Optical Evidence for the Assembly of Sensors Based on Reduced Graphene Oxide and Polydiphenylamine for the Detection of Epidermal Growth Factor Receptor
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
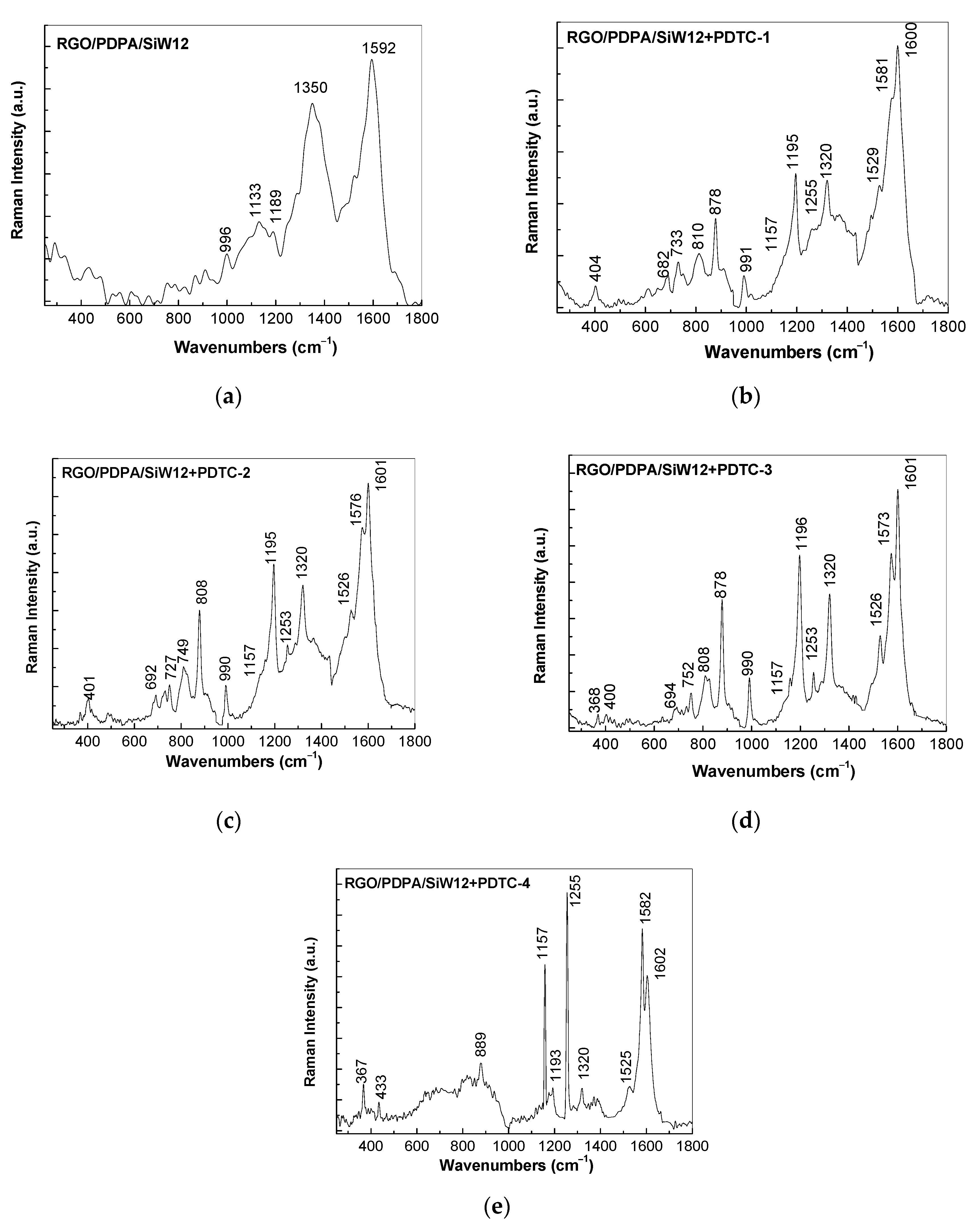
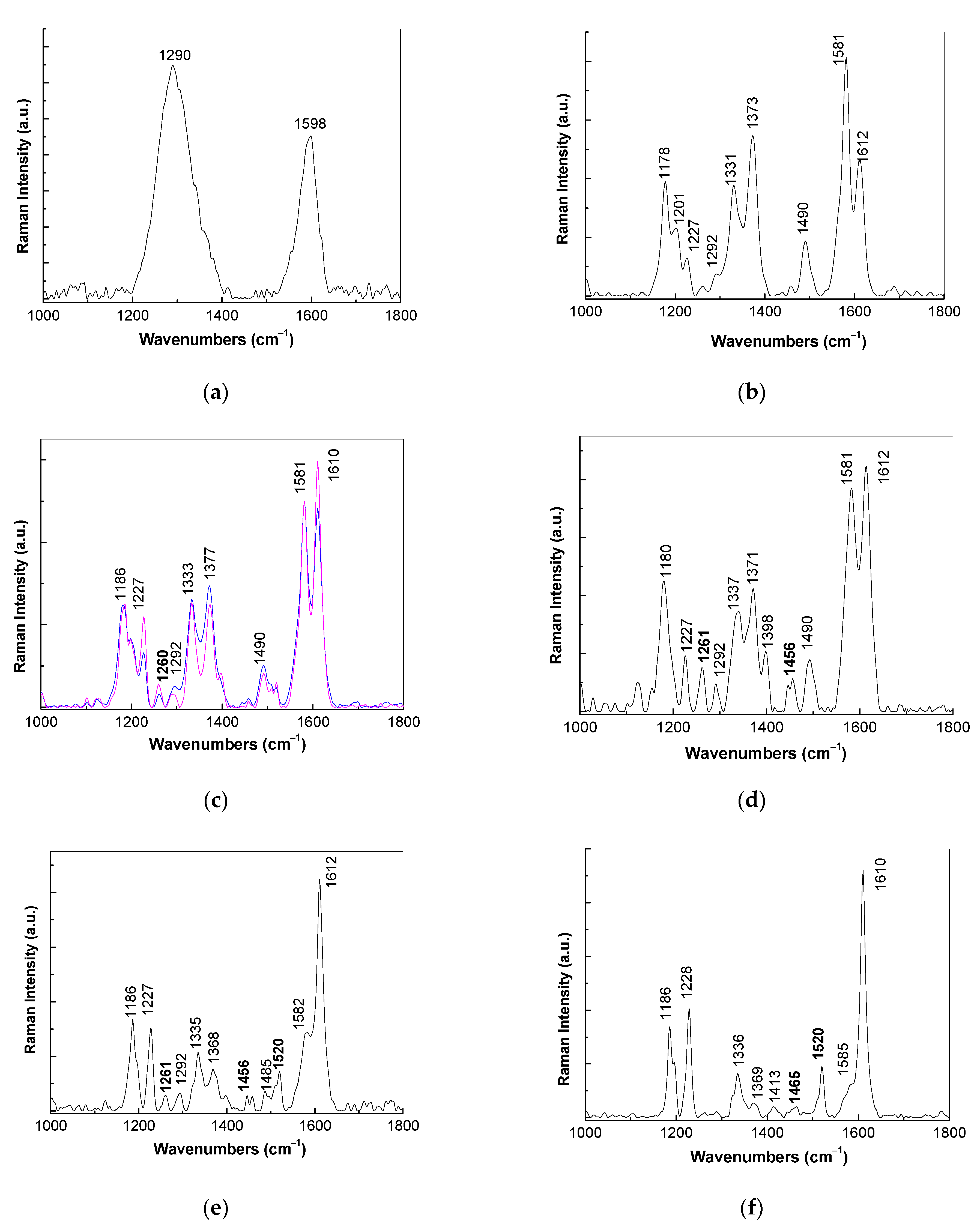
