Synthesis and Frost Suppression Performance of PDMS-SiO2/PFA Hybrid Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PDMS-SiO2 Hybrid and PFA Emulsion
2.3. Synthesis of PDMS-SiO2/PFA Hybrid Coating
2.4. Film Thickness and Adhesion Test
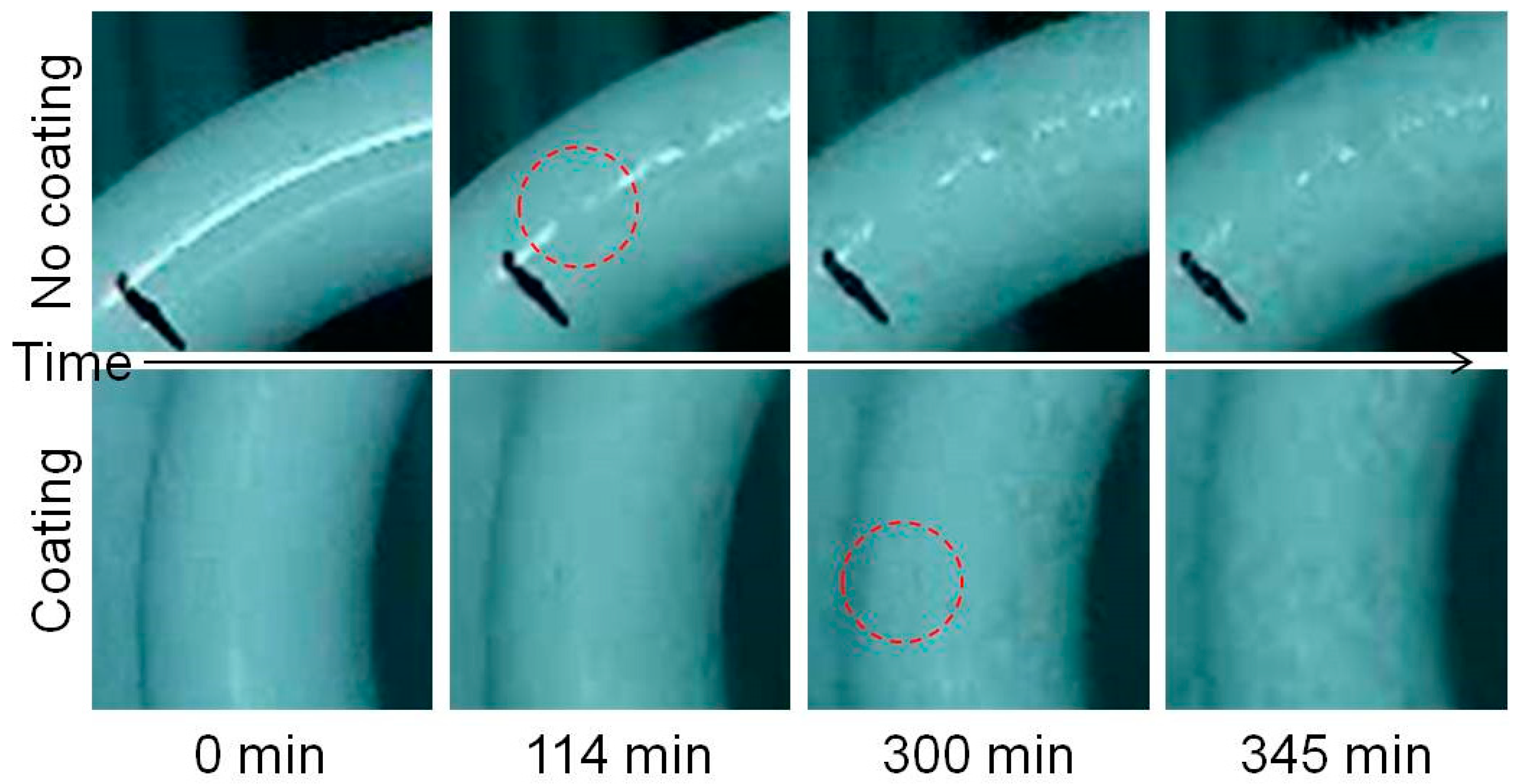
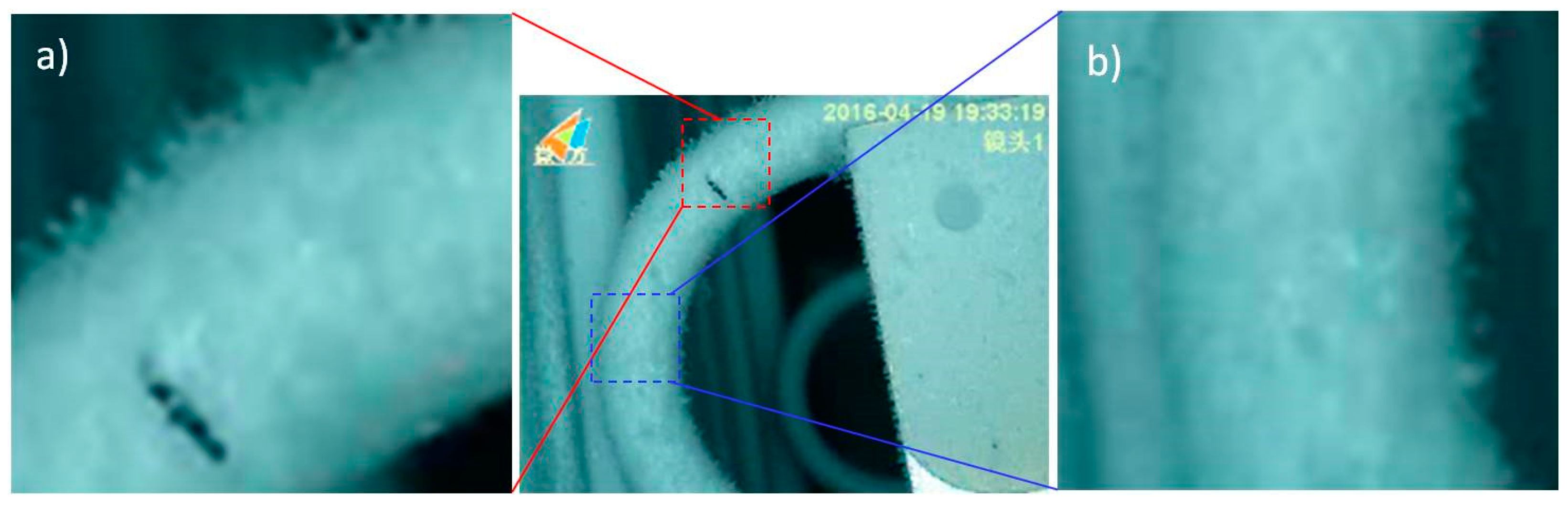
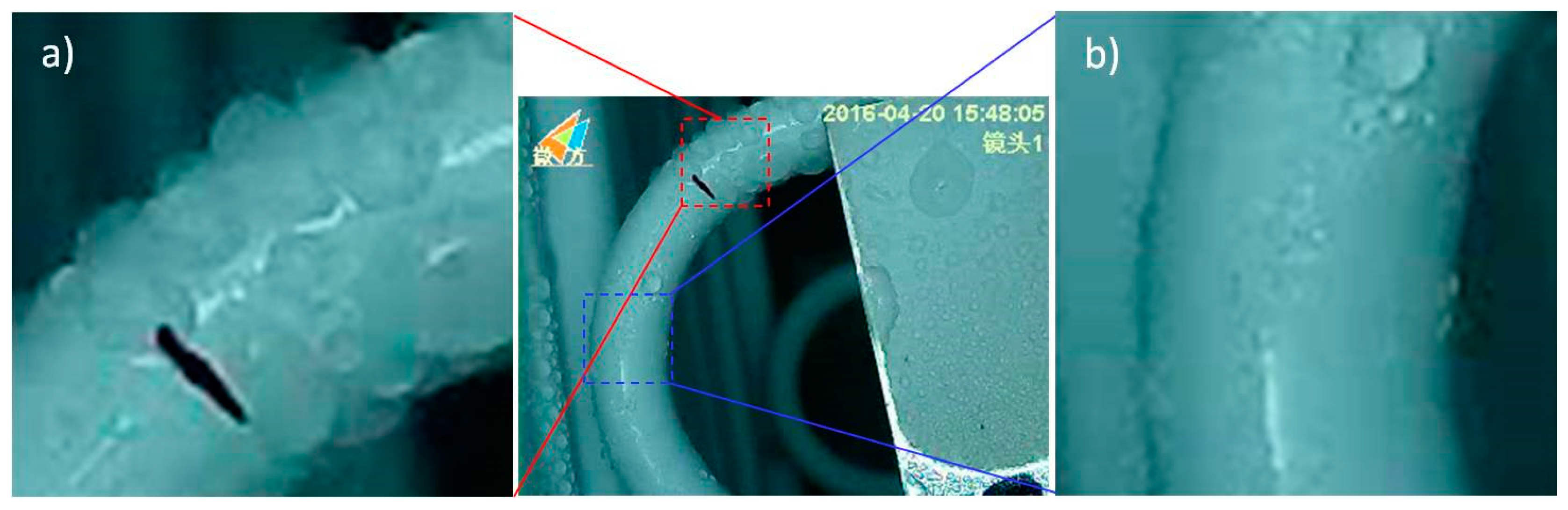
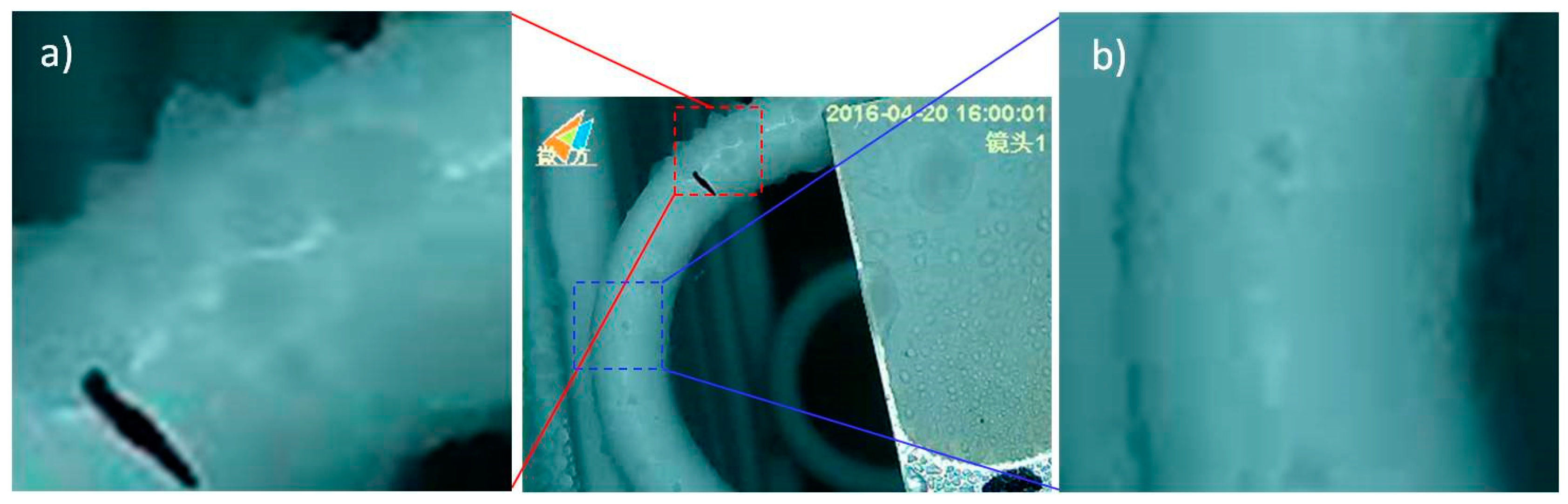
2.5. Frosting Test
2.6. Characterization
3. Results and Discussion
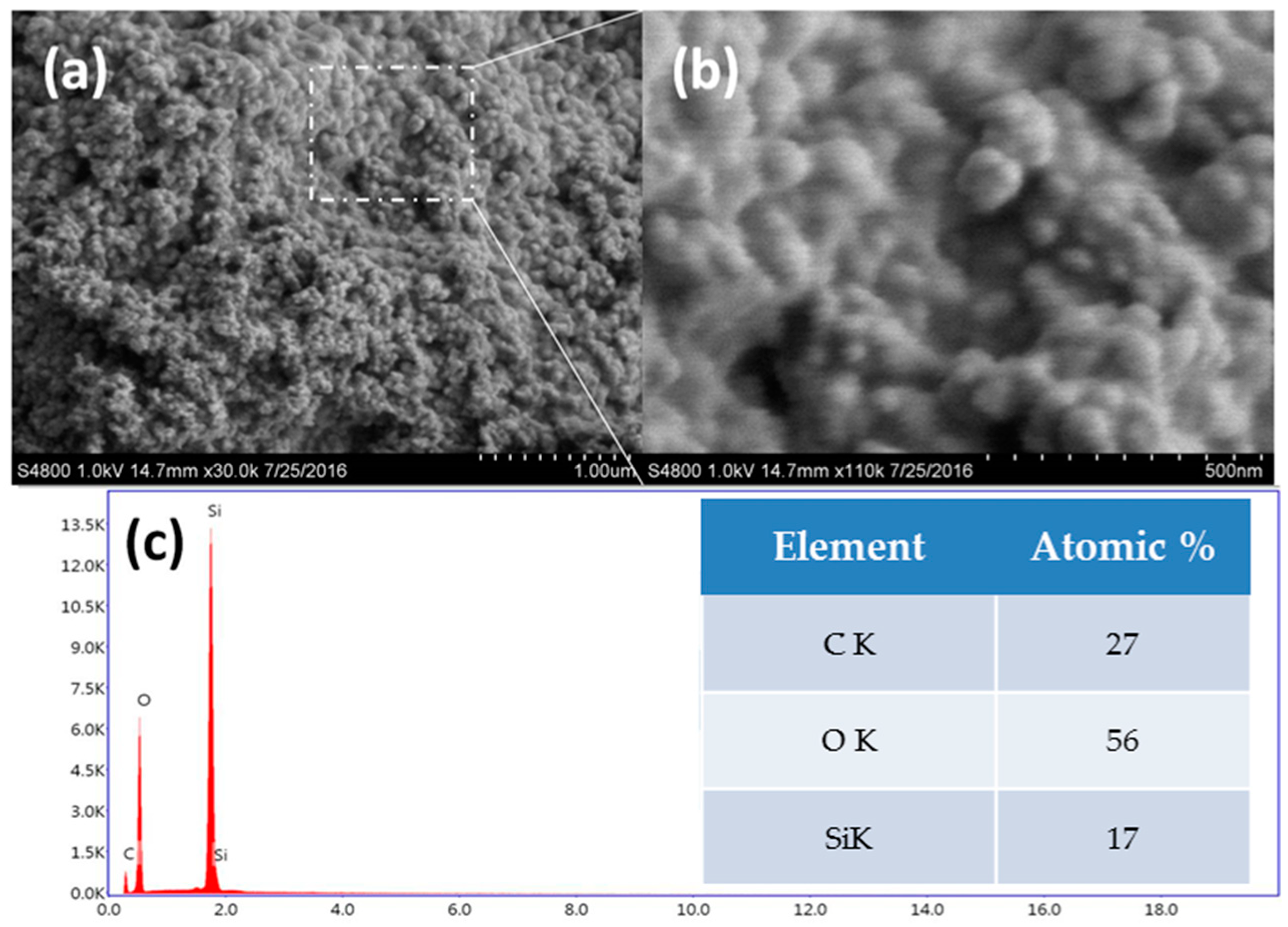
