Synthesis, Luminescent Properties and White LED Fabrication of Sm3+ Doped Lu2WMoO9
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
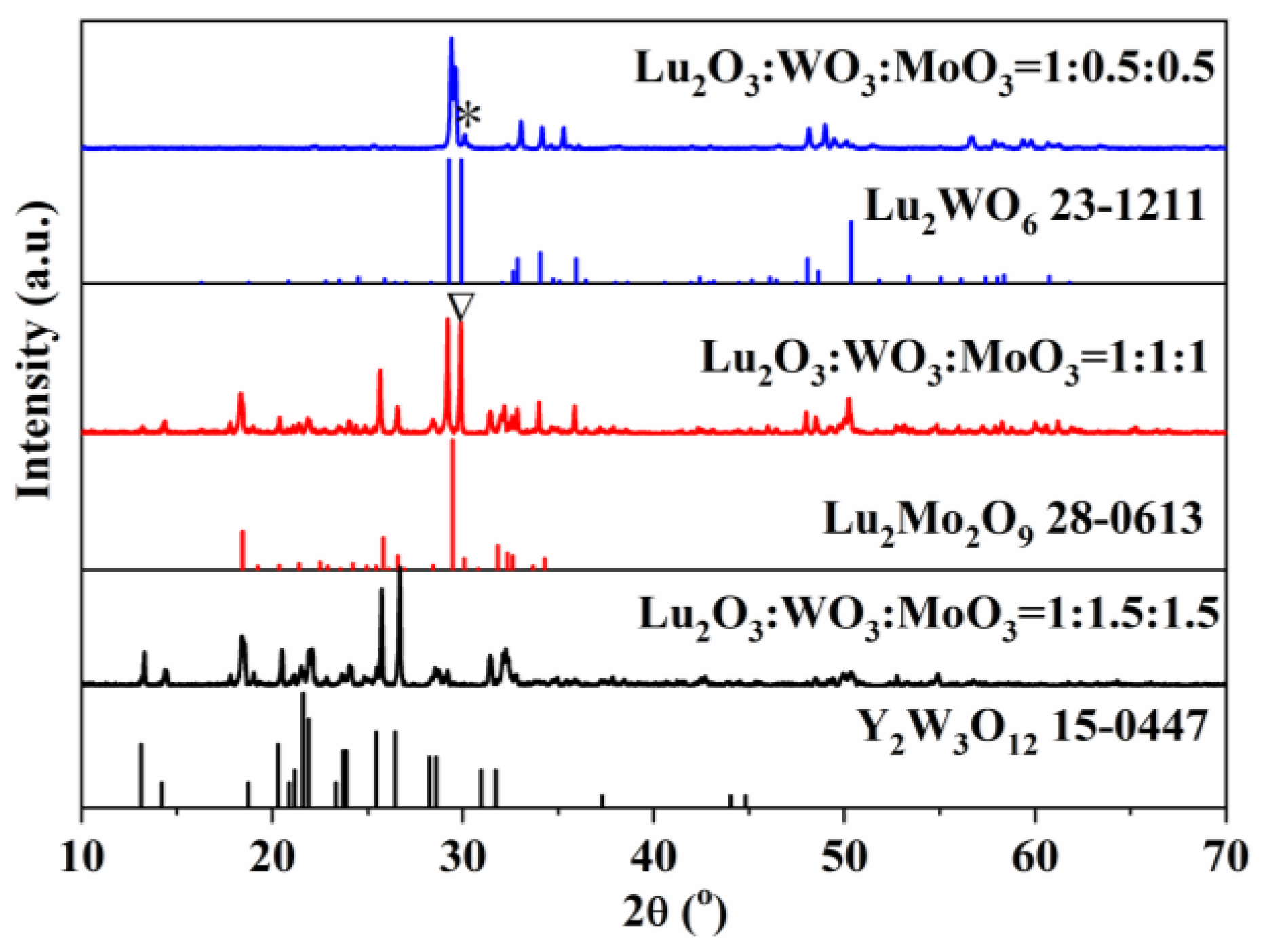
3.1. Crystalline and Morphology
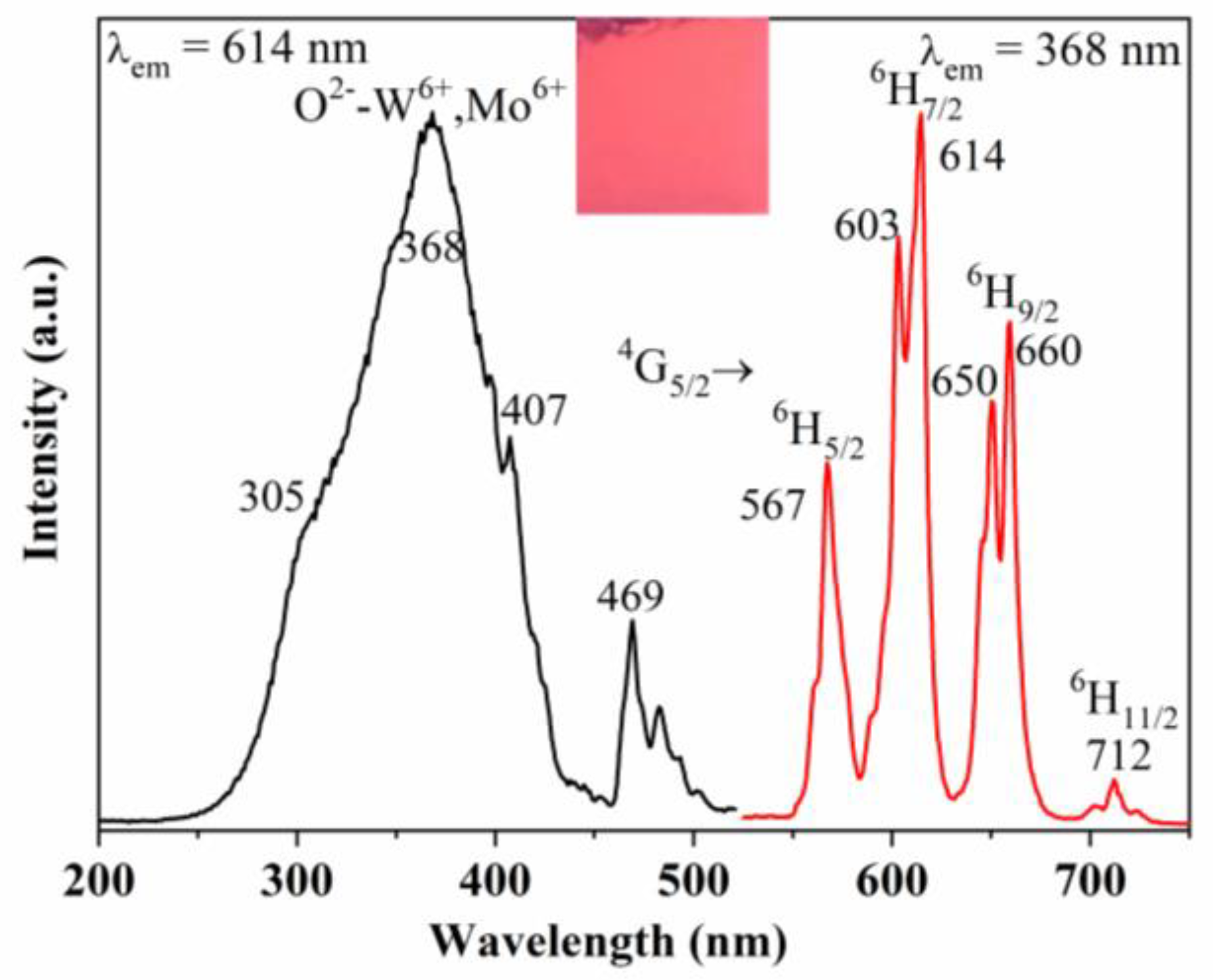
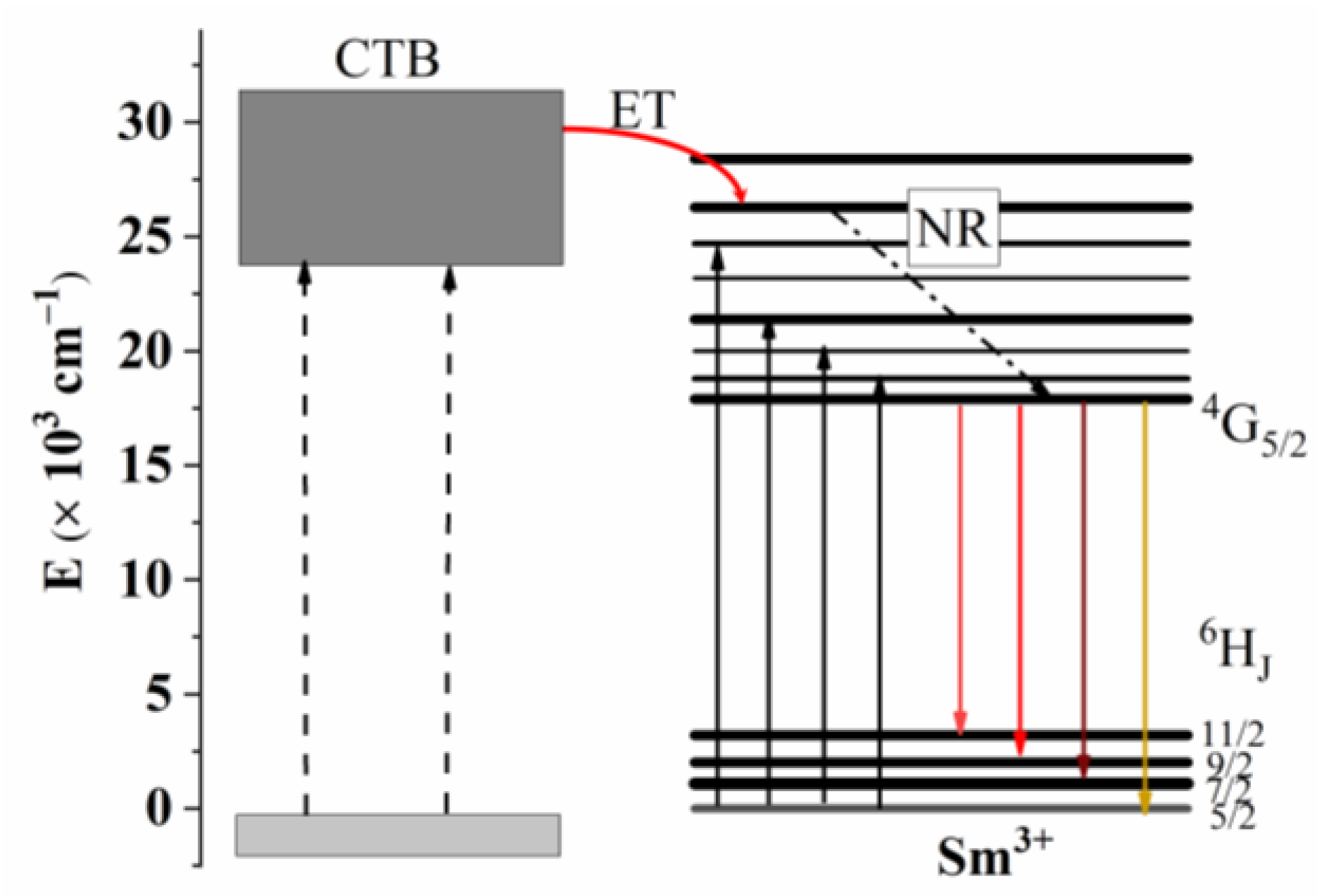
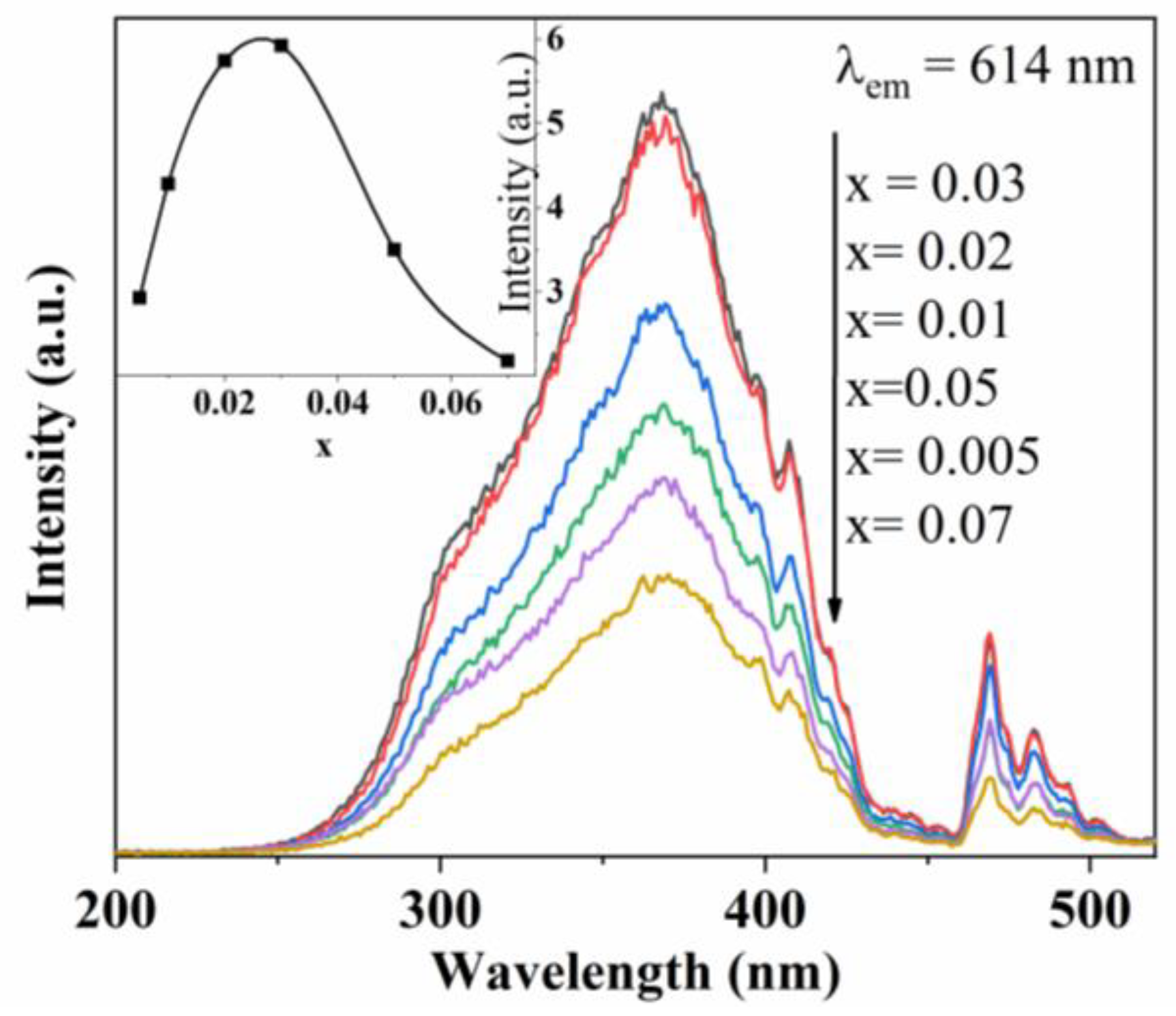
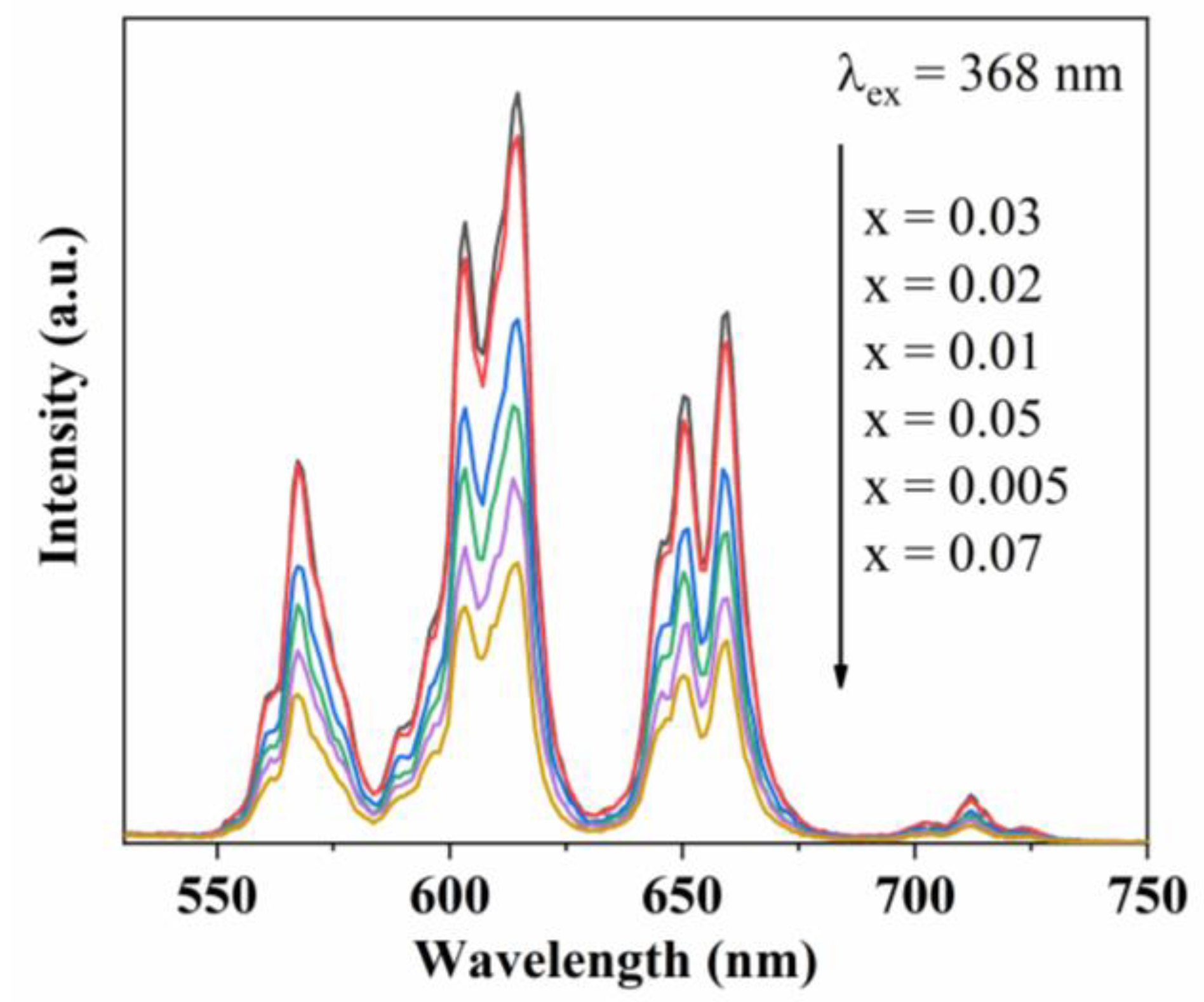
3.2. PL Spectra
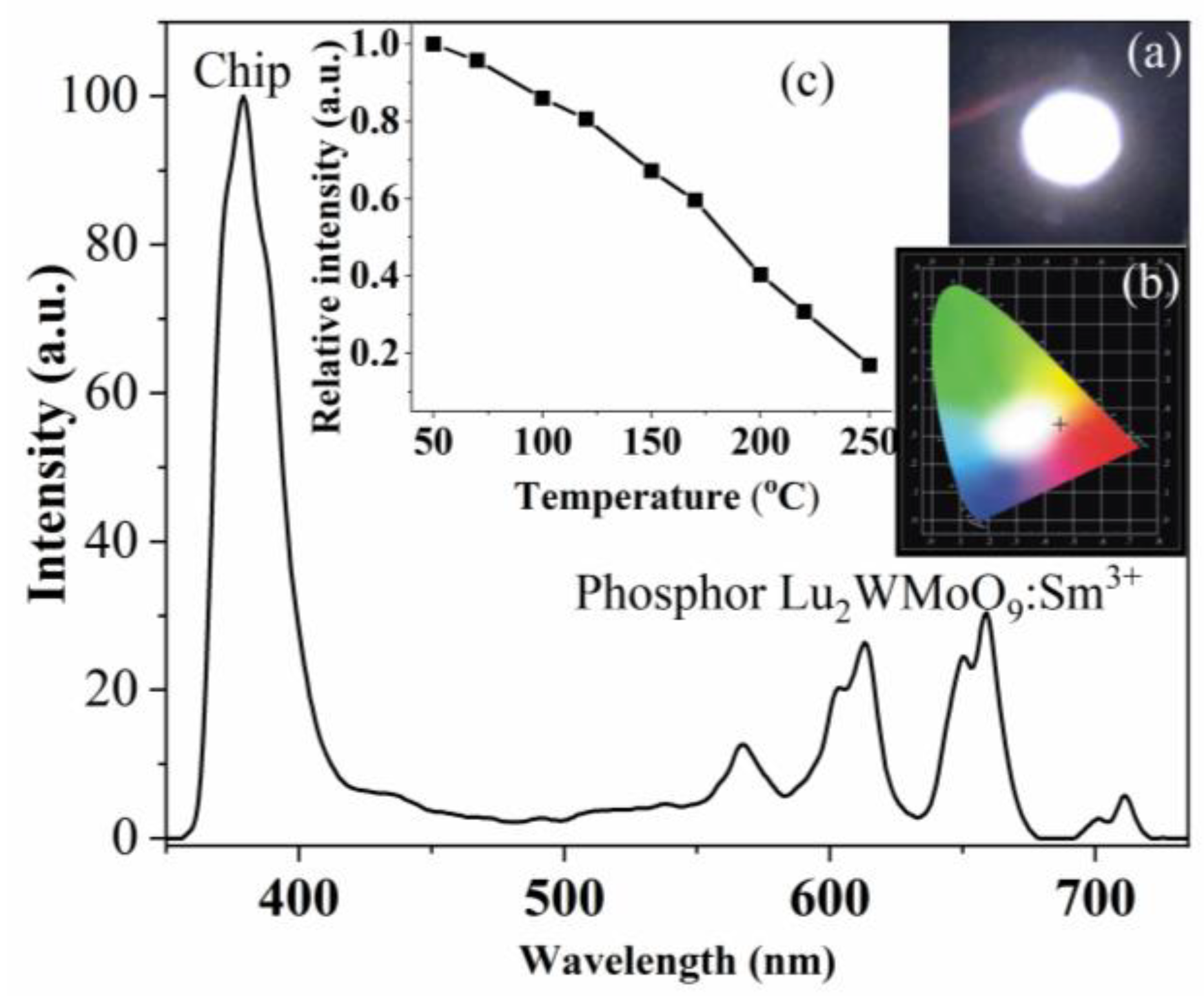
3.3. W-LED Fabrication and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daicho, H.; Iwasaki, T.; Enomoto, K.; Sasaki, Y.; Maeno, Y.; Shinomiya, Y.; Aoyagi, S.; Nishibori, E.; Sakata, M.; Sawa, H.; et al. A novel phosphor for glareless white light-emitting diodes. Nat. Commun. 2012, 3, 1132. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, W.; Li, W.; Ren, C.; Jiang, H.; Shi, J. Sr2ZnWO6:Eu3+,Bi3+,Li+: A potential white-emitting phosphor for near-ultraviolet white light-emitting diodes. J. Lumin. 2015, 31, 665–670. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, H.; Yu, Y.; Chen, D.; Xu, J.; Wang, Y. A new-generation color converter for high-power white LED: Transparent Ce3+: YAG phosphor-in-glass. Laser Photon. Rev. 2014, 8, 158–164. [Google Scholar] [CrossRef]
- Lian, H.; Huang, Q.; Chen, Y.; Li, K.; Liang, S.; Shang, M.; Liu, M.; Lin, J. Resonance Emission Enhancement (REE) for Narrow Band Red-Emitting A2GeF6:Mn4+ (A = Na, K, Rb, Cs) Phosphors Synthesized via a Precipitation–Cation Exchange Route. Inorg. Chem. 2017, 56, 11900–11910. [Google Scholar] [CrossRef]
- Khan, S.A.; Zhong, H.; Ji, W.; Hao, L.-Y.; Abadikhah, H.; Xu, X.; Khan, N.Z.; Agathopoulos, S. Single-Phase White Light-Emitting CaxBa(9–x)Lu2Si6O24:Eu2+/Mn2+ Phosphors. ACS Omega 2017, 2, 6270–6277. [Google Scholar] [CrossRef]
- Li, J.; Liang, Q.; Hong, J.-Y.; Yan, J.; Dolgov, L.; Meng, Y.; Xu, Y.; Shi, J.; Wu, M. White Light Emission and Enhanced Color Stability in a Single-Component Host. ACS Appl. Mater. Interfaces 2018, 10, 18066–18072. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Liu, Y.; Yang, T.; Huang, Z.; Fang, M. Crystal structure tailoring and luminescence tuning of Sr1-xBaxAl2Si2O8:Eu2+ phosphors for white-light-emitting diodes. J. Alloys Compd. 2019, 776, 554–559. [Google Scholar] [CrossRef]
