A Review on the Processing Technologies for Corrosion Resistant Thermoelectric Oxide Coatings
Abstract
1. Introduction
2. Thermoelectric Oxide Coating Processing Technologies
2.1. Vapor Phase Deposition
2.2. Liquid Phase Deposition
2.3. Nanocasting
2.4. Solid-State Processing
2.5. Energy Beam Techniques
3. Perspectives and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Klochko, N.P.; Klepikova, K.S.; Khrypunova, I.V.; Zhadan, D.O.; Petrushenko, S.I.; Kopach, V.R.; Dukarov, S.V.; Sukhov, V.M.; Kirichenko, M.V.; Khrypunova, A.L. Flexible thermoelectric module based on zinc oxide thin film grown via SILAR. Curr. Appl. Phys. 2021, 21, 121–133. [Google Scholar] [CrossRef]
- Kumar, A.; Sivaprahasam, D.; Thakur, A.D. Colossal Seebeck coefficient in Aurivillius phase-perovskite oxide composite. J. Alloys Compd. 2021, 853, 157001. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L.X.; Li, H.Q.; Li, D.; Yang, N.; Jiang, X.B.; Qiu, S.Z. Performance analysis of alkali metal thermoelectric converter and segmented thermoelectric generator hybrid system based on a comprehensive model. Appl. Thermal. Eng. 2021, 183, 116206. [Google Scholar] [CrossRef]
- Tian, X.X.; Asaadi, S.; Moria, H.; Kaood, A.; Pourhedayat, S.; Jermsittiparsert, K. Proposing tube-bundle arrangement of tubular thermoelectric module as a novel air cooler. Energy 2020, 208, 118428. [Google Scholar] [CrossRef]
- Gan, Y.X. Nanomaterials for Thermoelectric Devices, 1st ed.; Jenny Stanford Publishing: Singapore, 2018; pp. 6–8. [Google Scholar]
- Boulat, L.; Viennois, R.; Oliviero, E.; Dadras, M.; Fréty, N. Study of TaN and TaN-Ta-TaN thin films as diffusion barriers in CeFe4Sb12 skutterudite. J. Appl. Phys. 2019, 126, 125306. [Google Scholar] [CrossRef]
- Appel, O.; Zaharoni, T.; Breuer, G.; Beeri, O.; Gelbstein, Y. Thermoelectric properties of Ti0.3Zr0.35Hf0.35Ni1.005Sn Half-Heusler alloy. J. Appl. Phys. 2019, 126, 085110. [Google Scholar] [CrossRef]
- Zeier, W.G.; Schmitt, J.; Hautier, G.; Aydemir, U.; Gibbs, Z.M.; Felser, C.; Snyder, G.J. Engineering Half-Heusler thermoelectric materials using Zintl chemistry. Nat. Rev. Mater. 2016, 1, 16032. [Google Scholar] [CrossRef]
- Landmann, D.; Tang, Y.; Kunz, B.; Huber, R.; Widner, D.; Rickhaus, P.; Widmer, R.N.; Elsener, H.R.; Battaglia, C. Fabrication, characterization, and application-matched design of thermoelectric modules based on Half-Heusler FeNbSb and TiNiSn. J. Appl. Phys. 2019, 126, 085113. [Google Scholar] [CrossRef]
- Rogl, G.; Ghosh, S.; Wang, L.; Bursik, J.; Grytsiv, A.; Kerber, M.; Bauer, E.; Mallik, R.C.; Chen, X.Q.; Zehetbauer, M.; et al. Half-Heusler alloys: Enhancement of ZT after severe plastic deformation (ultra-low thermal conductivity). Acta Mater. 2020, 183, 285–300. [Google Scholar] [CrossRef]
- Gürth, M.; Rogl, G.; Romaka, V.V.; Grytsiv, A.; Bauer, E.; Rogl, P. Thermoelectric high ZT Half-Heusler alloys Ti1−x−yZrxHfyNiSn. Acta Mater. 2016, 104, 210–222. [Google Scholar] [CrossRef]
- Tavassoli, A.; Failamani, F.; Grytsiv, A.; Rogl, G.; Heinrich, P.; Müller, H.; Bauer, E.; Zehetbauer, M.; Rogl, P. On the Half-Heusler compounds Nb1−x{Ti,Zr,Hf}xFeSb: Phase relations, thermoelectric properties at low and high temperature, and mechanical properties. Acta Mater. 2017, 135, 263–276. [Google Scholar] [CrossRef]
- Shi, Y.; Sturm, C.; Kleinke, H. Chalcogenides as thermoelectric materials. J. Solid State Chem. 2019, 270, 273–279. [Google Scholar] [CrossRef]
- Gelbstein, Y.; Dashevsky, Z.; Dariel, M.P. High performance n-type PbTe-based materials for thermoelectric applications. Phys. B 2005, 363, 196–205. [Google Scholar] [CrossRef]