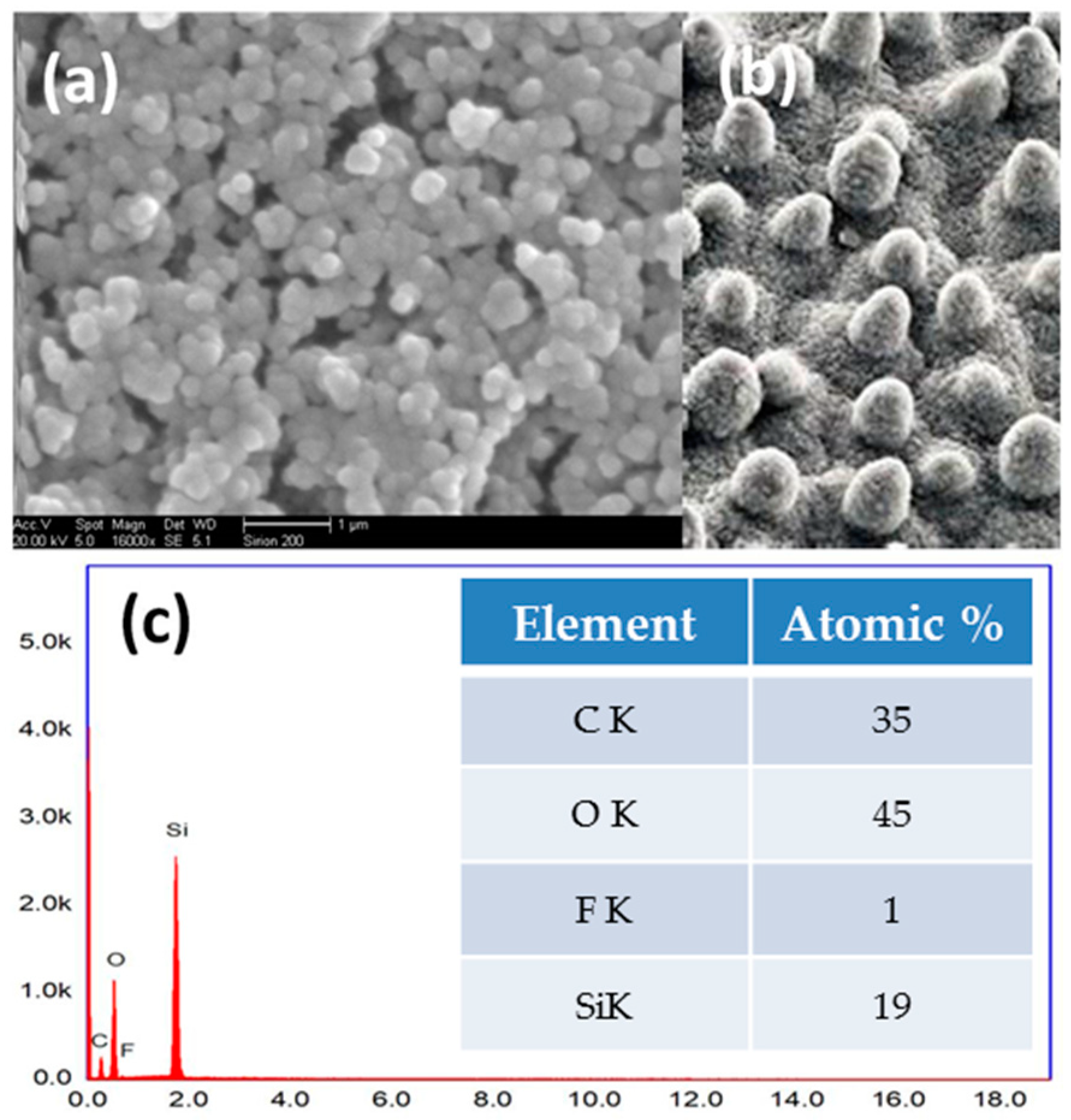
3.1. Surface Topography
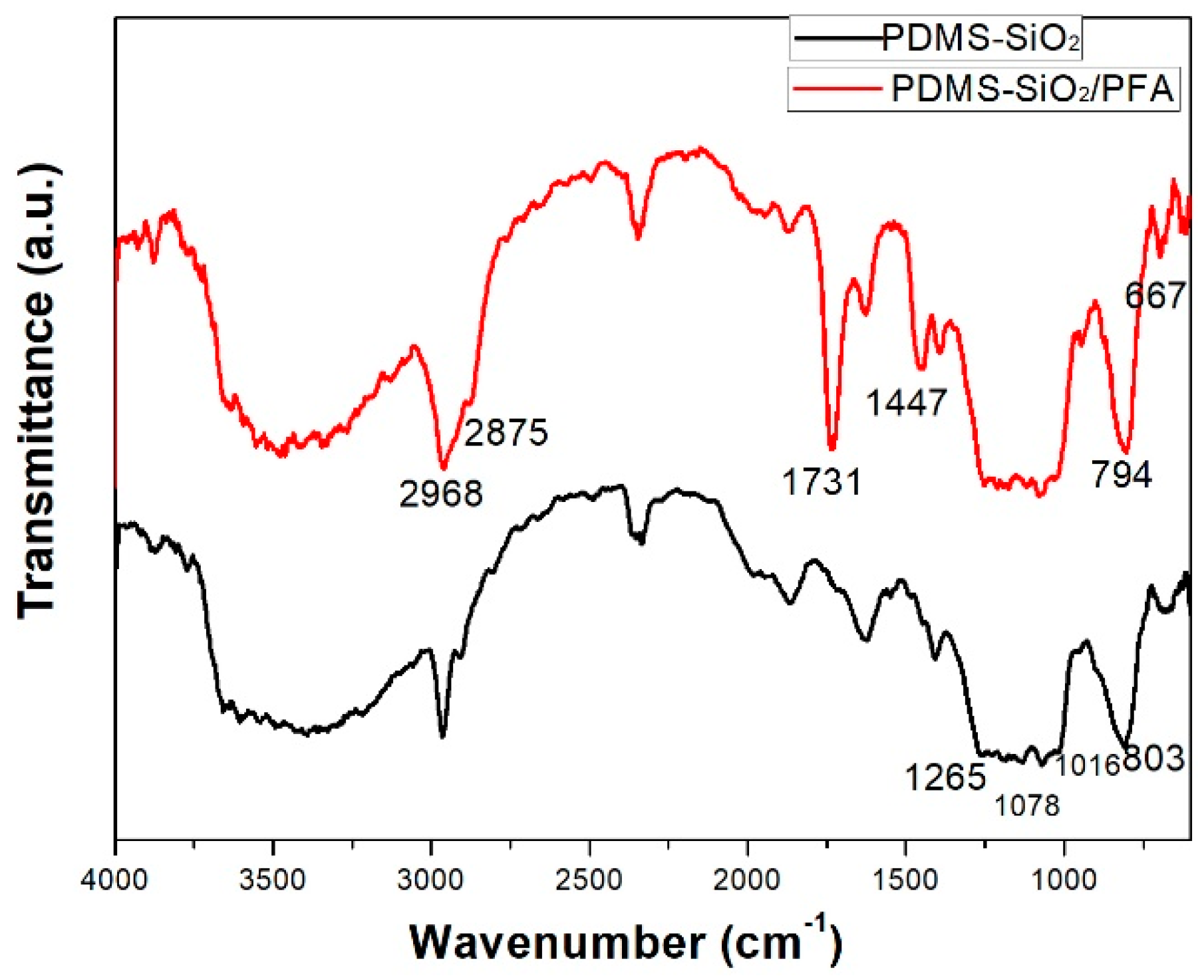
3.2. FTIR
3.3. Surface Wettability
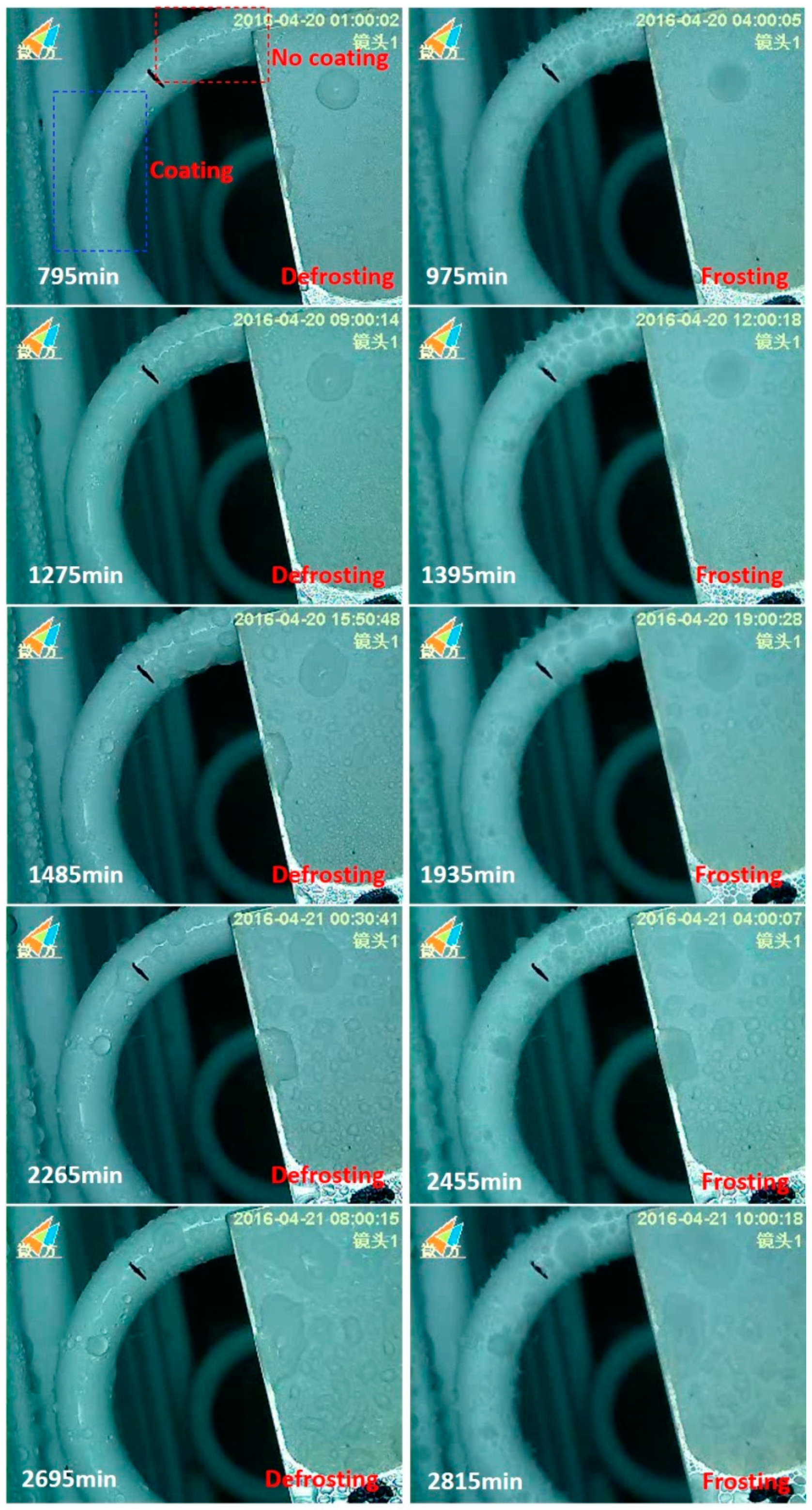
3.4. Frosting Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piening, W. The heat transfer-on tubes with free flow taking into account the formation of condensation and mature. Health Eng. 1933, 56, 493–501. [Google Scholar]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of supercooled droplet freezing on surfaces. Nat. Commun. 2012, 3, 615–623. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.; Tian, J.; Zhu, J.; Gao, X.F. Energy-effective frost-free coatings based on superhydrophobic aligned nanocones. ACS Appl. Mater. Interfaces 2014, 6, 8976–8980. [Google Scholar] [CrossRef]
- Ruan, M.; Li, W.; Wang, B.S.; Deng, B.W.; Ma, F.M.; Yu, Z.L. Preparation and anti-icing behavior of superhydrophobic surfaces on aluminum alloy substrates. Langmuir 2013, 29, 8482–8491. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Zhang, X.M.; Liu, Z.Y.; Huo, K.F.; Chu, P.K.; Zhai, J.; Jiang, L. Regulating water adhesion on superhydrophobic TiO2 nanotube arrays. Adv. Funct. Mater. 2014, 24, 6381–6388. [Google Scholar] [CrossRef]
- Wang, L.; Wen, M.X.; Zhang, M.Q.; Jiang, L.; Zheng, Y.M. Ice-phobic gummed tape with nano-cones on microspheres. J. Mater. Chem. A 2014, 2, 3312–3316. [Google Scholar] [CrossRef]
- Croutch, V.K.; Hartley, R.A. Adhesion of ice to coatings and the performance of ice release coatings. J. Coat. Technol. 1992, 64, 41–53. [Google Scholar]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.S.; Chakradhar, R.P.S.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Ling, E.J.Y.; Uong, V.; Renault-Crispo, J.S.; Kietzig, A.M.; Servio, P. Reducing ice adhesion on nonsmooth metallic surfaces: Wettability and topography effects. ACS Appl. Mater. Interfaces 2016, 8, 8789–8800. [Google Scholar] [CrossRef]
- He, Y.; Jiang, C.Y.; Cao, X.B.; Chen, J.; Tian, W.; Yuan, W.Z. Reducing ice adhesion by hierarchical micro-nano-pillars. Appl. Surf. Sci. 2014, 305, 589–595. [Google Scholar] [CrossRef]