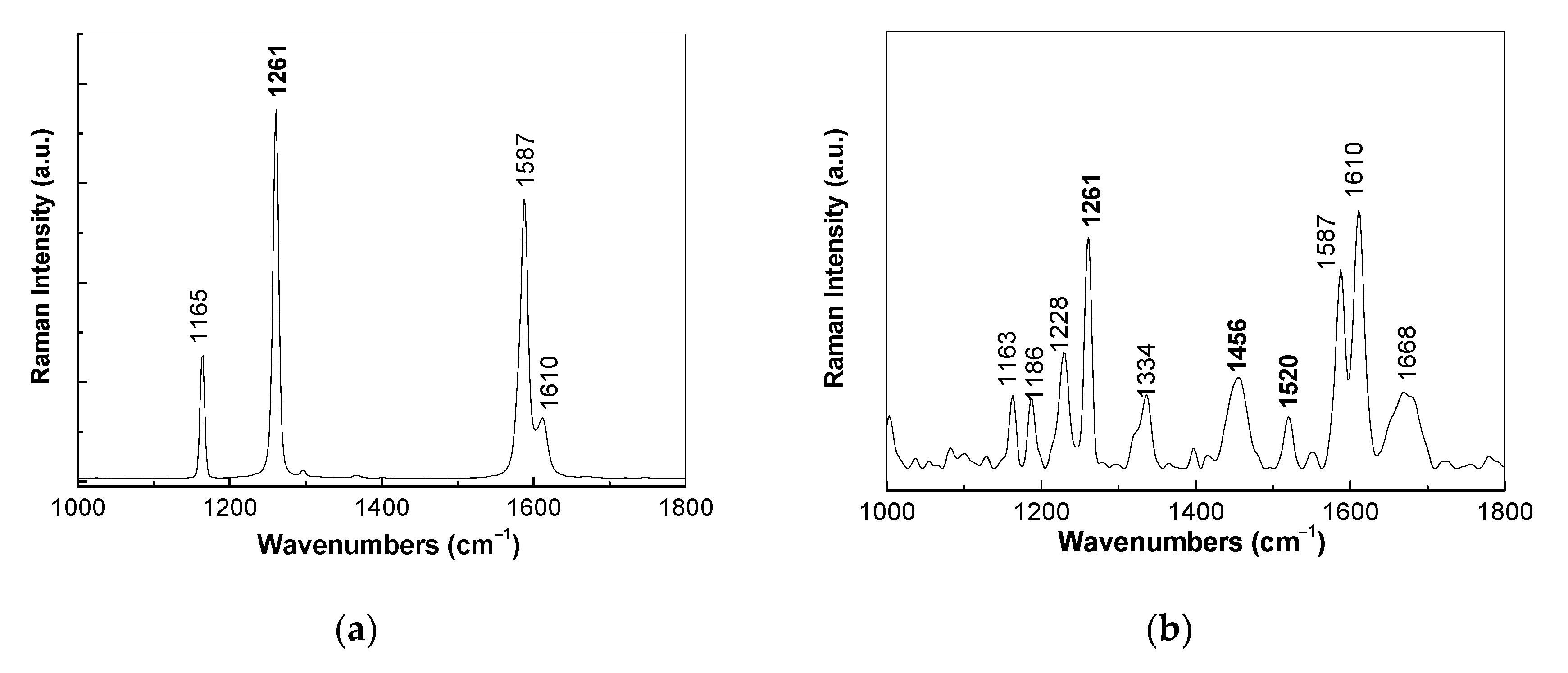
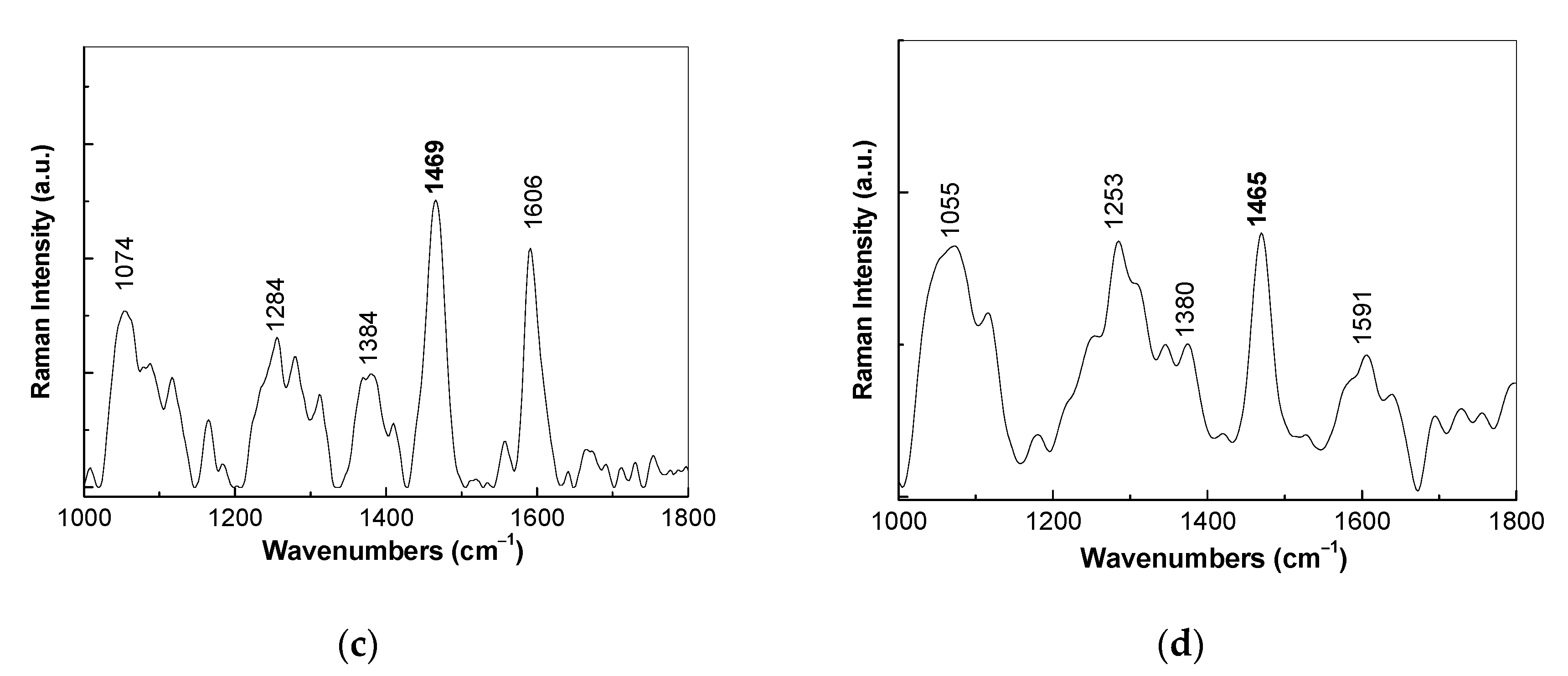
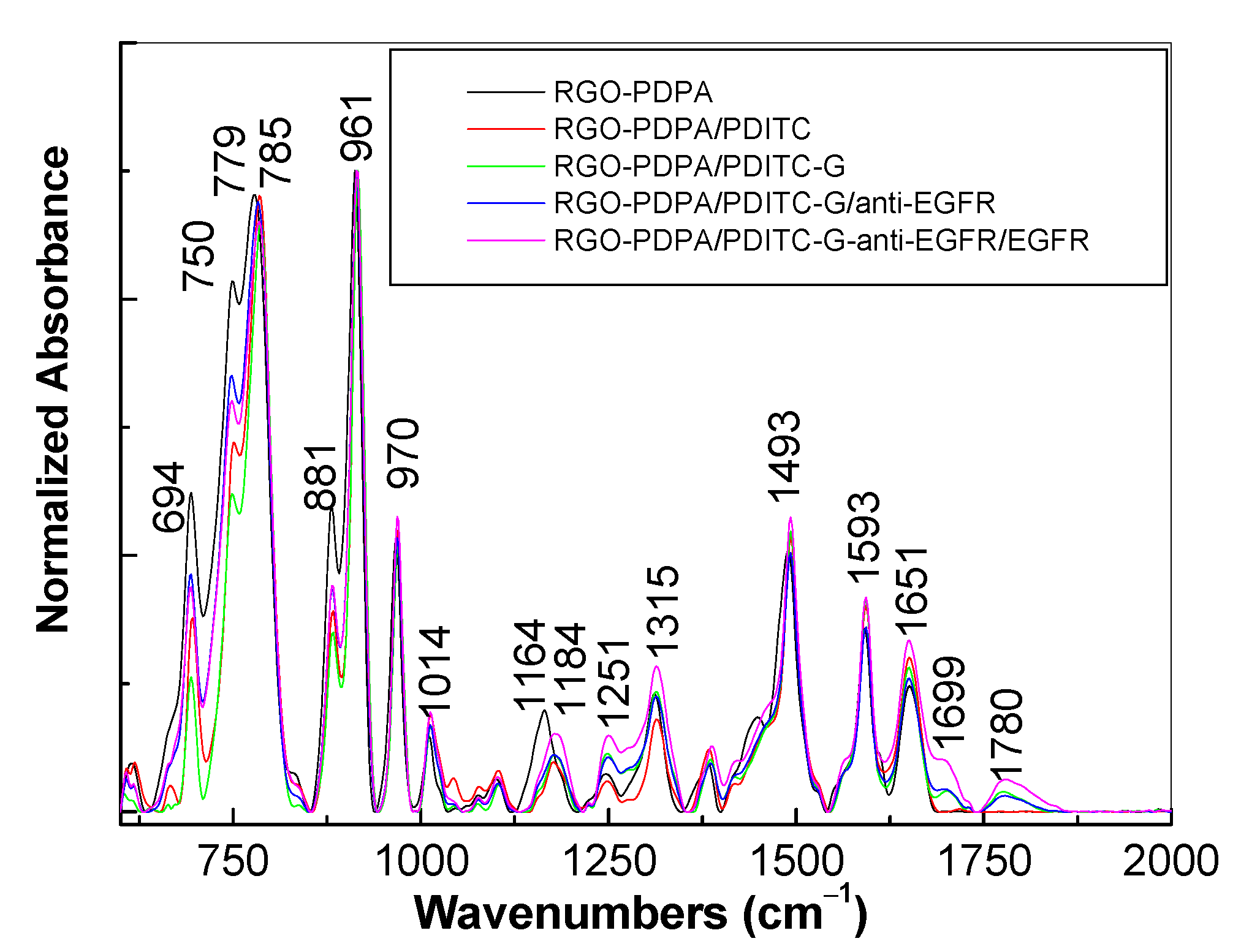
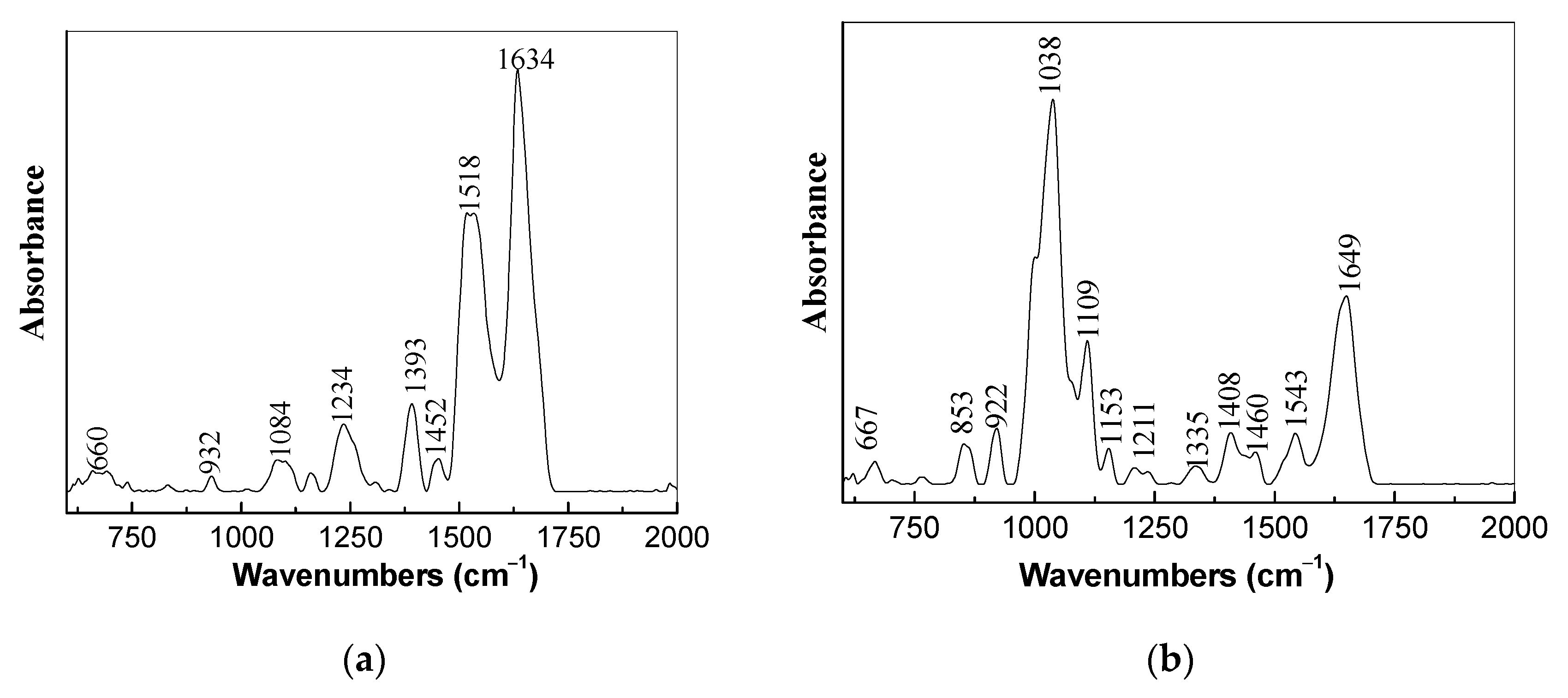