- Guo, Q.; Assoud, A.; Kleinke, H. Improved bulk materials with thermoelectric figure-of-merit>1: Tl10−xSnxTe6 and Tl10−xPbxTe6. Adv. Energy Mater. 2014, 4, 1400348. [Google Scholar] [CrossRef]
- Zhao, L.D.; Chang, C.; Tan, G.; Kanatzidis, M.G. SnSe: A remarkable new thermoelectric material. Energy Environ. Sci. 2016, 9, 3044–3060. [Google Scholar] [CrossRef]
- Zhao, L.D.; Lo, S.H.; Zhang, Y.S.; Sun, H.; Tan, G.H.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef]
- Liu, H.; Shi, X.; Xu, F.; Zhang, L.; Zhang, W.; Chen, L.; Li, Q.; Uher, C.; Day, T.; Snyder, G.J. Copper ion liquid-like thermoelectrics. Nat. Mater. 2021, 11, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Assoud, A.; Thomas, S.; Sutherland, B.; Zhang, H.; Tritt, T.M.; Kleinke, H. Thermoelectric properties of the new polytelluride Ba3Cu14−δTe12. Chem. Mater. 2006, 18, 3866–3872. [Google Scholar] [CrossRef]
- Shi, Y.X.; Assoud, A.; Sankar, C.R.; Kleinke, H. TlAg12Se7: A new pnp conduction switching material with extraordinarily low thermal conductivity. Chem. Mater. 2017, 29, 9565–9571. [Google Scholar] [CrossRef]
- Snedaker, M.L.; Zhang, Y.; Birkel, C.S.; Wang, H.; Day, T.; Shi, Y.; Ji, X.; Kraemer, S.; Mills, C.E.; Moosazadeh, A.; et al. Silicon-based thermoelectrics made from a boron-doped silicon dioxide nanocomposite. Chem. Mater. 2013, 25, 4867–4873. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, M. Record low thermal conductivity of polycrystalline Si nanowire: Breaking the Casimir limit by severe suppression of propagons. Nano Lett. 2016, 16, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Argemi, L.; Yu, Z.; Lee, J. Effects of metal silicide inclusion interface and shape on thermal transport in silicon nanocomposites. J. Appl. Phys. 2019, 126, 035106. [Google Scholar] [CrossRef]
- Nakasawa, H.; Hayashi, K.; Takamatsu, T.; Miyazaki, Y. Lattice dynamics and lattice thermal conductivity of CrSi2 calculated from first principles and the phonon Boltzmann transport equation. J. Appl. Phys. 2019, 126, 025105. [Google Scholar] [CrossRef]
- Murphy-Armando, F. Enhancement of the electronic thermoelectric properties of bulk strained silicon-germanium alloys using the scattering relaxation times from first principles calculations. J. Appl. Phys. 2019, 126, 215103. [Google Scholar] [CrossRef]
- Antonov, A.; Ivanov, Y.; Konstantinov, P.; Kuznetsova, V.; Novikov, S.; Ovchinnikov, A.; Pshenay-Severin, D.; Burkov, A. Thermoelectric and galvanomagnetic properties of topologically non-trivial (Co-M)Si semimetals (M = Fe, Ni) at high temperatures. J. Appl. Phys. 2019, 126, 245103. [Google Scholar] [CrossRef]
- Khalil, M.; Moll, A.; Godfroy, M.; Letrouit-Lebranchu, A.; Villeroy, B.; Alleno, E.; Viennois, R.; Beaudhuin, M. Thermoelectric properties and stability of nanostructured chromium disilicide CrSi2. J. Appl. Phys. 2019, 126, 135103. [Google Scholar] [CrossRef]
- Nonoguchi, Y.; Tani, A.; Kitano, T.; Kawai, T. Enhanced thermoelectric properties of semiconducting carbon nanotube films by UV/ozone treatment. J. Appl. Phys. 2019, 126, 135108. [Google Scholar] [CrossRef]
- Kim, G.; Kim, S.W.; Rim, H.J.; Lee, H.; Kim, J.; Roh, J.W.; Kim, B.W.; Lee, K.H.; Lee, W. Improved trade-off between thermoelectric performance and mechanical reliability of Mg2Si by hybridization of few-layered reduced graphene oxides. Script. Mater. 2019, 162, 402–407. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.C.; Liu, R.; Zhou, Z.F.; Liu, C.; Lan, J.L.; Zhang, Q.H.; Lin, Y.H.; Nan, C.W. Synergistical enhancement of thermoelectric properties in n-type Bi2O2Se by carrier engineering and hierarchical microstructure. Adv. Energy Mater. 2019, 9, 1900354. [Google Scholar] [CrossRef]