- Wang, X.-M.; Wang, C.-H.; Kuang, X.-J.; Zou, R.-Q.; Wang, Y.-X.; Jing, X.-P. Promising Oxonitridosilicate Phosphor Host Sr3Si2O4N2: Synthesis, Structure, and Luminescence Properties Activated by Eu2+ and Ce3+/Li+ for pc-LEDs. Inorg. Chem. 2012, 51, 3540–3547. [Google Scholar] [CrossRef]
- Huang, C.-H.; Luo, L.; Yeh, Y.-T.; Jang, S.-M.; Liu, W.-R. Novel red-emitting garnet Na2CaTi2Ge3O12:Pr3+,Na+ phosphors. RSC Adv. 2014, 4, 5513. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Zhang, Z.; Liu, Z. The synthesis and luminescent properties of Dy/Re (Re = Tb or Eu) co-doped Gd2(WO4)3 phosphor with tunable color via energy transfer. J. Lumin- 2019, 207, 114–122. [Google Scholar] [CrossRef]
- Baur, F.; Jüstel, T. Eu3+ activated molybdates–Structure property relations. Opt. Mate. X 2019, 1, 100015. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, Q. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Lan, Y.M.; Wang, D.; Xie, D.L.; Tan, J.H.; Li, B.W.; Zhang, M.; Chen, Y. Preparation of Red Phosphor Sr2Si5N8: Eu2+ by Pellet Method and Its Optical Characteristics. Coatings 2021, 11, 283. [Google Scholar] [CrossRef]
- Farooq, U.; Zhao, Z.; Sui, Z.L.; Gao, C.; Dai, R.C.; Wang, Z.P.; Zhang, Z.M. Tm3+/Dy3+/Eu3+ (Sm3+) tri-activated Y2WO6 as one potential single-phase phosphor for WLEDs. J. Alloys Compd. 2019, 778, 942–950. [Google Scholar] [CrossRef]
- Mu, Z.; Hu, Y.; Chen, L.; Wang, X.; Ju, G.; Yang, Z.; Jin, Y. A single-phase, color-tunable, broadband-excited white light-emitting phosphor Y2WO6: Sm3+. J. Lumin. 2014, 146, 33–36. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, B.; Ke, Y.; Shu, S.; Liu, R.; Yu, R. Spectroscopic investigation of La7Ta3W4O30:Sm3+ orange-red phosphor for white LEDs. Arab. J. Chem. 2020, 13, 5581–5592. [Google Scholar] [CrossRef]
- Cao, C.; Wei, S.; Zhu, Y.; Liu, T.; Xie, A.; Noh, H.M.; Jeong, J.H. Synthesis, optical properties, and packaging of Dy3+ doped Y2WO6, Y2W3O12, and Y6WO12 phosphors. Mater. Res. Bull. 2020, 126, 110846. [Google Scholar] [CrossRef]
