In-Situ Investigation of the Oxidation Behaviour of Chemical Vapour Deposited Zr(C,N) Hard Coatings Using Synchrotron X-ray Diffraction
Abstract
1. Introduction
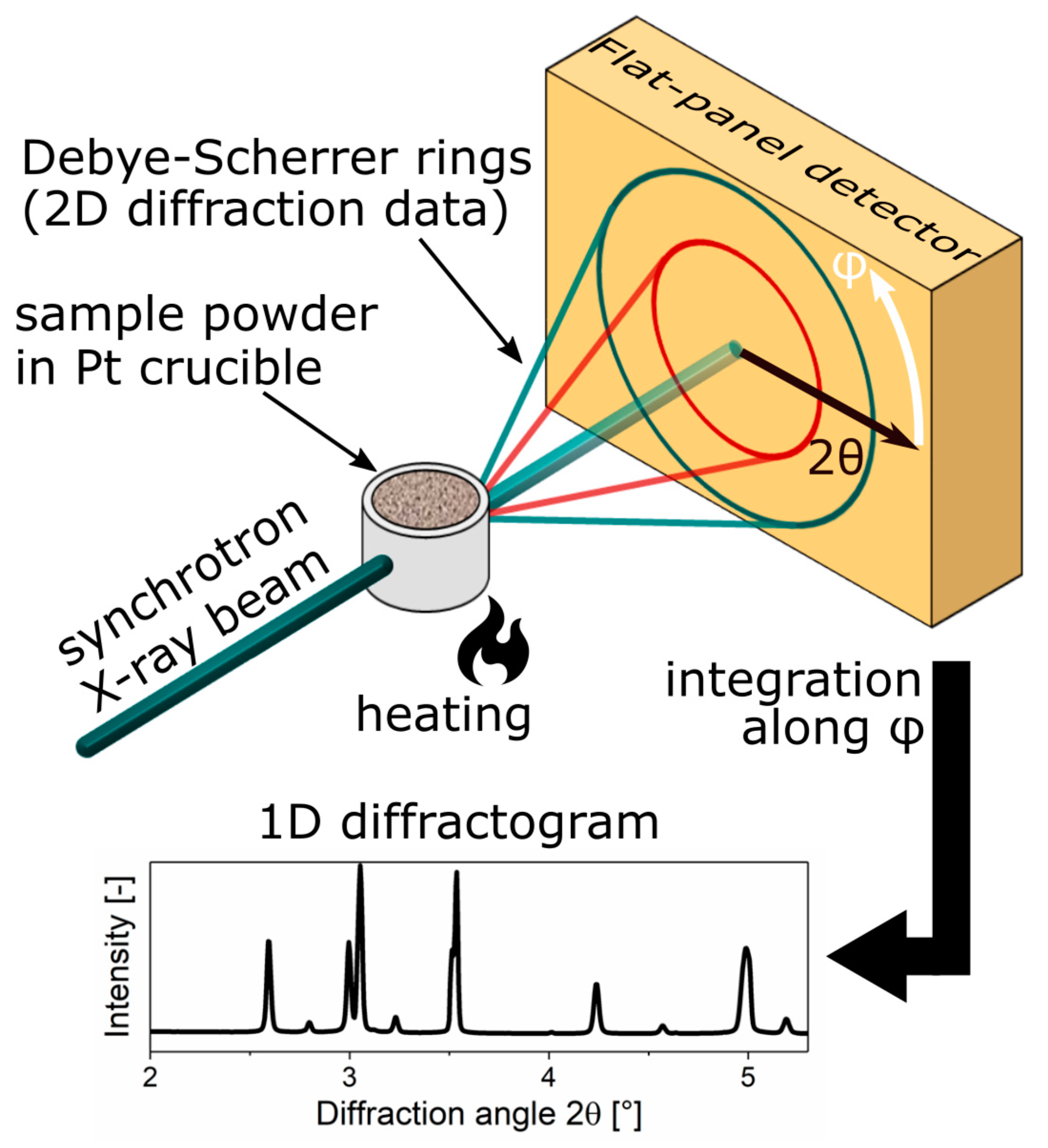
2. Materials and Methods
2.1. Coating Deposition
2.2. Coating Characterisation
2.3. Synchrotron Data Evaluation—Parametric Rietveld Refinement
3. Results and Discussion
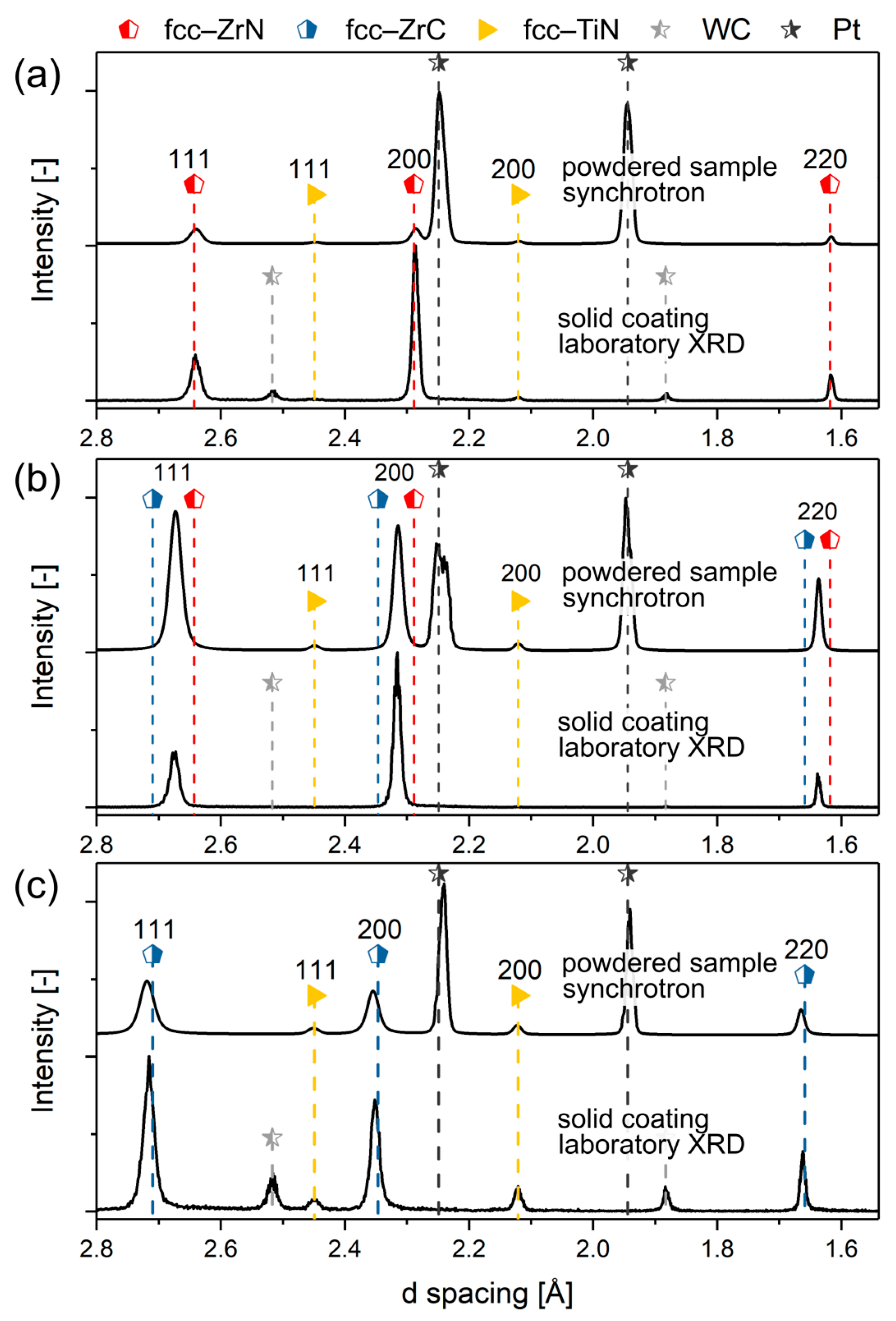
3.1. As-Deposited Microstructure
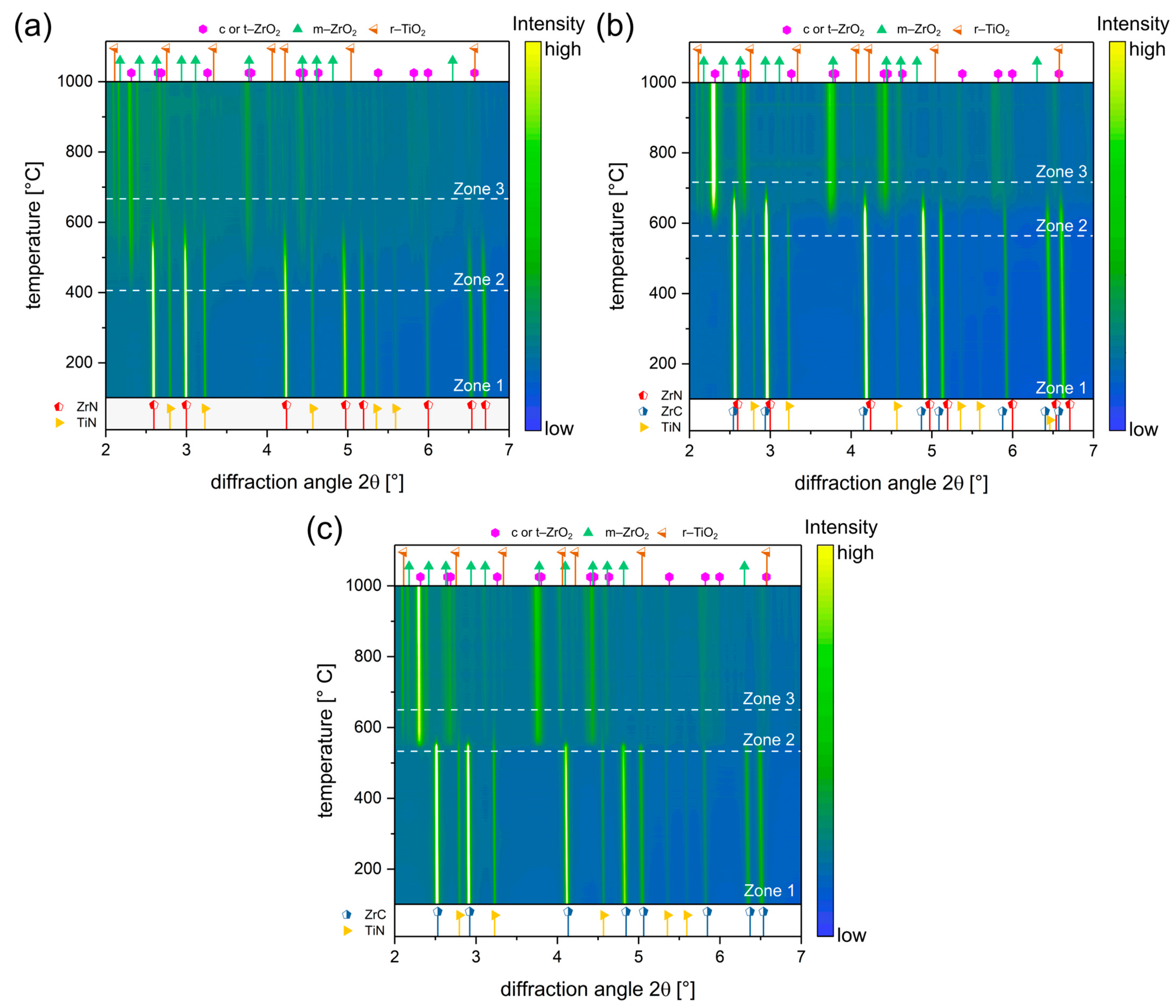
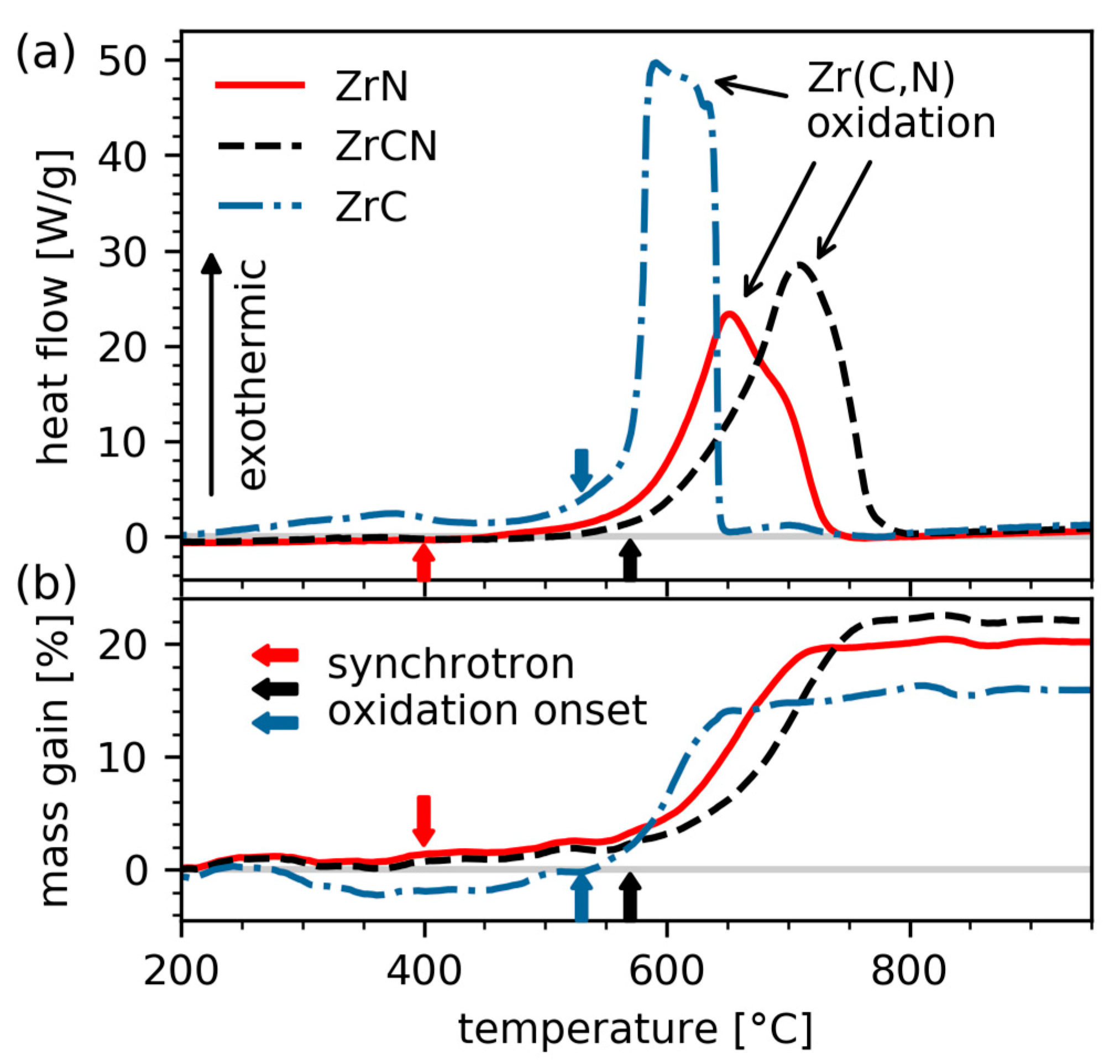
3.2. In-Situ Monitoring of the Oxidation Behaviour
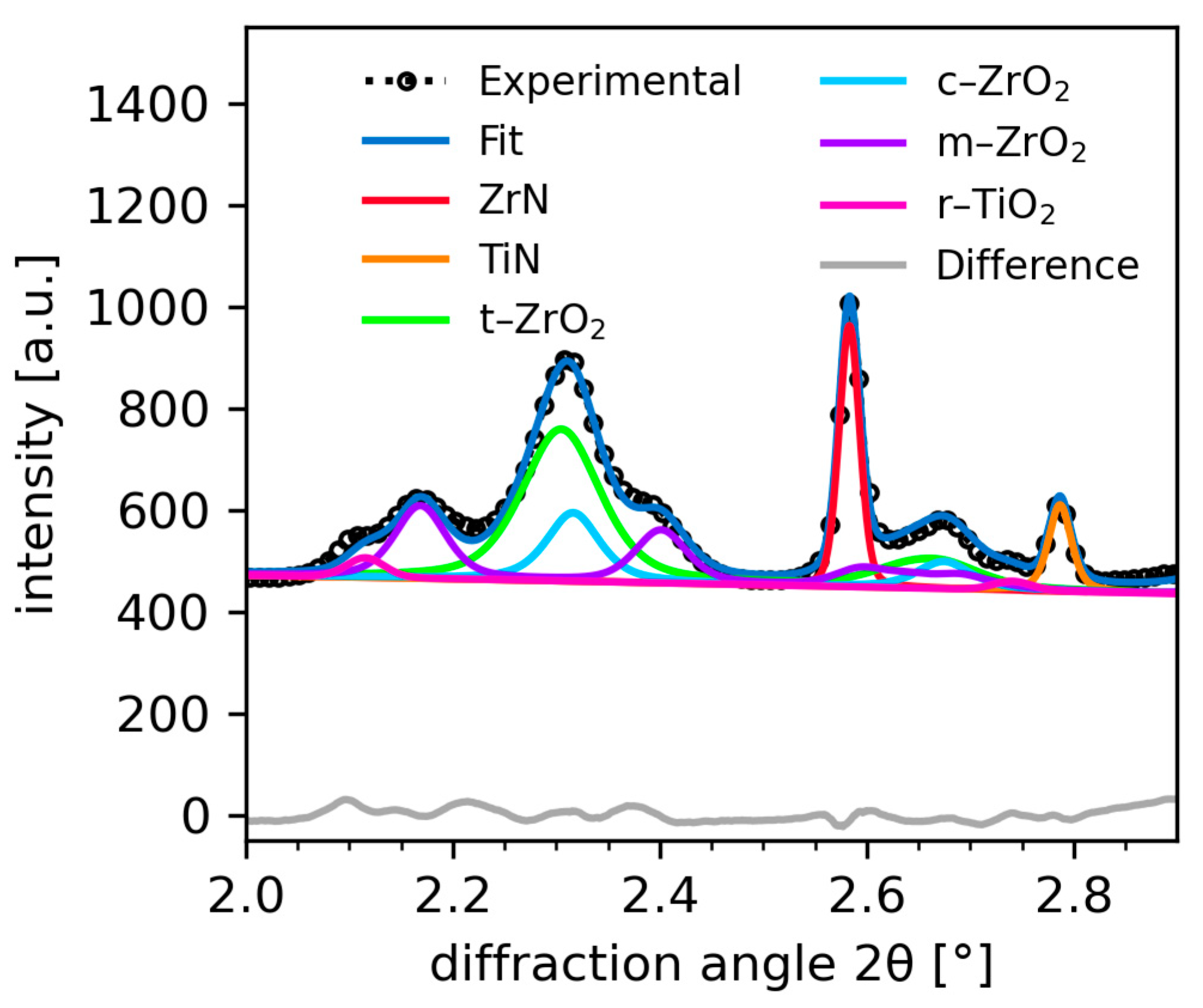
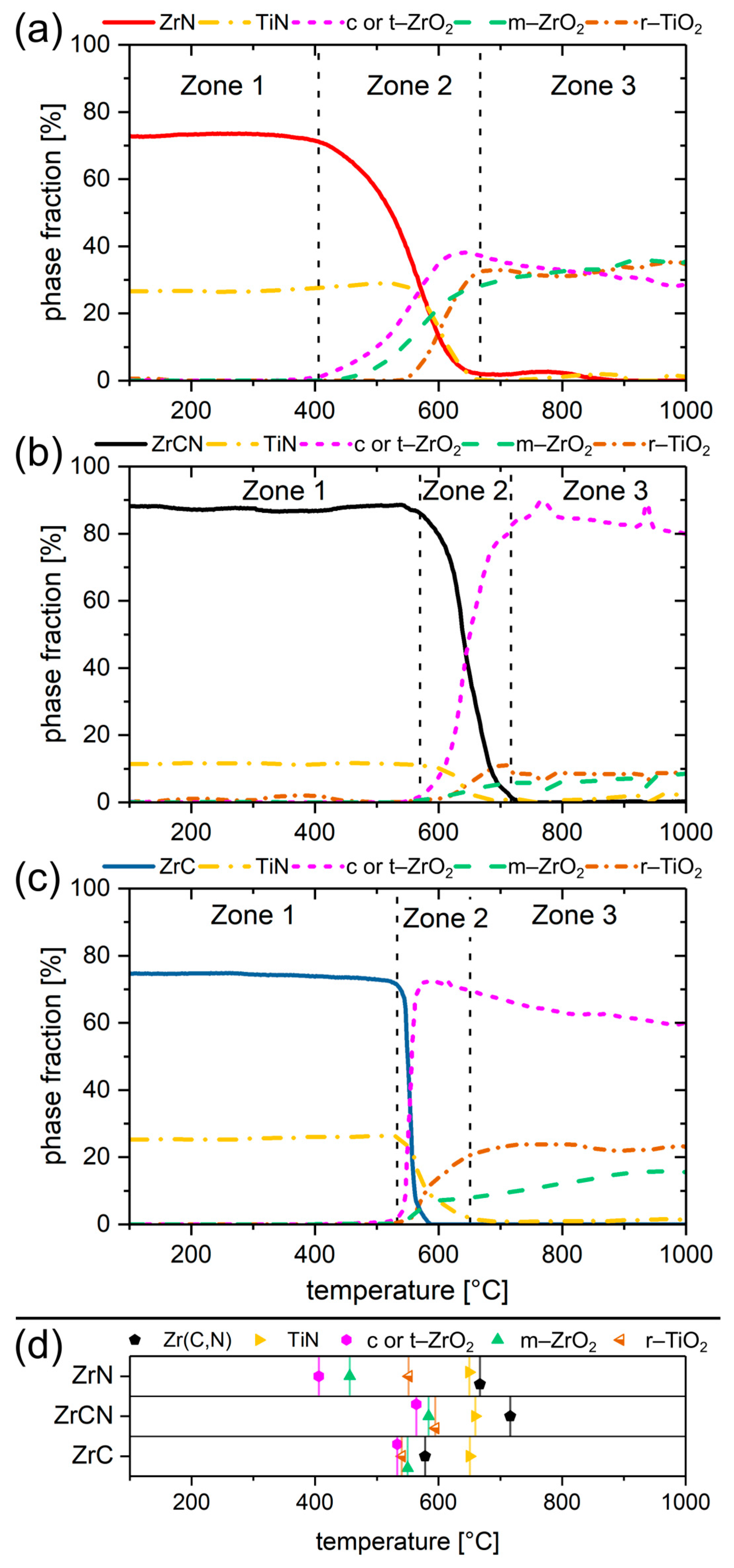
3.3. Quantification of the Temperature Dependant Phase Evolution with Parametric Rietveld Refinement
3.4. Influence of Carbon on the Oxidation Onset Temperature
3.5. Ex-Situ Raman and XRD Investigations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sundgren, J.E. Structure and properties of TiN coatings. Thin Solid Films 1985, 128, 21–44. [Google Scholar] [CrossRef]
- Rebenne, H.E.; Bhat, D.G. Review of CVD TiN coatings for wear-resistant applications: Deposition processes, properties and performance. Surf. Coat. Technol. 1994, 63, 1–13. [Google Scholar] [CrossRef]