- Kenfaui, D.; Lenoir, B.; Chateigner, D.; Ouladdiaf, B.; Gomina, M.; Noudem, J.G. Development of multilayer textured Ca3Co4O9 materials for thermoelectric generators: Influence of the anisotropy on the transport properties. J. Eur. Ceram. Soc. 2012, 32, 2405–2414. [Google Scholar] [CrossRef]
- Yin, Y.; Tudu, B.; Tiwari, A. Recent advances in oxide thermoelectric materials and modules. Vacuum 2017, 146, 356–374. [Google Scholar] [CrossRef]
- Baranovskiy, A.; Amouyal, Y. Structural stability of calcium-manganate based CaO(CaMnO3)m (m = 1, 2, 3, ∞) compounds for thermoelectric applications. J. Alloys Compd. 2016, 687, 562–569. [Google Scholar] [CrossRef]
- Tauc, J. The thermal photo-electric phenomenon in semi-conductors. Cechoslov. Fiz. Zurnal (Czechoslov. J. Phys.) 1955, 5, 528–535. [Google Scholar] [CrossRef]
- Kwok, H.B.; Bube, R.H. Thermoelectric and photothermoelectric effects in semiconductors: CdS single crystals. J. Appl. Phys. 1973, 44, 138. [Google Scholar] [CrossRef]
- Harper, J.G.; Matthews, H.E.; Bube, R.H. Photothermoelectric Effects in semiconductors: N- and p-type silicon. J. Appl. Phys. 1970, 41, 765. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Tanabe, K.; Taniguchi, H.; Okazaki, R.; Terasaki, I. Interplay between quantum paraelectricity and thermoelectricity in the photo-Seebeck effect in a single crystal. J. Appl. Phys. 2019, 126, 045111. [Google Scholar] [CrossRef]
- Okazaki, R.; Horikawa, A.; Yasui, Y.; Terasaki, I. Photo-Seebeck effect in ZnO. J. Phys. Soc. Jpn. 2012, 81, 114722. [Google Scholar] [CrossRef]
- Horikawa, A.; Igarashi, T.; Terasaki, I.; Okazaki, R. Photo-Seebeck effect in polycrystalline ZnO. J. Appl. Phys. 2015, 118, 095101. [Google Scholar] [CrossRef]
- Tanabe, K. Influence of carrier diffusion on photo-Seebeck effect in zinc oxide. J. Appl. Phys. 2018, 124, 035108. [Google Scholar] [CrossRef]
- Suzuki, K.; Watanabe, T.; Kakemoto, H.; Irie, H. Photo- and gas-tuned, reversible thermoelectric properties and anomalous photo-thermoelectric effects of platinum-loaded tungsten trioxide. J. Appl. Phys. 2016, 119, 245109. [Google Scholar] [CrossRef]
- Azuma, C.; Kawano, T.; Kakemoto, H.; Irie, H. Photo-controllable thermoelectric properties with reversibility and photo-thermoelectric effects of tungsten trioxide accompanied by its photochromic phenomenon. J. Appl. Phys. 2014, 116, 173502. [Google Scholar] [CrossRef]
- Mondal, P.S.; Okazaki, R.; Taniguchi, H.; Terasaki, I. Photo-Seebeck effect in tetragonal PbO single crystals. J. Appl. Phys. 2013, 114, 173710. [Google Scholar] [CrossRef]
- Diez, J.C.; Torres, M.A.; Rasekh, S.; Constantinescu, G.; Madre, M.A.; Sotelo, A. Enhancement of Ca3Co4O9 thermoelectric properties by Cr for Co substitution. Ceram. Int. 2013, 39, 6051–6056. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Wang, C.; Xia, S. Ce1−xSrxZnSbO: New thermoelectric materials formed between intermetallics and oxides. J. Alloys Compd. 2016, 688, 849–853. [Google Scholar] [CrossRef]
- Zhang, Y.; Sugo, K.; Cho, H.J.; Ohta, H. Thermoelectric phase diagram of the SrTiO3-LaTiO3 solid-solution system through a metal to Mott insulator transition. J. Appl. Phys. 2019, 126, 075104. [Google Scholar] [CrossRef]
- Bresch, S.; Mieller, B.; Schoenauer-Kamin, D.; Moos, R.; Giovanelli, F.; Rabe, T. Influence of pressure assisted sintering and reaction sintering on microstructure and thermoelectric properties of bi-doped and undoped calcium cobaltite. J. Appl. Phys. 2019, 126, 075102. [Google Scholar] [CrossRef]