- Liu, G.K.; Jacquier, B. Spectroscopic Properties of Rare Earths in Optical Materials; Springer: Beijing, China, 2005. [Google Scholar]
- Llanos, J.; Olivares, D.; Manríquez, V.; Espinoza, D.; Brito, I. Synthesis and luminescent properties of two different Y2WO6:Eu3+ phosphor phases. J. Alloys Compd. 2015, 628, 352–356. [Google Scholar] [CrossRef]
- Wang, J.; Bu, Y.Y.; Wang, X.F.; Seo, H.J. Optical thermometry in low temperature through manipulating the energy transfer from WO66−to Ho3+ in Y2WO6:Ho3+ phosphors. Opt. Mater. 2018, 84, 778–785. [Google Scholar] [CrossRef]
- Wu, T.-C.; Chi, Y.-C.; Wang, H.-Y.; Tsai, C.-T.; Chang, J.-K.; Chen, L.-Y.; Cheng, W.-H.; Lin, G.-R. White-Lighting Communication With a Lu3Al5O12:Ce3+/CaAlSiN3:Eu2+ Glass Covered 450-nm InGaN Laser Diode. J. Lightwave Technol. 2018, 36, 1634–1643. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Zhang, Y.; Zhao, Y.; Liang, X.; Chen, G.; Liu, Y.; Xiang, W. Designed glass frames full color in white light-emitting diodes and laser diodes lighting. Chem. Eng. J. 2021, 414, 128754. [Google Scholar] [CrossRef]