- Holzschuh, H. Deposition of Ti–B–N (single and multilayer) and Zr–B–N coatings by chemical vapor deposition techniques on cutting tools. Thin Solid Films 2004, 469–470, 92–98. [Google Scholar] [CrossRef]
- Wagner, J.; Mitterer, C.; Penoy, M.; Michotte, C.; Wallgram, W.; Kathrein, M. The effect of deposition temperature on microstructure and properties of thermal CVD TiN coatings. Int. J. Refract. Met. Hard Mater. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Kainz, C.; Schalk, N.; Tkadletz, M.; Winkler, M.; Czettl, C. Microstructure, mechanical and thermo-physical properties of CVD TiCxN1−x coatings on cemented carbide substrates grown with C2H6 as C feeding precursor. Surf. Coat. Technol. 2020. [Google Scholar] [CrossRef]
- Garcia, J.; Moreno, M.F.; Östby, J.; Persson, J.; Pinto, H.C. Design of coated cemented carbides with improved comb crack resistance. In Proceedings of the 19th Plansee Seminar, Reutte, Tirol, Austria, 29 May–2 June 2017; pp. 1–8. [Google Scholar]
- El Azhari, I.; Garcia, J.; Zamanzade, M.; Soldera, F.; Pauly, C.; Llanes, L.; Mücklich, F. Investigations on micro-mechanical properties of polycrystalline Ti(C,N) and Zr(C,N) coatings. Acta Mater. 2018, 149, 364–376. [Google Scholar] [CrossRef]
- El Azhari, I.; Barrirero, J.; García, J.; Soldera, F.; Llanes, L.; Mücklich, F. Atom Probe Tomography investigations on grain boundary segregation in polycrystalline Ti(C,N) and Zr(C,N) CVD coatings. Scr. Mater. 2019, 162, 335–340. [Google Scholar] [CrossRef]