- Jerič, M.; de Boor, J.; Jančar, B.; Ceh, M. An enhanced thermoelectric figure of merit for Sr(Ti0.8Nb0.2)O3 based on a Ruddlesden–Popper-polytype-induced microstructure. J. Eur. Ceram. Soc. 2016, 36, 1177–1182. [Google Scholar] [CrossRef]
- Han, J.; Sun, Q.; Li, W.; Song, Y. Microstructure and thermoelectric properties of La0.1Dy0.1SrxTiO3 ceramics. Ceram. Int. 2017, 43, 5557–5563. [Google Scholar] [CrossRef]
- Tiginyanu, I.M.; Lupan, O.; Ursaki, V.V.; Chow, L.; Enachi, M. Nanostructures of metal oxides. In Comprehensive Semiconductor Science and Technology; Bhattacharya, P., Fornari, R., Kamimura, H., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 3, pp. 396–479. [Google Scholar]
- Matsuo, J.; Katsumata, H.; Minami, E.; Yamada, I. O2 cluster ion-assisted deposition for tin-doped indium oxide films. Nucl. Instrum. Methods Phys. Res. Sec. B Beam Interact. Mater. At. 2000, 161–163, 952–957. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Electrochemical synthesis of metal oxides and hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ning, H.; Busbee, J.; Shen, Z.; Kiggins, C.; Hua, Y.; Eaves, J.; Davis III, J.; Shi, T.; Shao, Y.T.; et al. Electroplating lithium transition metal oxides. Sci. Adv. 2017, 3, e1602427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, R.; Meng, J.; Liu, X.; Wang, X.; Li, Q.; Mai, L. Facile electrospinning formation of carbon-confined metal oxide cube-in-tube nanostructures for stable lithium storage. Chem. Commun. 2017, 53, 8284–8287. [Google Scholar] [CrossRef] [PubMed]
- Tsukuma, K.; Akiyama, T.; Imai, H. Liquid phase deposition film of tin oxide. J. Non Cryst. Solids 1997, 210, 48–54. [Google Scholar] [CrossRef]
- Deng, X.; Chen, K.; Tüysüz, H. Protocol for the nanocasting method: Preparation of ordered mesoporous metal oxides. Chem. Mater. 2017, 29, 40–52. [Google Scholar] [CrossRef]
- Ozegowski, M.; Meteva, K.; Metev, S.; Sepold, G. Pulsed laser deposition of multicomponent metal and oxide films. Appl. Surf. Sci. 1999, 138–139, 68–74. [Google Scholar] [CrossRef]
- Kathalingam, A.; Shanmugam, K.; Park, H.C.; Kim, H.S. Fabrication of arrayed metal oxide structures by electrochemical local oxidation using metallic tip with electric field and humidity. J. Mater. Proc. Technol. 2018, 252, 304–312. [Google Scholar] [CrossRef]
- Hsu, M.C.; Leu, I.C.; Sun, Y.M.; Hon, M.H. Fabrication of CdS@TiO2 coaxial composite nanocables arrays by liquid-phase deposition. J. Cryst. Growth 2005, 285, 642–648. [Google Scholar] [CrossRef]
- Wu, J.M.; Wu, W.T.; Shih, H.C. Characterization of single-crystalline TiO2 nanowires grown by thermal evaporation. J. Electrochem. Soc. 2005, 152, G613. [Google Scholar] [CrossRef]
- Wang, Z.L. Functional oxide nanobelts: Materials, properties and potential applications in nanosystems and biotechnology. Annu. Rev. Phys. Chem. 2004, 55, 159–196. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Yang, L.; Chen, Y. Synthesis and ultraviolet luminescence properties of half-wall Al2O3 nanotube arrays. J. Phys. D Appl. Phys. 2009, 42, 225106. [Google Scholar] [CrossRef]
- Li, P.G.; Lei, M.; Tang, W.H.; Guo, X.; Wang, X. Facile route to straight SnO2 nanowires and their optical properties. J. Alloys Compd. 2009, 477, 515–518. [Google Scholar] [CrossRef]
- Jiang, X.; Herricks, T.; Xia, Y. CuO nanowires can be synthesized by heating copper substrates in air. Nano Lett. 2002, 2, 1333–1338. [Google Scholar] [CrossRef]
- Xiang, X.; Cao, C.B.; Guo, Y.; Zhu, H.S. A simple method to synthesize gallium oxide nanosheets and nanobelts. Chem. Phys. Lett. 2003, 378, 660. [Google Scholar] [CrossRef]