3.1. Optical Evidences by Raman Scattering and FTIR Spectroscopy Studies Concerning the Assembling of the Sensorial Platforms for EGFR Detection
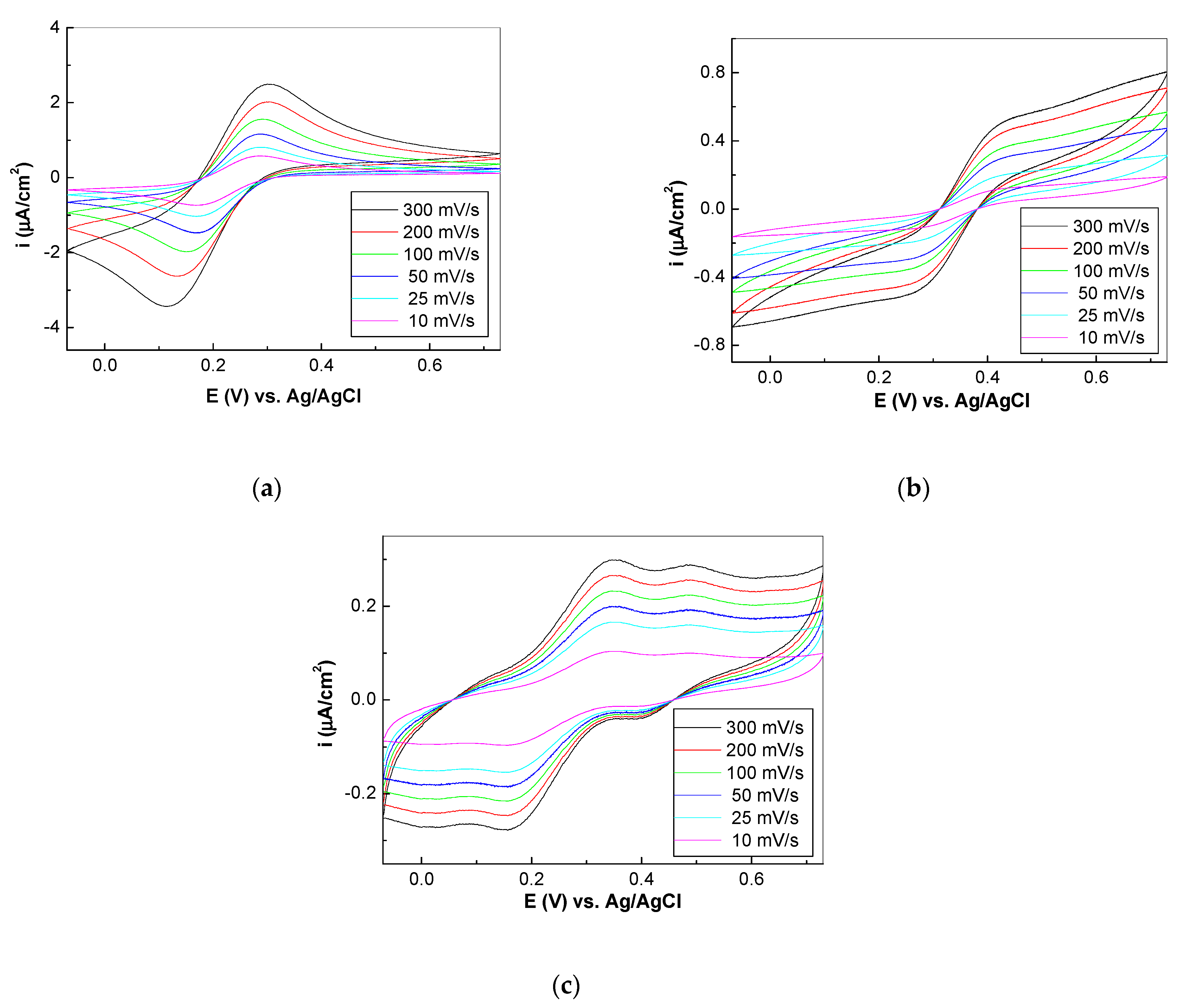
3.2. The Electrochemical Properties of the Platforms SPCE-RGO/PDPA-PDITC-G-EGFR Antibodies-EGFR
4. Conclusions
- i.
