.; Lim, D.V. Antibiotic-conjugated polyacrylate NPs: New opportunities for development of anti-MRSA agents. Bioorg. Med. Chem. Lett. 2007, 17, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Espuelas, M.S.; Legrand, P.; Loiseau, P.M.; Cbories, C.; Barratt, G.; Irache, J.M. In vitro antileishmanial activity of amphotericin B loaded in poly(1-Caprolactone) nanospheres. J. Drug Target 2002, 10, 593–599. [Google Scholar] [CrossRef]
- Forestier, F.; Gerrier, P.; Chaumard, C.; Quero, A.M.; Convreur, P.; Labarre, C. Effect of nanoparticle-bound ampicillin on the survival of Listeria monocytogenes in mouse peritoneal macrophages. J. Antimicrob. Chemother. 1992, 30, 173–179. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Smith, A. Evaluation of poly (lactic acid) as a biodegradable drug delivery system for parenteral administration. Int. J. Pharm. 1986, 30, 215–220. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Shi, W.; Ma, Q.; Cui, H.; Zhang, L. Application of Nanotechnology in Immunity against Infection. Coatings 2021, 11, 430. https://doi.org/10.3390/coatings11040430
Zhang J, Shi W, Ma Q, Cui H, Zhang L. Application of Nanotechnology in Immunity against Infection. Coatings. 2021; 11(4):430. https://doi.org/10.3390/coatings11040430
Chicago/Turabian StyleZhang, Jingxin, Weiyue Shi, Qiang Ma, Haixin Cui, and Liang Zhang. 2021. "Application of Nanotechnology in Immunity against Infection" Coatings 11, no. 4: 430. https://doi.org/10.3390/coatings11040430
APA StyleZhang, J., Shi, W., Ma, Q., Cui, H., & Zhang, L. (2021). Application of Nanotechnology in Immunity against Infection. Coatings, 11(4), 430. https://doi.org/10.3390/coatings11040430