- Cha, H.G.; Kim, C.W.; Kim, Y.H.; Jung, M.H.; Ji, E.S.; Das, B.K.; Kim, J.C.; Kang, Y.S. Preparation and characterization of α-Fe2O3 nanorod-thin film by metal-organic chemical vapor deposition. Thin Solid Film. 2009, 517, 1853–1856. [Google Scholar] [CrossRef]
- Basharat, S.; Carmalt, C.J.; Barnett, S.A.; Tocher, D.A.; Davies, H.O. Aerosol assisted chemical vapor deposition of In2O3 films from Me3In and donor functionalized alcohols. Inorg. Chem. 2007, 46, 9473–9480. [Google Scholar] [CrossRef]
- Terasako, T.; Ohmae, K.; Yamane, M.; Shirakata, S. Carrier transport in undoped CdO films grown by atmospheric-pressure chemical vapor deposition. Thin Solid Film. 2014, 572, 20–27. [Google Scholar] [CrossRef]
- Stetsovych, V.; Pagliuca, F.; Dvořák, F.; Duchoň, T.; Vorokhta, M.; Aulická, M.; Lachnitt, J.; Schernich, S.; Matolínová, I.; Veltruská, K.; et al. Epitaxial cubic Ce2O3 films via Ce-CeO2 interfacial reaction. J. Phys. Chem. Lett. 2013, 4, 866–871. [Google Scholar] [CrossRef]
- Ahmoum, H.; Li, G.J.; Belakry, S.; Boughrara, M.; Suait, M.S.; Kerouad, M.; Wang, Q. Structural, morphological and transport properties of Ni doped ZnO thin films deposited by thermal co-evaporation method. Mater. Sci. Semicond. Proc. 2021, 123, 105530. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering physical vapour deposition (PVD) coatings: A critical review on process improvement and market trend demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Perednis, D.; Gauckler, L.J. Thin film deposition using spray pyrolysis. J. Electroceram. 2005, 14, 103–111. [Google Scholar] [CrossRef]
- Arca, E.; Fleischer, K.; Shvets, I.V. Influence of the precursors and chemical composition of the solution on the properties of ZnO thin films grown by spray pyrolysis. J. Phys. Chem. C 2009, 113, 21074–21081. [Google Scholar] [CrossRef]
- Hateef, A.A. Effect of the thickness on electrical properties of TiO2 thin films, prepared by thermal chemical spray pyrolysis deposition. Int. J. Innov. Res. Sci. Eng. Technol. (Irjesti) 2012, 1, 175–179. [Google Scholar]
- Andronic, L.; Manolache, S.; Duta, A. TiO2 thin films prepared by spray pyrolysis deposition (SPD) and their photocatalytic activities. J. Optoelectron. Adv. Mater. 2007, 9, 1403–1406. [Google Scholar]
- Boyadzhiev, S.; Georgieva, V.; Rassovska, M. Characterization of reactive sputtered TiO2 thin films for gas sensor applications. J. Phys. Conf. Ser. 2010, 253, 012040. [Google Scholar] [CrossRef]
- Hussian, H.A.R.A.; Hassan, M.A.M.; Agool, I.R. Synthesis of titanium dioxide (TiO2) nanofiber and nanotube using different chemical method. Optik 2016, 127, 2996–2999. [Google Scholar] [CrossRef]
- Ennaceri, H.; Boujnah, M.; Taleb, A.; Khaldoun, A.; Sáez-Araoz, R.; Ennaoui, A.; Kenz, A.E.; Benyoussef, A. Thickness effect on the optical properties of TiO2-anatase thin films prepared by ultrasonic spray pyrolysis: Experimental and ab initio study. Int. J. Hydrog. Energy 2017, 42, 19467–19480. [Google Scholar] [CrossRef]
- Oja, I.; Mere, A.; Krunks, M.; Solterbeck, C.H.; Es-Souni, M. Properties of TiO2 films prepared by the spray pyrolysis method. In Functional Nanomaterials for Optoelectronics and Other Applications; Lojkowski, W., Blizzard, J.R., Eds.; Solid State Phenom; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2004; Volume 99–100, pp. 259–264. [Google Scholar] [CrossRef]
- Castaneda, L.; Alonso, J.C.; Ortiz, A.; Andrade, E.; Saniger, J.M.; Banuelos, J.G. Spray pyrolysis deposition and characterization of titanium oxide thin films. Mater. Chem. Phys. 2003, 77, 938–944. [Google Scholar] [CrossRef]