- the interaction of the SPCE-RGO /PDPA platform with PDITC leads to a covalent functionalization of this platform, evidenced by an up-shift of the Raman line, from 1176 to 1189 cm−1, and the appearance of a new Raman line at 1133 cm−1;
- ii.
- the successive interactions of the SPCE-RGO/PDPA-PDITC with protein G, EGFR antibodies, and EGFR were highlighted by (a) new Raman lines at 1260, 1456, 1465, and 1520 cm−1 belonging to PDITC, protein G, EGFR antibodies, and EGFR, respectively, and (b) the IR spectra by the appearance of new IR bands at 1699 and 1780 cm−1; and
- iii.
- at the interface of the SPCE-RGO/PDPA-PDITC-G-EGFR antibodies/EGFR platform with K3[Fe(CN)6]/K4[Fe(CN)6] solution in PB with a pH equal to 7.4, the irreversible processes were reported.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, R.; Gee, J.; Harper, M. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37, 9–15. [Google Scholar] [CrossRef]
- Arteaga, C.L. Epidermal growth factor receptor dependence in human tumors: More than just expression? Oncologist 2002, 7, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Dziadziuszko, R.; Thatcher, N.; Mann, H.; Wartkins, C.; Parums, D.V.; Speake, G.; Holloway, B.; Bunn, P.A.; Franklin, W.A. Epidermal growth factor receptor immunohistochemistry: Comparison of antibodies and cutoff points to predict benefit from gefitinib in a phase 3 placebo-controlled study in advanced non-small-cell lung cancer. Cancer 2008, 112, 1114–1121. [Google Scholar] [CrossRef]
- Buffet, W.; Geboes, K.P.; Dehertogh, G. EGFR-immunohistochemistry in colorectal cancer and non-small cell lung cancer: Comparison of 3 commercially available EGFR-antibodies. Acta Gastroenterol. Belg. 2008, 71, 213–218. [Google Scholar] [PubMed]
- Thariat, J.; Etienne-Grimaldi, M.C.; Grall, D.; Bensadoun, R.J.; Cayre, A.; Penault-Llorca, F.; Verracini, L.; Francoual, M.; Formento, J.L.; Dassonville, O.; et al. Epidermal growth factor receptor protein detection in head and neck cancer patients: A many-faceted picture. Clin. Cancer Res. 2012, 18, 1313–1322. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Nexo, E.; Bentzen, S.M.; Clausen, P.P.; Andersen, K.W.; Rose, C.D. Enzyme-linked immunosorbent assay of epidermal growth factor receptor in lung cancer: Comparisons with immunohistochemistry, clinicopathological features and prognosis. Br. J. Cancer 1998, 78, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Weigum, S.E.; Floriano, P.N.; Redding, P.N.; Yeh, S.W.; Westbrook, S.D.; McGuff, S.; Lin, H.; Miller, A.; Vllarreal, F.R.; Rowan, F.; et al. Nano-bio-chip sensor platform for examination of oral exfoliative cytology. Cancer Prev. Res. 2010, 3, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Weigum, S.E.; Floriano, P.N.; Christodoulides, N.; McDevitt, J.T. Cell-based sensor for analysis of EGFR biomarker expression in oral cancer. Lab Chip 2007, 7, 995–1003. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Surface plasmon resonance scattering and absorption of anti-egfr antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005, 5, 829–834. [Google Scholar] [CrossRef]