- Harrison, R.W.; Lee, W.E. Mechanism and kinetics of oxidation of ZrN ceramics. J. Am. Ceram. Soc. 2015, 98, 2205–2213. [Google Scholar] [CrossRef]
- Qi, Z.B.; Wu, Z.T.; Liang, H.F.; Zhang, D.F.; Wang, J.H.; Wang, Z.C. In Situ and Ex Situ studies of microstructure evolution during high-temperature oxidation of ZrN hard coating. Scr. Mater. 2015, 97, 9–12. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Y.; Kulczyk-Malecka, J.; Kelly, P.; Zeng, Y.; Zhang, X.; Li, C.; Liu, H.; Rohbeck, N.; Xiao, P. Comparison of the oxidation behavior of a zirconium nitride coating in water vapor and air at high temperature. Corros. Sci. 2018, 138, 242–251. [Google Scholar] [CrossRef]
- Krusin-Elbaum, L.; Wittmer, M. Oxidation kinetics of ZrN thin films. Thin Solid Films 1983, 107, 111–116. [Google Scholar] [CrossRef]
- Shimada, S.; Ishil, T. Oxidation kinetics of Zirconium Carbide at relatively low temperatures. J. Am. Ceram. Soc. 1990, 73, 2804–2808. [Google Scholar] [CrossRef]
- Rao, G.A.R.; Venugopal, V. Kinetics and mechanism of the oxidation of ZrC. J. Alloys Compd. 1994, 206, 237–242. [Google Scholar] [CrossRef]