- Vautey, C.; Burgos, M.; Langlet, M. Aerosol-gel deposition and low temperature heat-treatment of SiO2 layers. Thin Solid Film. 1999, 347, 184–194. [Google Scholar] [CrossRef]
- Djaoued, Y.; Taj, R.; Brüning, R.; Badilescu, S.; Ashrit, P.V.; Bader, G.; Vo-Van, T. Study of the phase transition and the thermal nitridation of nanocrystalline sol–gel titania films. J. Non Cryst. Solids 2002, 297, 55–66. [Google Scholar] [CrossRef]
- Burgos, M.; Langlet, M. The sol-gel transformation of TIPT coatings: A FTIR study. Thin Solid Film. 1999, 349, 19–23. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and characterization of TiO2 thin films by sol-gel method. J. Sol Gel Sci. Technol. 2002, 25, 137–145. [Google Scholar] [CrossRef]
- Yao, T.; Uchimoto, Y.; Sugiyama, T.; Nagai, Y. Synthesis of (La, Sr)MeO3 (Me = Cr, Mn, Fe, Co) solid solutions from aqueous solutions. Solid State Ion. 2000, 135, 359–364. [Google Scholar] [CrossRef]
- Deki, S.; Iizuka, S.; Akamatsu, K.; Mizuhata, M.; Kajinami, A. Novel fabrication method for Si1−xTixO2 thin films with graded composition profiles by liquid phase deposition. J. Mater. Chem. 2001, 11, 984–986. [Google Scholar] [CrossRef]
- Koumoto, K.; Seo, S.; Sugiyama, T.; Seo, W.S.; Dressick, W.J. Micropatterning of titanium dioxide on self-assembled monolayers using a liquid-phase deposition process. Chem. Mater. 1999, 11, 2305–2309. [Google Scholar] [CrossRef]
- Deki, S.; Aoi, Y.; Asaoka, Y.; Kajinami, A.; Mizuhata, M. Preparation and characterization of Au-dispersed TiO2 thin films by a liquid-phase deposition method. J. Mater. Chem. 1996, 7, 733–736. [Google Scholar] [CrossRef]
- Gao, Y.; Masuda, Y.; Yonezawa, T.; Koumoto, K. Preparation of SrTiO3 thin films by the liquid phase deposition method. Mater. Sci. Eng. B 2003, 99, 290–293. [Google Scholar] [CrossRef]
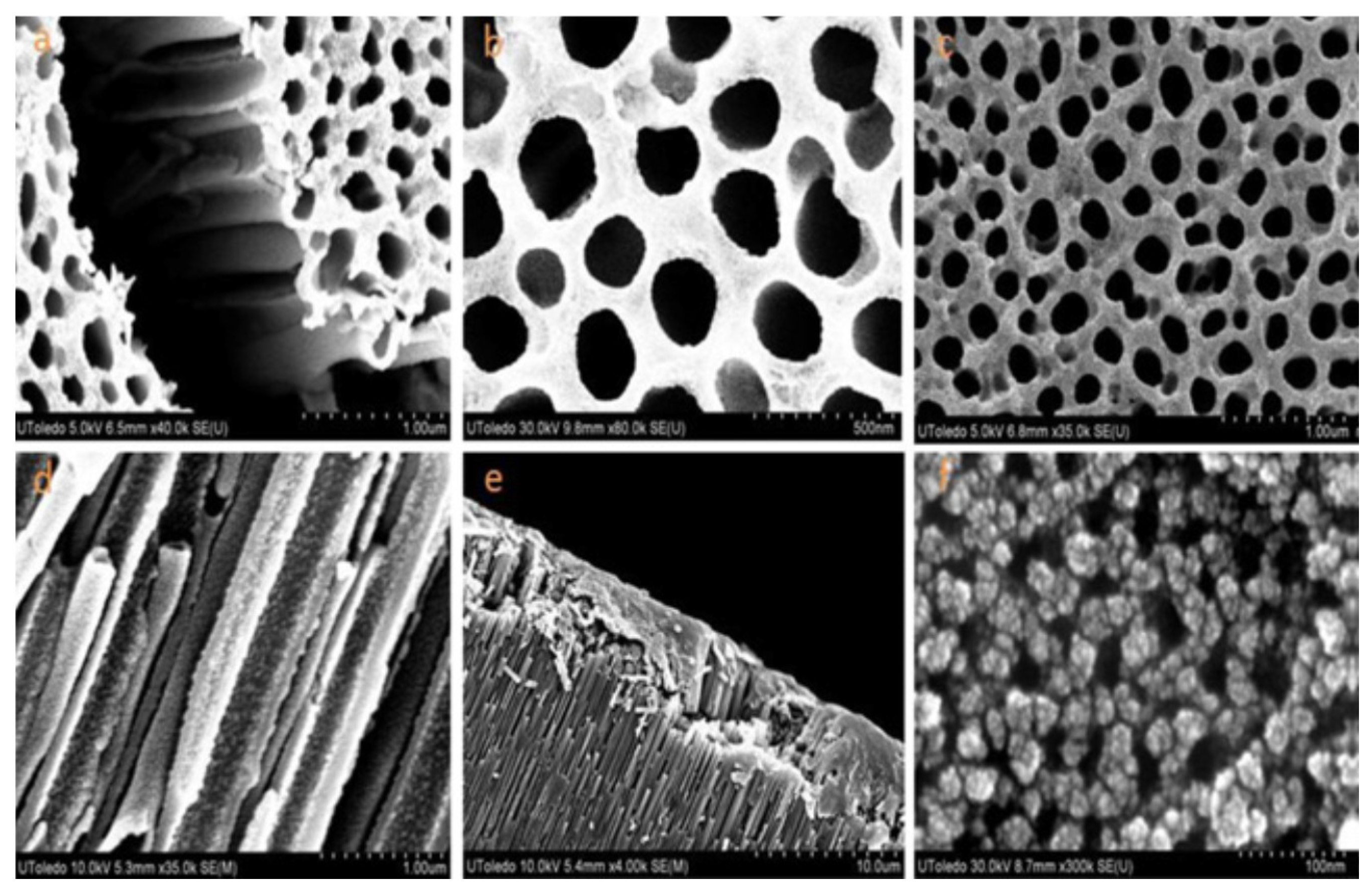
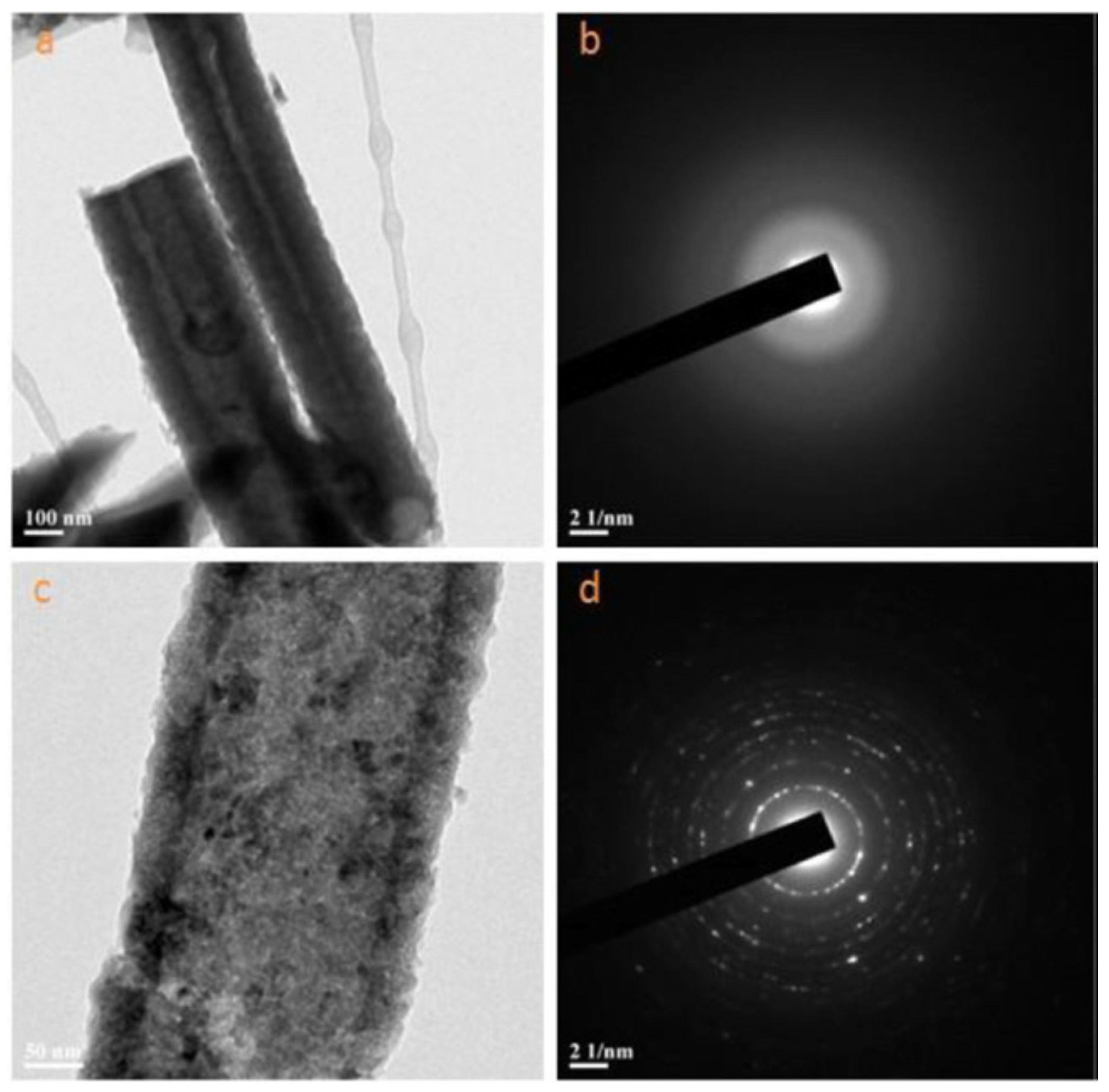
- Su, L.; Gan, Y.X.; Zhang, L. Thermoelectricity of nanocomposites containing TiO2-CoO coaxial nanocables. Script. Mater. 2011, 64, 745–748. [Google Scholar] [CrossRef]
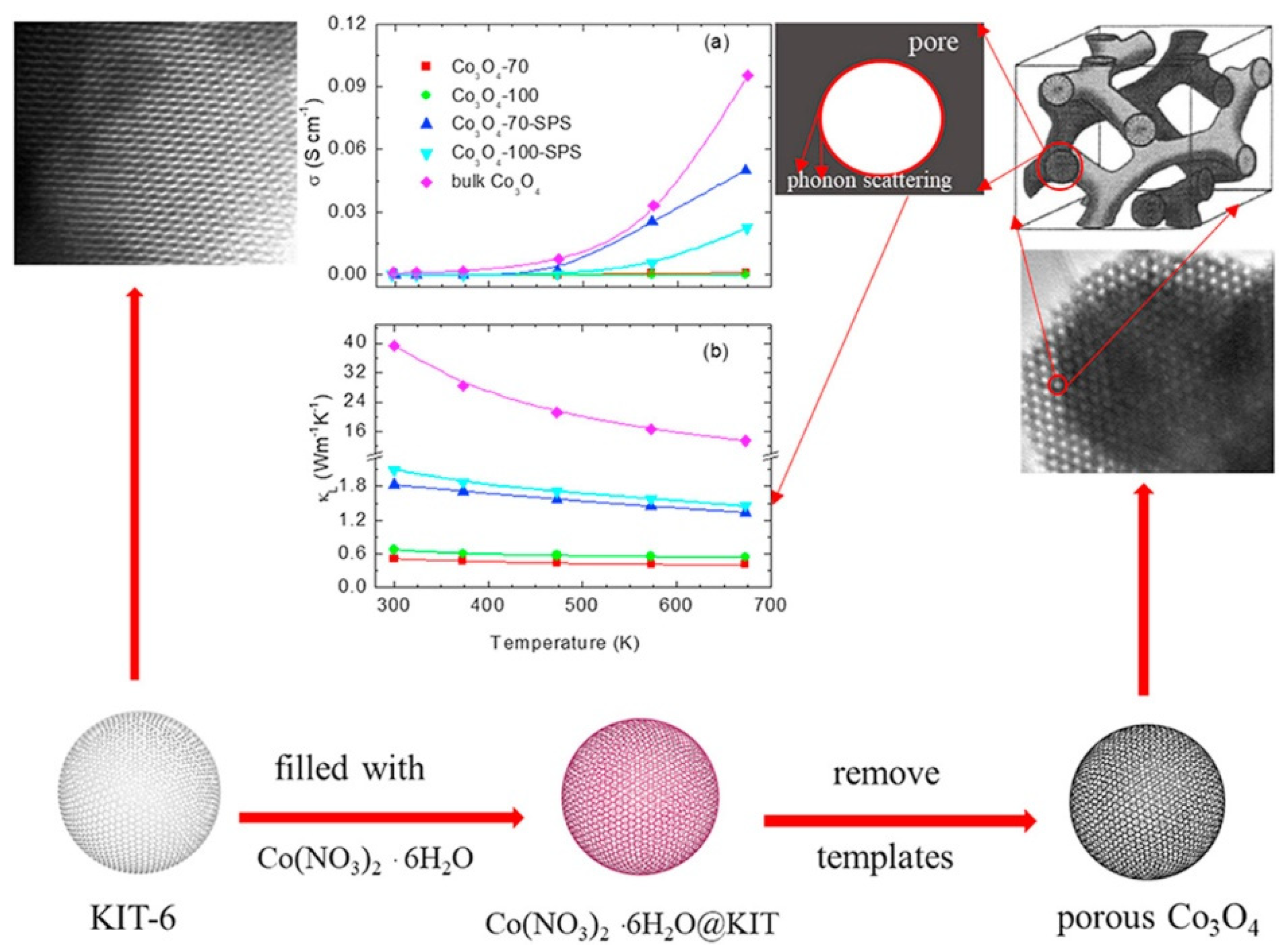
- Chen, L.L.; Zhang, Z.Y.; Qi, N.; Zhou, B.; Deng, S.P.; Chen, Z.Q.; Wu, Y.C.; Tang, X.F. Giant reduction in thermal conductivity of Co3O4 with ordered mesopore structures. Microporous Mesoporous Mater. 2020, 296, 109969. [Google Scholar] [CrossRef]
- Zhou, B.; Li, C.Y.; Qi, N.; Jiang, M.; Wang, B.; Chen, Z.Q. Pore structure of mesoporous silica (KIT-6) synthesized at different temperatures using positron as a nondestructive probe. Appl. Surf. Sci. 2018, 450, 31–37. [Google Scholar] [CrossRef]
- Sun, S.; Gao, Q.; Wang, H.; Zhu, J.; Guo, H. Influence of textural parameters on the catalytic behavior for CO oxidation over ordered mesoporous Co3O4. Appl. Catal. B Environ. 2010, 97, 284–291. [Google Scholar] [CrossRef]
- Jiang, D.; Ekren, D.; Azough, F.; Day, S.J.; Chen, K.; Mahajan, A.; Kepaptsoglou, D.M.; Ramasse, Q.M.; Reece, M.J.; Freer, R. The structure and thermoelectric properties of tungsten bronze Ba6Ti2Nb8O30. J. Appl. Phys. 2019, 126, 125115. [Google Scholar] [CrossRef]
- Presečnik, M.; Bernik, S. Microstructural and thermoelectric properties of WO3-doped Ca3Co4O9 ceramics. Ceram. Int. 2016, 42, 16103–16108. [Google Scholar] [CrossRef]
- Kahraman, F.; Madre, M.A.; Rasekh, S.; Salvador, C.; Bosque, P.; Torres, M.A.; Diez, J.C.; Sotelo, A. Enhancement of mechanical and thermoelectric properties of Ca3Co4O9 by Ag addition. J. Eur. Ceram. Soc. 2015, 35, 3835–3841. [Google Scholar] [CrossRef]