- Cao, C.Y.; Yang, X.L.; Chen, X.; Xie, A. Enhanced emission of Eu3+ in lutetium tungsten molybdenum oxide phosphors: Syn-thesis, optical properties, thermal behavior, and LED packaging, J. Lumin. 2020, 223, 117269. [Google Scholar] [CrossRef]
- Xue, J.P.; Li, H.P.; Noh, H.M.; Choi, B.C.; Park, S.H.; Jeong, J.H.; Kim, J.H. Molybdenum substitution induced luminescence enhancement in Gd2W1-xMoxO6:Eu3+ phosphors for near ultraviolet based solid-state lighting. J. Lumin. 2018, 202, 97–106. [Google Scholar] [CrossRef]
- Huang, J.; Hou, B.; Ling, H.; Liu, J.; Yu, X. Crystal Structure, Electronic Structure, and Photoluminescence Properties of La3BW1–xMoxO9:Eu3+ Red Phosphor. Inorg. Chem. 2014, 53, 9541–9547. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xie, A.; Zhou, T.; Zhong, H.; Lu, X.; Xie, A.; Noh, H.M.; Jeong, J.H. Eu3+ doped lutetium molybdenum oxides: Synthesis, optical properties, thermal behavior, and LED packaging. J. Lumin. 2020, 217, 116759. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Liu, H.; Pan, K.; Wang, W.; Zeng, X.; Chen, X. Preparation and negative thermal expansion properties of Y2W3O12 thin films grown by pulsed laser deposition. Ceram. Int. 2016, 42, 18902–18906. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, Z.; Wang, J.; Hei, Z.; Li, M.; Noh, H.M.; Jeong, J.H.; Yu, R. Photoluminescence properties of a new orange–red emitting Sm3+-doped Y2Mo4O15 phosphor. J. Solid State Chem. 2015, 228, 110–116. [Google Scholar] [CrossRef]
- Xiong, F.; Guo, D.; Lin, H.; Wang, L.J.; Shen, H.X.; Zhu, W. High-color-purity red-emitting phosphors RE2WO6:Pr3+ (RE = Y., Gd) for blue LED. J. Alloys Compd. 2015, 647, 1121–1127. [Google Scholar] [CrossRef]
- Deng, Y.; Yi, S.; Huang, J.; Xian, J.; Zhao, W. White light emission and energy transfer in Dy3+/Eu3+ co-doped BaLa2WO7 phosphors. Mater. Res. Bull. 2014, 57, 85–90. [Google Scholar] [CrossRef]
- Hou, D.; Pan, X.; Li, J.; Zhou, W.; Ye, X. Structure and luminescence properties of Sm 3+ doped Y 2 MoO 6 phosphor under near ultraviolet light excitation. J. Rare Earths 2017, 35, 335–340. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Xu, H.; Cao, C.; Chen, X.; Zhang, M.; Jian, M.; Li, Y.; Xie, A. Synthesis, Luminescent Properties and White LED Fabrication of Sm3+ Doped Lu2WMoO9. Coatings 2021, 11, 403. https://doi.org/10.3390/coatings11040403
Chen Z, Xu H, Cao C, Chen X, Zhang M, Jian M, Li Y, Xie A. Synthesis, Luminescent Properties and White LED Fabrication of Sm3+ Doped Lu2WMoO9. Coatings. 2021; 11(4):403. https://doi.org/10.3390/coatings11040403
Chicago/Turabian StyleChen, Zijun, Huiyi Xu, Chunyan Cao, Xiaoting Chen, Min Zhang, Minkun Jian, Yuechan Li, and An Xie. 2021. "Synthesis, Luminescent Properties and White LED Fabrication of Sm3+ Doped Lu2WMoO9" Coatings 11, no. 4: 403. https://doi.org/10.3390/coatings11040403
APA StyleChen, Z., Xu, H., Cao, C., Chen, X., Zhang, M., Jian, M., Li, Y., & Xie, A. (2021). Synthesis, Luminescent Properties and White LED Fabrication of Sm3+ Doped Lu2WMoO9. Coatings, 11(4), 403. https://doi.org/10.3390/coatings11040403