- Tamura, K.; Ogawa, T.; Fukuda, K. The oxidation behavior of ZrC coating and powder studied by laser Raman spectroscopy and X-ray diffraction. J. Nucl. Mater. 1990, 175, 266–269. [Google Scholar] [CrossRef]
- Shimada, S.; Inagaki, M.; Suzuki, M. Microstructural observation of the ZrC/ZrO2 interface formed by oxidation of ZrC. J. Mater. Res. 1996, 11, 2594–2597. [Google Scholar] [CrossRef]
- Harrison, R.W.; Lee, W.E. Processing and properties of ZrC, ZrN and ZrCN ceramics: A review. Adv. Appl. Ceram. 2015, 115, 294–307. [Google Scholar] [CrossRef]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed; Wiley: New York, NY, USA, 1976; ISBN 0471478601. [Google Scholar]
- Shimada, S. A thermoanalytical study on the oxidation of ZrC and HfC powders with formation of carbon. Solid State Ion. 2002, 149, 319–326. [Google Scholar] [CrossRef]
- Shevchenko, A.S.; Lyutikov, R.A.; Andrievskii, R.A.; Terekhova, V.A. Oxidation of zirconium and niobium carbides. Sov. Powder Metall. Met. Ceram. 1980, 19, 48–52. [Google Scholar] [CrossRef]
- Shimada, S.; Nishisako, M.; Inagaki, M.; Yamamoto, K. Formation and microstructure of Carbon-containing oxide scales by oxidation of single crystals of Zirconium Carbide. J. Am. Ceram. Soc. 1995, 78, 41–48. [Google Scholar] [CrossRef]
- Shimada, S. Interfacial reaction on oxidation of carbides with formation of carbon. Solid State Ion. 2001, 141–142, 99–104. [Google Scholar] [CrossRef]