- Demirel, S.; Altin, E.; Oz, E.; Altin, S.; Bayri, A. An enhancement ZT and spin state transition of Ca3Co4O9 with Pb doping. J. Alloys Compd. 2015, 627, 430–437. [Google Scholar] [CrossRef]
- Yu, J.; Li, T.; Sun, Q. Single-layer BiOBr: An effective p-type 2D thermoelectric material. J. Appl. Phys. 2019, 125, 205111. [Google Scholar] [CrossRef]
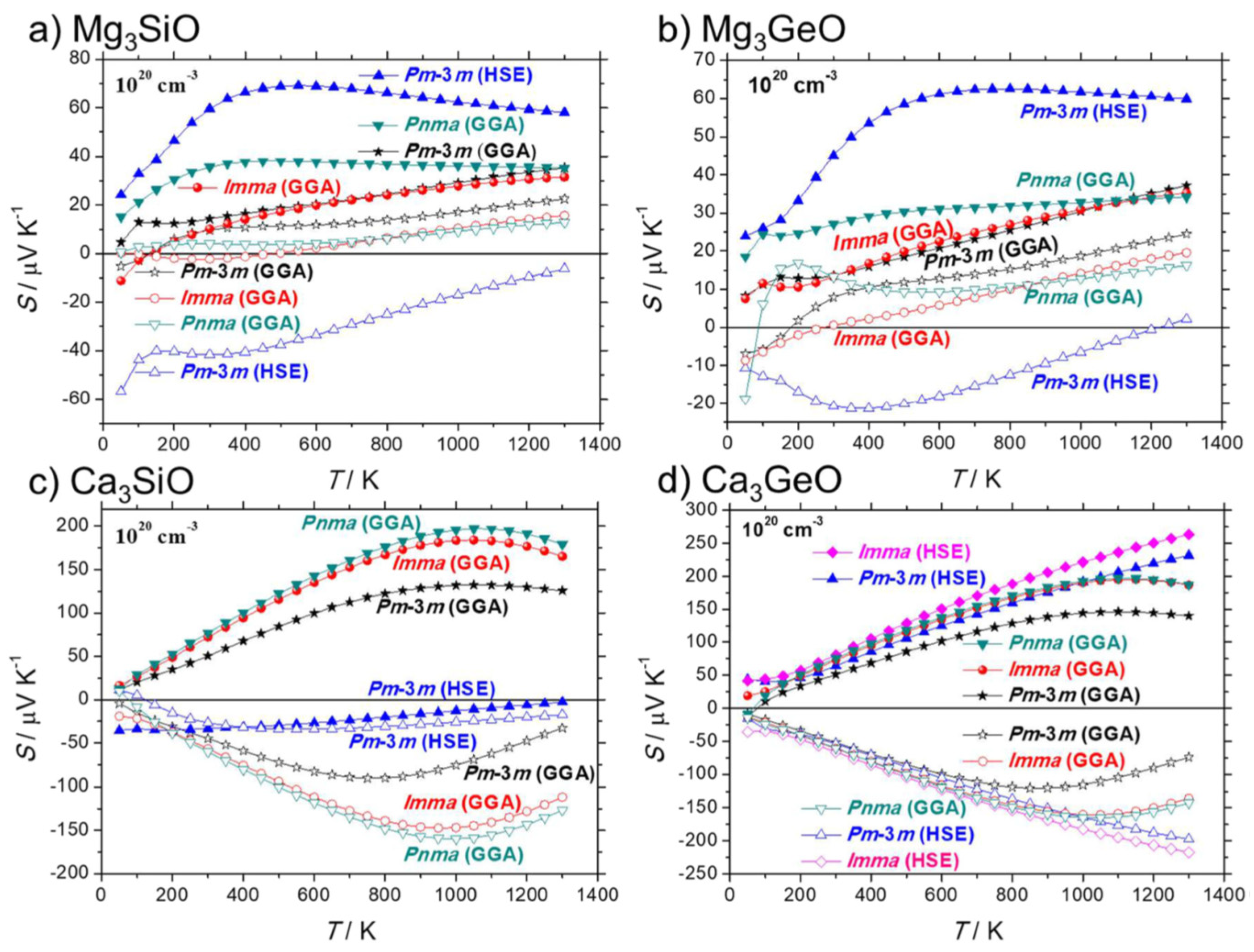
- Pöhls, J.H.; Mar, A. Thermoelectric properties of inverse perovskites A3TtO (A = Mg, Ca; Tt = Si, Ge): Computational and experimental investigations. J. Appl. Phys. 2019, 126, 025110. [Google Scholar] [CrossRef]
- Madre, M.A.; Rasekh, S.; Diez, J.C.; Sotelo, A. New solution method to produce high performance thermoelectric ceramics: A case study of Bi-Sr-Co-O. Mater. Lett. 2010, 64, 2566–2568. [Google Scholar] [CrossRef]
- Sotelo, A.; Rasekh, S.; Madre, M.A.; Guilmeau, E.; Marinel, S.; Diez, J.C. Solution-based synthesis routes to thermoelectric Bi2Ca2Co1.7Ox. J. Eur. Ceram. Soc. 2011, 31, 1763–1769. [Google Scholar] [CrossRef]
- Griesser, A.; Kraus, T.; Klein, O.; Karl, H. Low-resistance electrical contact between epitaxially grown thermoelectric oxide material (Ca2CoO3)(0.62)CoO2 and iridium. Thin Solid Film. 2021, 717, 138420. [Google Scholar] [CrossRef]
- Jones, J.G.; Boeckl, J.J.; Smith, S.R.; Landis, G.R.; Murphy, N.R.; Hu, Z.Q.; Bowers, C.T.; Stutz, C.E. Al2O3-BaTiO3 nanolaminates fabricated by multistationary target pulsed laser deposition with in situ ellipsometry. J. Nanophotonics 2017, 11, 043506. [Google Scholar] [CrossRef]
- Gutierrez-Llorente, A.; Iglesias, L.; Rodriguez-Gonzalez, B.; Rivadulla, F. Epitaxial stabilization of pulsed laser deposited Srn+1IrnO3n+1 thin films: Entangled effect of growth dynamics and strain. APL Mater. 2018, 6, 091101. [Google Scholar] [CrossRef]
- Kulik, P.; Yu, C.J.; Sokolov, A.; Liang, W.T.; Harris, V.G. BaFe12O19 magnetoplumbite films grown on SiO2/Si substrates for widescale magnetic film semiconductor systems integration. Scr. Mater. 2020, 188, 190–194. [Google Scholar] [CrossRef]
- Xiao, T.T.; Yang, Q.; Yu, J.; Xiong, Z.W.; Wu, W.D. Annealing condition effects on the structural properties of FePt nanoparticles embedded in MgO via pulsed laser deposition. Nanomaterials 2021, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Cesaria, M.; Lorusso, A.; Caricato, A.P.; Finocchiaro, P.; Amaducci, S.; Martino, M.; Aziz, M.R.; Calcagnile, L.; Perrone, A.; Quarta, G. B-10-based films grown by pulsed laser deposition for neutron conversion applications. Appl. Phys. A Mater. Sci. Proc. 2020, 126, 404. [Google Scholar] [CrossRef]
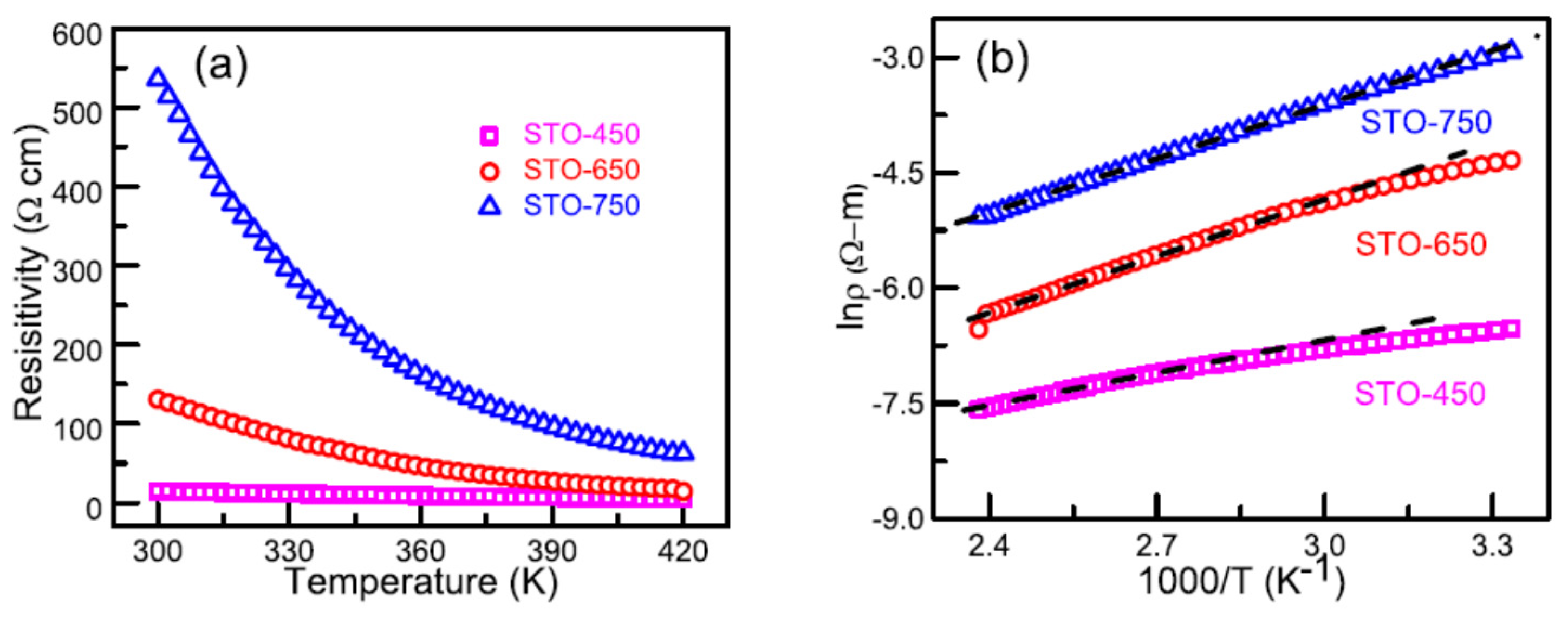
- Bhogra, A.; Masarrat, A.; Meena, R.; Hasina, D.; Bala, M.; Dong, C.L.; Chen, C.L.; Kumar, T.A.; Kandasami, A. Tuning the electrical and thermoelectric properties of N ion implanted SrTiO3 thin films and their conduction mechanisms. Sci. Rep. 2019, 9, 14486. [Google Scholar] [CrossRef] [PubMed]
- Bhogra, A.; Masarrat, A.; Hasina, D.; Meena, R.; Umapathy, G.R.; Kumar, A.; Som, T.; Dong, C.L.; Chen, C.L.; Kandasami, A. Significant role of substrate temperature on the morphology, electronic structure and thermoelectric properties of SrTiO3 films deposited by pulsed laser deposition. Surf. Coat. Technol. 2021, 407, 126740. [Google Scholar] [CrossRef]
- Christopher, B.; Rao, A.; Deka, U.; Prasad, K.S.; Okram, G.S.; Sanjeev, G.; Petwal, V.C.; Verma, V.P.; Dwivedi, J. Electrical, thermal and magnetic studies on 7.5 MeV electron beam irradiated PrCoO3 polycrystalline samples. Phys. B Condens. Matter 2018, 540, 26–32. [Google Scholar] [CrossRef]