- Vasudev, A.; Kaushik, A.; Bhansali, S. Electrochemical immunosensor for label free epidermal growth factor receptor (EGFR) detection. Biosens. Bioelectron. 2013, 39, 300–305. [Google Scholar] [CrossRef]
- Baibarac, M.; Daescu, M.; Socol, M.; Bartha, C.; Negrila, C.; Fejer, S.N. Influence of reduced graphene oxide on the electropolymerization of 5-amino-1-naphthol and the interaction of 1,4-phenylene diisothiocyanate with the poly(5-amino-1-naphtol)/reduced graphene oxide composite. Polymers 2020, 12, 1299. [Google Scholar] [CrossRef]
- Baibarac, M.; Stroe, M.; Fejer, S.N. Vibrational and photoluminescence properties of polydiphenylamine doped with silicotungstic acid heteropolyanions and their composites with reduced graphene oxide. J. Mol. Struct. 2019, 1184, 25–35. [Google Scholar] [CrossRef]
- Smaranda, I.; Benito, A.M.; Maser, W.K.; Baltog, I.; Baibarac, M. Electrochemical grafting of reduced graphene oxide with polydiphenylamine doped with heteropolyanions and its optical properties. J. Phys. Chem. C 2014, 118, 25704–25717. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Guo, Y.; Li, K.; Yu, X.; Clark, J.H. Mesoporous H3PW12O40-silica composite: Efficient and reusable solid acid catalyst for the synthesis of diphenolic acid from levulinic acid. Appl. Catal. B Environ. 2008, 81, 182–191. [Google Scholar] [CrossRef]
- Baibarac, M.; Baltog, I.; Lefrant, S.; Gómez-Romero, P. Polydiphenylamine/carbon nanotube composites for applications in rechargeable lithium batteries. Mater. Sci. Eng. B 2011, 176, 110–120. [Google Scholar] [CrossRef]
- Legagneux, N.; Basset, J.M.; Thomas, A.; Lefebvre, F.; Goguet, A.; Sa, J.; Hardacre, C. Characterization of silica-supported dodecatungstic heteropolyacids as a function of their dihydroxylation temperature. Dalton Trans. 2009, 12, 2235–2240. [Google Scholar]
- Kvarnström, C.; Petr, A.; Damlin, P.; Lindfors, T.; Ivaska, A.; Dunsch, L. Raman and FTIR spectroscopic characterization of electrochemically synthesized poly(triphenylamine), PTPA. J. Solid State Electrochem. 2002, 6, 505–512. [Google Scholar] [CrossRef]
- Quillard, S.; Louarn, G.; Lefrant, S.; MacDiarmid, A.G. Vibrational analysis of polyaniline—a comparative study of leucoemeraldine, emeraldine and pernigranilien bases. Phys. Rev. B 1994, 50, 12496–12508. [Google Scholar] [CrossRef] [PubMed]
- Janik, I.; Carmichael, I.; Tripathi, G. Transient Raman spectra, structure and thermochemistry of the thiocyanate dimer radical anion in water. J. Chem. Phys. 2017, 146, 214305. [Google Scholar] [CrossRef]
- Moritz, A. Infra-red and Raman spectra of methyl thiocyanate and methyl-d3 thiocyanate. Spectrochim. Acta 1966, 22, 1021–1028. [Google Scholar] [CrossRef]
- Hill, W.; Wehling, B. Potential- and pH-dependent surface-enhanced Raman scattering of p-mercapto aniline on silver and gold substrates. J. Phys. Chem. 1993, 97, 9451–9455. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Cui, C.; Jiang, J. In situ surface-enhanced Raman spectroscopy study of thiocyanate ions adsorbed on silver nanoparticles under high pressure. Chem. Phys. 2019, 516, 1–5. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, Y.; Tan, T.; Lv, Y. Targeted live cell raman imaging and visualization of cancer biomarkers with thermal-stimuli responsive imprinted nanoprobes. Part. Part. Syst. Charact. 2018, 35, 1800390. [Google Scholar] [CrossRef]