- Voitovich, R.F.; Pugach, E.A. High-temperature oxidation of ZrC and HfC. Sov. Powder Metall. Met. Ceram. 1973, 12, 916–921. [Google Scholar] [CrossRef]
- Jackson, H.F.; Lee, W.E. Properties and Characteristics of ZrC; Konings, R.J.M., Ed.; Elsevier Inc.: Oxford, UK, 2012; ISBN 9780080560335. [Google Scholar]
- Katoh, Y.; Vasudevamurthy, G.; Nozawa, T.; Snead, L.L. Properties of Zirconium Carbide for nuclear fuel applications. J. Nucl. Mater. 2013, 441, 718–742. [Google Scholar] [CrossRef]
- Gasparrini, C.; Chater, R.J.; Horlait, D.; Vandeperre, L.; Lee, W.E. Zirconium carbide oxidation: Kinetics and oxygen diffusion through the intermediate layer. J. Am. Ceram. Soc. 2018, 101, 2638–2652. [Google Scholar] [CrossRef]
- Gasparrini, C.; Podor, R.; Horlait, D.; Chater, R.; Lee, W.E. Zirconium Carbide oxidation: Maltese cross formation and interface characterization. Oxid. Met. 2017, 88, 509–519. [Google Scholar] [CrossRef]
- Huang, J.H.; Kuo, K.L.; Yu, G.P. Oxidation behavior and corrosion resistance of vacuum annealed ZrN-coated stainless steel. Surf. Coat. Technol. 2019, 358, 308–319. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S. Mechanisms of room temperature metastable tetragonal phase stabilisation in Zirconia. Int. Mater. Rev. 2005, 50, 45–64. [Google Scholar] [CrossRef]