- Kennedy, J.; Murmu, P.P.; Leveneur, J.; Williams, G.V.W.; Moody, R.L.; Maity, T.; Chong, S.V. Enhanced power factor and increased conductivity of aluminum doped zinc oxide thin films for thermoelectric applications. J. Nanosci. Nanotechnol. 2018, 18, 1384–1387. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, P.; Yu, K.Q.; Zhao, X.L.; Ding, G.F. ITO film prepared by ion beam sputtering and its application in high-temperature thermocouple. Vacuum 2017, 146, 31–34. [Google Scholar] [CrossRef]
- Zhu, C.H.; Li, Z.Z.; An, H.P.; Tang, G.D.; Hou, D.L. Enhancing the thermoelectric properties of Ca3Co4O9 thin films by the addition of a nanoscale NbNx second phase. J. Electron. Mater. 2014, 43, 3666–3671. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Takai, O.; Saito, N. Fabrication and characterization of epitaxial SrTiO3/Nb-doped SrTiO3 superlattices by double ECR ion beam sputter deposition. Vacuum 2013, 89, 35–39. [Google Scholar] [CrossRef]
- Waller, G.; Stein, A.; Abiade, J.T. Nanofabrication of doped, complex oxides. J. Vac. Sci. Technol. B 2012, 30, 011804. [Google Scholar] [CrossRef]
- Beke, S. A review of the growth of V2O5 films from 1885 to 2010. Thin Solid Film 2011, 519, 1761–1771. [Google Scholar] [CrossRef]
- Ramana, C.V.; Hussain, O.M.; Naidu, B.S.; Reddy, P.J. Influence of substrate temperature on the composition and structural properties of electron beam evaporated V2O5 thin films. Vacuum 1997, 48, 431–434. [Google Scholar] [CrossRef]
- Ramana, C.V.; Hussain, O.M.; Uthanna, S.; Naidu, B.S. Influence of oxygen partial pressure on the optical properties of electron beam evaporated vanadium pentoxide thin films. Opt. Mater. 1998, 10, 101–107. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.; Kulkarni, N.; Kaur, D. Structural and optical studies of nanocrystalline V2O5 thin films. Thin Solid Film 2008, 516, 912–918. [Google Scholar] [CrossRef]
- Gan, Y.X.; Gan, J.B. Measuring the electrical and photonic properties of cobalt oxide-containing composite carbon fibers. J. Compos. Sci. 2020, 4, 156. [Google Scholar] [CrossRef]
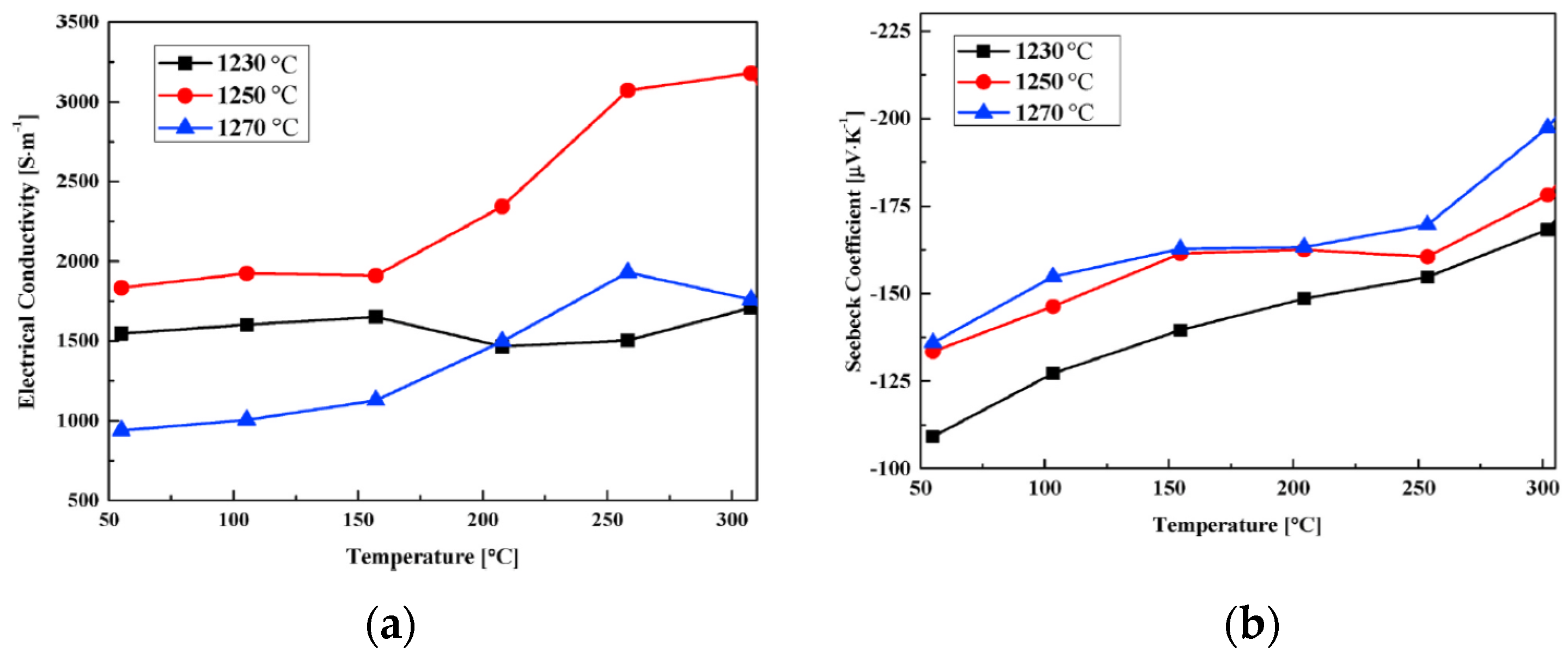
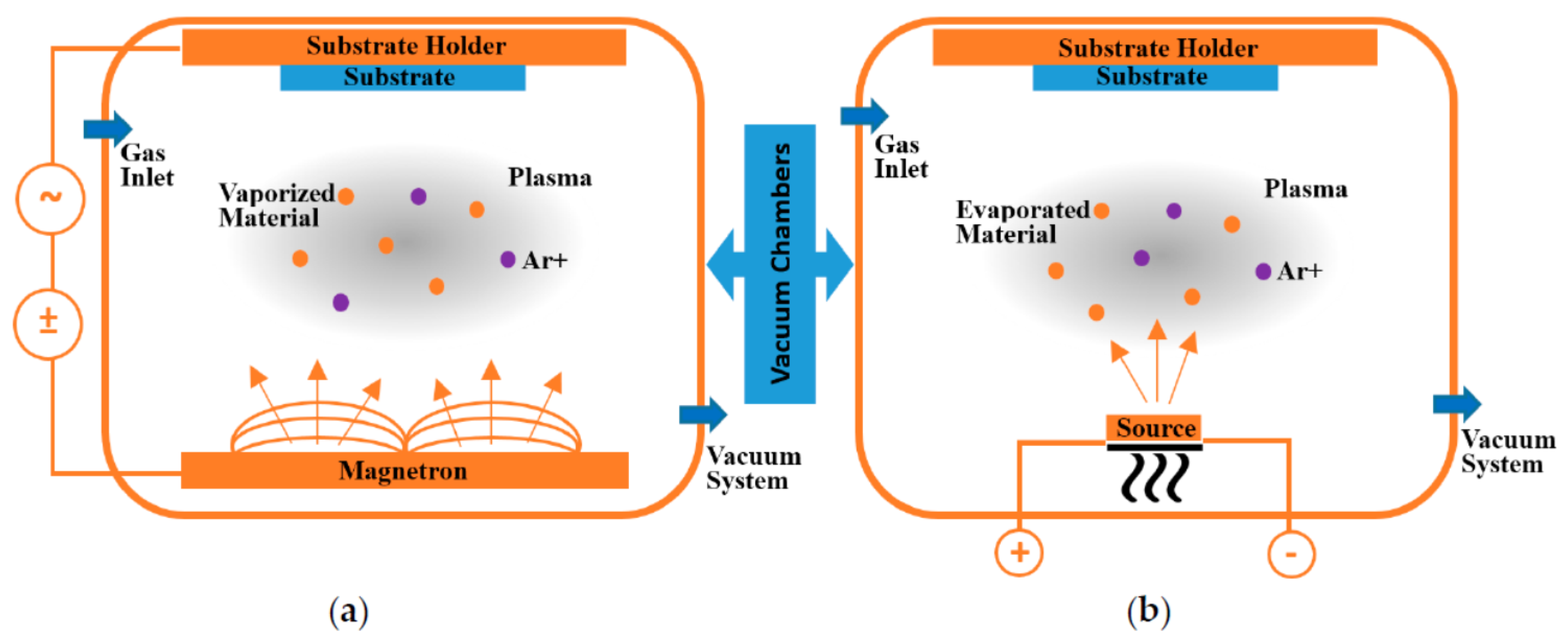
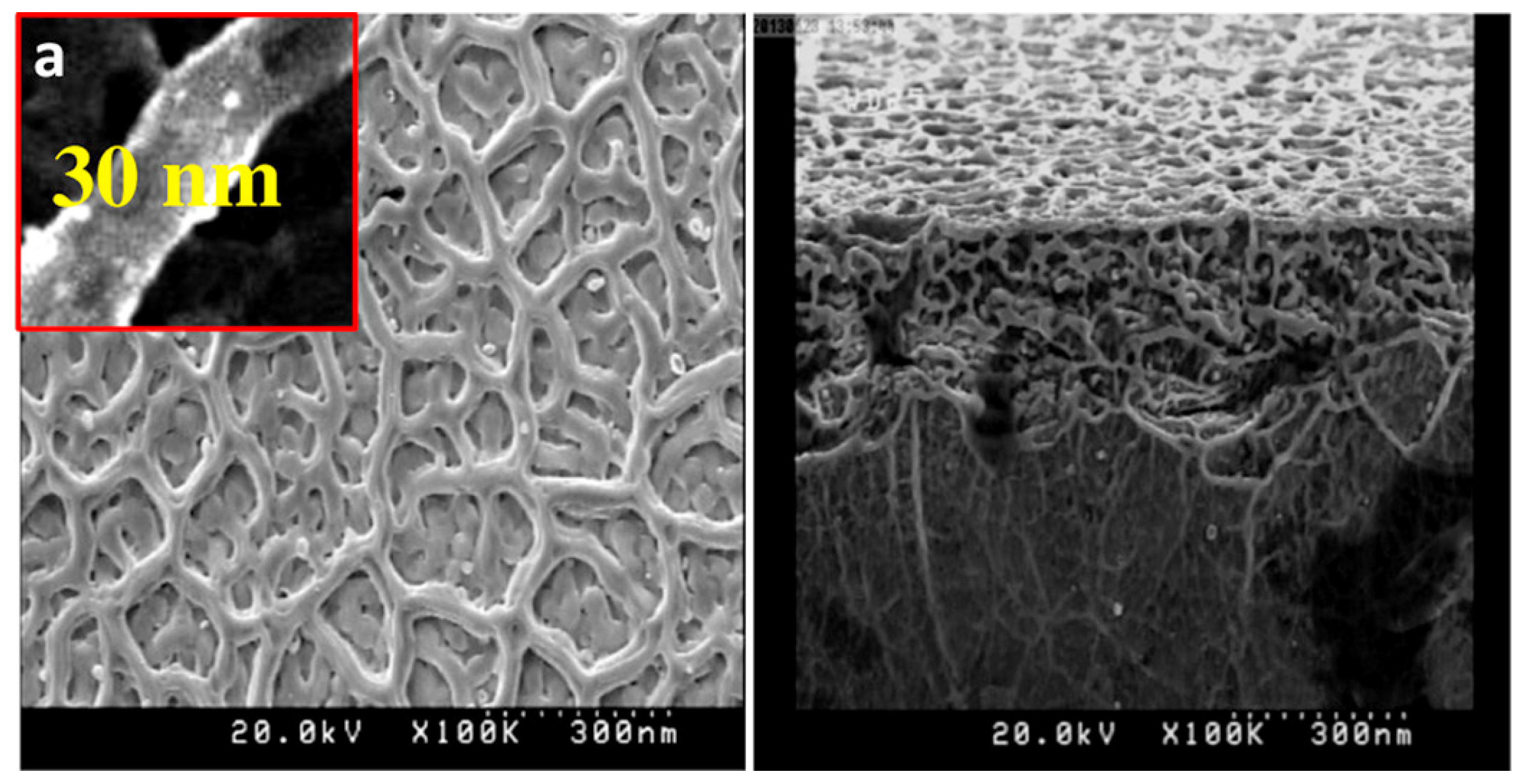
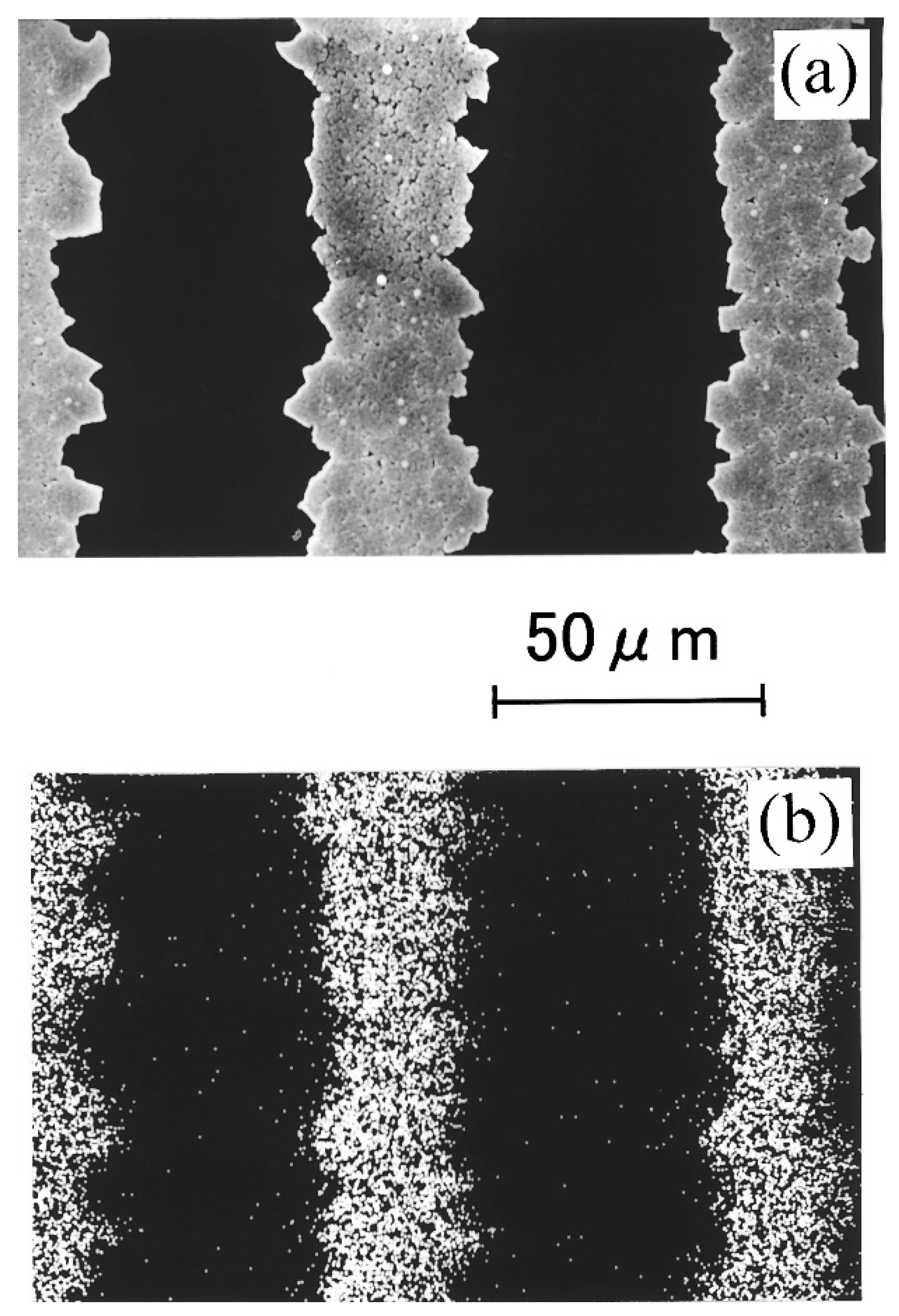

| Temperature | TiO2 (µV/K) | CoO | TiO2 + Ag | TiO2 + Ag + CoO |
|---|---|---|---|---|
| ΔV/ΔT@40 °C | −220 | −93.33 | −393.33 | −300 |
| ΔV/ΔT@45 °C | −210 | −90 | −320 | −255 |
| ΔV/ΔT@50 °C | −172 | −68 | −296 | −208 |
| ΔV/ΔT@55 °C | −153.33 | −80 | −316.67 | −200 |
| ΔV/ΔT@60 °C | −125.71 | −71.43 | −274.29 | −162.86 |
| ΔV/ΔT@70 °C | −106.67 | −66.67 | −266.67 | −166.67 |
| ΔV/ΔT@80 °C | −98.18 | −60 | −245.45 | −129.09 |
| ΔV/ΔT@90 °C | −101.54 | −60 | −27.69 | −113.85 |
| ΔV/ΔT@110 °C | −78.82 | −56.47 | −207.06 | −108.24 |
| ΔV/ΔT@130 °C | −90.48 | −34.29 | −153.33 | −107.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.X. A Review on the Processing Technologies for Corrosion Resistant Thermoelectric Oxide Coatings. Coatings 2021, 11, 284. https://doi.org/10.3390/coatings11030284
Gan YX. A Review on the Processing Technologies for Corrosion Resistant Thermoelectric Oxide Coatings. Coatings. 2021; 11(3):284. https://doi.org/10.3390/coatings11030284
Chicago/Turabian StyleGan, Yong X. 2021. "A Review on the Processing Technologies for Corrosion Resistant Thermoelectric Oxide Coatings" Coatings 11, no. 3: 284. https://doi.org/10.3390/coatings11030284
APA StyleGan, Y. X. (2021). A Review on the Processing Technologies for Corrosion Resistant Thermoelectric Oxide Coatings. Coatings, 11(3), 284. https://doi.org/10.3390/coatings11030284