- Sui, C.; Li, C.; Guo, X.; Cheng, T.; Gao, Y.; Zhou, G.; Gong, J.; Du, J. Facile synthesis of silver nanoparticles-modified PVA/H4SiW12O40 nanofibers-based electrospinning to enhance photocatalytic activity. Appl. Surf. Sci. 2012, 258, 7105–7111. [Google Scholar] [CrossRef]
- Raj, M.R.; Anandan, S.; Zhou, M.; AshokKumar, M. A facile one-step synthesis of hollow polydiphenylamine. Int. J. Polym. Mater. 2013, 62, 23–27. [Google Scholar] [CrossRef]
- De Santana, H.; Dias, F. Characterization and properties of polydiphenylamine electrochemically modified by iodide species. Mater. Chem. Phys. 2003, 82, 882–886. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. et Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
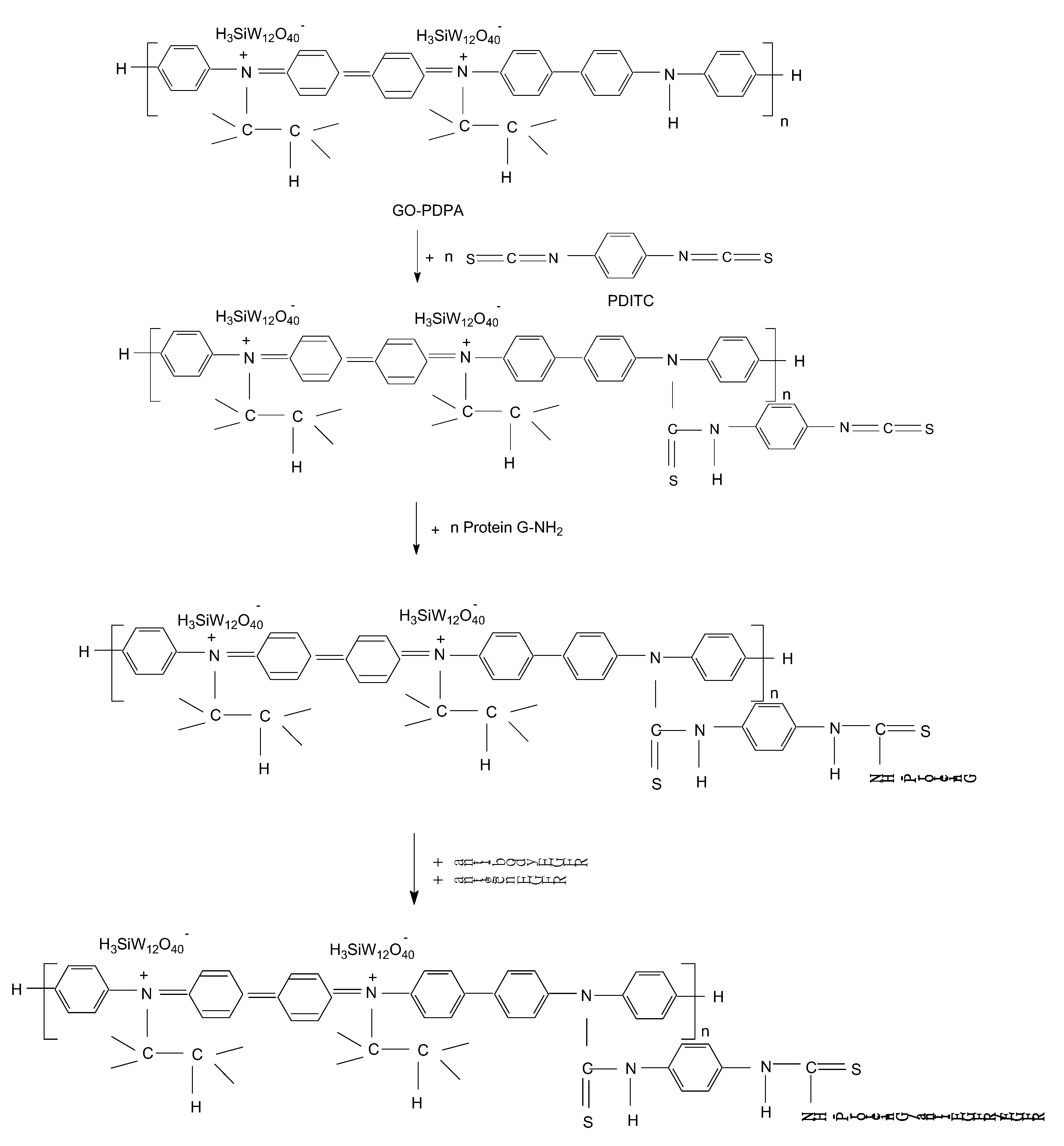
| Electrode | v (mV s−1) | Epa (mV) | Epc (mV) | ΔE (mV) | ipa (µA cm−2) | ipc (µA cm−2) | ipa/ipc |
|---|---|---|---|---|---|---|---|
| 0 | 300 | 300 | 114 | 186 | 2.49 | 3.43 | 0.72 |
| 200 | 295 | 131 | 164 | 2.01 | 2.63 | 0.76 | |
| 100 | 284 | 144 | 140 | 1.55 | 1.97 | 0.78 | |
| 50 | 283 | 167 | 116 | 1.16 | 1.47 | 0.79 | |
| 25 | 281 | 166 | 115 | 0.81 | 1.03 | 0.79 | |
| 10 | 283 | 168 | 115 | 0.58 | 0.74 | 0.78 | |
| SPCE-RGO | 300 | 435 | 258 | 177 | 0.52 | 0.50 | 1.04 |
| 200 | 428 | 254 | 174 | 0.45 | 0.45 | 1 | |
| 100 | 421 | 258 | 163 | 0.35 | 0.44 | 1.03 | |
| 50 | 417 | 264 | 153 | 0.28 | 0.29 | 0.96 | |
| 25 | 416 | 270 | 146 | 0.19 | 0.19 | 1 | |
| 10 | 410 | 279 | 131 | 0.11 | 0.11 | 1 | |
| SPCE-RGO/PDPA-PDITC-G-anti-EGFR/ EGFR | 300 | 345 | 147 | 198 | 0.3 | 0.28 | 1.07 |
| 200 | 343 | 148 | 195 | 0.26 | 0.25 | 1.04 | |
| 100 | 341 | 150 | 191 | 0.23 | 0.21 | 1.09 | |
| 50 | 338 | 152 | 186 | 0.19 | 0.18 | 1.06 | |
| 25 | 334 | 155 | 179 | 0.16 | 0.15 | 1.07 | |
| 10 | 330 | 160 | 170 | 0.10 | 0.09 | 1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baibarac, M.; Daescu, M.; Fejer, S.N. Optical Evidence for the Assembly of Sensors Based on Reduced Graphene Oxide and Polydiphenylamine for the Detection of Epidermal Growth Factor Receptor. Coatings 2021, 11, 258. https://doi.org/10.3390/coatings11020258
Baibarac M, Daescu M, Fejer SN. Optical Evidence for the Assembly of Sensors Based on Reduced Graphene Oxide and Polydiphenylamine for the Detection of Epidermal Growth Factor Receptor. Coatings. 2021; 11(2):258. https://doi.org/10.3390/coatings11020258
Chicago/Turabian StyleBaibarac, Mihaela, Monica Daescu, and Szilard N. Fejer. 2021. "Optical Evidence for the Assembly of Sensors Based on Reduced Graphene Oxide and Polydiphenylamine for the Detection of Epidermal Growth Factor Receptor" Coatings 11, no. 2: 258. https://doi.org/10.3390/coatings11020258
APA StyleBaibarac, M., Daescu, M., & Fejer, S. N. (2021). Optical Evidence for the Assembly of Sensors Based on Reduced Graphene Oxide and Polydiphenylamine for the Detection of Epidermal Growth Factor Receptor. Coatings, 11(2), 258. https://doi.org/10.3390/coatings11020258