- Schell, N.; King, A.; Beckmann, F.; Ruhnau, H.U.; Kirchhof, R.; Kiehn, R.; Müller, M.; Schreyer, A. The High Energy Materials Science Beamline (HEMS) at PETRA III. AIP Conf. Proc. 2010, 1234, 391–394. [Google Scholar] [CrossRef]
- Saringer, C.; Tkadletz, M.; Stark, A.; Schell, N.; Czettl, C.; Schalk, N. In-situ investigation of the oxidation behavior of metastable CVD Ti1−xAlxN using a novel combination of synchrotron radiation XRD and DSC. Surf. Coat. Technol. 2019, 374, 617–624. [Google Scholar] [CrossRef]
- Baerlocher, C.; Cheetham, A.K.; David, W.I.F.; de Keijser, T.H.; Delhez, R.; Hill, R.J.; Izumi, F.; Jorgensen, J.D.; Langford, J.I.; Louër, D.; et al. The Rietveld Method; Young, R.A., Ed.; Oxford University Press: Oxford, UK, 1993; Volume 1, ISBN 9780198559122. [Google Scholar]
- NIST 660c Certificate. Available online: https://www-s.nist.gov/srmors/view_detail.cfm?srm=660c (accessed on 25 August 2020).
- Stinton, G.W.; Evans, J.S.O. Parametric Rietveld refinement. J. Appl. Crystallogr. 2007, 40, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Dinnebier, R.E.; Leineweber, A.; Evans, J.S.O. Rietveld Refinement: Practical Powder Diffraction Pattern Analysis Using TOPAS; Walter de Gruyter GmbH & Co KG: Berlin, Germany; Boston, MA, USA, 2018; ISBN 3110461382. [Google Scholar]
- Crystallography Open Database. Available online: http://www.crystallography.net (accessed on 1 April 2019).
- Crystallography Open Database. COD-ID 1538058. Available online: http://www.crystallography.net/cod/1538058.html (accessed on 1 April 2019).
- Crystallography Open Database. COD-ID 1539496. Available online: http://www.crystallography.net/cod/1539496.html (accessed on 1 April 2019).
- Crystallography Open Database. COD-ID 1011322. Available online: http://www.crystallography.net/cod/1011322.html (accessed on 1 April 2019).
- Crystallography Open Database. COD-ID 1011099. Available online: http://www.crystallography.net/cod/1011099.html (accessed on 1 April 2019).
- Crystallography Open Database. COD-ID 1521753. Available online: http://www.crystallography.net/cod/1521753.html (accessed on 1 April 2019).
- Crystallography Open Database. COD-ID 1525705. Available online: http://www.crystallography.net/cod/1525705.html (accessed on 1 April 2019).
- Crystallography Open Database. COD-ID 2108450. Available online: http://www.crystallography.net/cod/2108450.html (accessed on 1 April 2019).
- Crystallography Open Database. COD-ID 9004141. Available online: http://www.crystallography.net/cod/9004141.html (accessed on 1 April 2019).
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Garvie, R.C. The occurrence of metastable tetragonal zirconia as a crystallite size effect. J. Phys. Chem. 1965, 69, 1238–1243. [Google Scholar] [CrossRef]
- Kanno, Y. Stability of metastable tetragonal ZrO2 in compound powders and nucleation arguments. J. Mater. Sci. 1990, 25, 1987–1990. [Google Scholar] [CrossRef]
- Reddy, G.L.N.; Ramana, J.V.; Kumar, S.; Kumar, S.V.; Raju, V.S. Investigations on the oxidation of zirconium nitride films in air by nuclear reaction analysis and backscattering spectrometry. Appl. Surf. Sci. 2007, 253, 7230–7237. [Google Scholar] [CrossRef]
- Gendre, M.; Maître, A.; Trolliard, G. Synthesis of zirconium oxycarbide (ZrCxOy) powders: Influence of stoichiometry on densification kinetics during spark plasma sintering and on mechanical properties. J. Eur. Ceram. Soc. 2011, 31, 2377–2385. [Google Scholar] [CrossRef]
- Garces, H.F.; Senturk, B.S.; Padture, N.P. In situ Raman spectroscopy studies of high-temperature degradation of thermal barrier coatings by molten silicate deposits. Scr. Mater. 2014, 76, 29–32. [Google Scholar] [CrossRef]
- Münz, W. Titanium aluminum nitride films: A new alternative to TiN coatings. J. Vac. Sci. Technol. Vac. Surf. Film. 1986, 4, 2717–2725. [Google Scholar] [CrossRef]
- Fateh, N.; Fontalvo, G.A.; Gassner, G.; Mitterer, C. Influence of high-temperature oxide formation on the tribological behaviour of TiN and VN coatings. Wear 2007, 262, 1152–1158. [Google Scholar] [CrossRef]
- Chen, L.; Paulitsch, J.; Du, Y.; Mayrhofer, P.H. Thermal stability and oxidation resistance of Ti-Al-N coatings. Surf. Coat. Technol. 2012, 206, 2954–2960. [Google Scholar] [CrossRef]
- Muñoz Tabares, J.A.; Anglada, M.J. Quantitative analysis of monoclinic phase in 3Y-TZP by Raman pectroscopy. J. Am. Ceram. Soc. 2010, 93, 1790–1795. [Google Scholar] [CrossRef]
- Gilman, J.J.; Galvanov, B.A.; Kindrachuk, V.M.; Zhang, L.C.; Cheong, W.C.D.; Ackland, G.J.; Levitas, V.I.; Fischer-Cripps, A.C.; Bhushan, B.; Xiaodong, L.; et al. High-Pressure Surface Science and Engineering, 1st ed.; Gogotsi, Y., Domnich, V., Eds.; Routledge: New York, NY, USA, 2004; ISBN 9780750308816. [Google Scholar]
- Naumenko, A.P.; Berezovska, N.I.; Biliy, M.M.; Shevchenko, O.V. Vibrational analysis and Raman spectra of tetragonal Zirconia. Phys. Chem. Solid State 2008, 9, 121–125. [Google Scholar]
- Rutile TiO2 Raman Peaks. Available online: https://rruff.info/rutile/display=default/R110109 (accessed on 2 November 2020).
- Krishnamurti, D. The Raman spectrum of rutile. Proc. Indian Acad. Sci. Sect. A 1962, 55, 290–299. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, F.; Tkadletz, M.; Saringer, C.; Stark, A.; Schell, N.; Deluca, M.; Czettl, C.; Schalk, N. In-Situ Investigation of the Oxidation Behaviour of Chemical Vapour Deposited Zr(C,N) Hard Coatings Using Synchrotron X-ray Diffraction. Coatings 2021, 11, 264. https://doi.org/10.3390/coatings11030264
Frank F, Tkadletz M, Saringer C, Stark A, Schell N, Deluca M, Czettl C, Schalk N. In-Situ Investigation of the Oxidation Behaviour of Chemical Vapour Deposited Zr(C,N) Hard Coatings Using Synchrotron X-ray Diffraction. Coatings. 2021; 11(3):264. https://doi.org/10.3390/coatings11030264
Chicago/Turabian StyleFrank, Florian, Michael Tkadletz, Christian Saringer, Andreas Stark, Norbert Schell, Marco Deluca, Christoph Czettl, and Nina Schalk. 2021. "In-Situ Investigation of the Oxidation Behaviour of Chemical Vapour Deposited Zr(C,N) Hard Coatings Using Synchrotron X-ray Diffraction" Coatings 11, no. 3: 264. https://doi.org/10.3390/coatings11030264
APA StyleFrank, F., Tkadletz, M., Saringer, C., Stark, A., Schell, N., Deluca, M., Czettl, C., & Schalk, N. (2021). In-Situ Investigation of the Oxidation Behaviour of Chemical Vapour Deposited Zr(C,N) Hard Coatings Using Synchrotron X-ray Diffraction. Coatings, 11(3), 264. https://doi.org/10.3390/coatings11030264