- Meuler, A.J.; McKinley, G.H.; Cohen, R.E. Exploiting topographical texture to impart icephobicity. ACS Nano 2010, 4, 7048–7052. [Google Scholar] [CrossRef]
- Ogawa, K.; Soga, M.; Takada, Y.; Nakayama, I. Development of a transparent and ultrahydrophobic glass plate. Jpn. J. Appl. Phys. 1993, 32, 614–615. [Google Scholar] [CrossRef]
- Kietzig, A.M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Li, M.Z.; Lv, T.; Wang, Q.J.; Chen, Q.M.; Ding, J.F. Influence of different chemical modifications on the icephobic properties of superhydrophobic surfaces in a condensate environment. J. Mater. Chem. A 2015, 3, 4967–4975. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B.; Bezdomnikov, A.A.; Chulkova, E.V.; Emelyanenko, K.A. Reinforced superhydrophobic coating on silicone rubber for longstanding anti -icing performance in severe conditions. ACS Appl. Mater. Interfaces 2017, 9, 24210–24219. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zheng, Y.M.; Wen, M.X.; Song, C.; Lin, Y.C.; Jiang, L. Icephobic/anti-icing properties of micro/nanostructured surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, Q.H.; Zhan, S.H.; Jiang, L.; Zheng, Y.M. Robust anti-icing performance of a flexible superhydrophobic surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef]
- Yeong, Y.H.; Wang, C.Y.; Wynne, K.J.; Gupta, M.C. Oil-infused superhydrophobic silicone material for low ice adhesion with long-term infusion stability. ACS Appl. Mater. Interfaces 2016, 8, 32050–32059. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yao, X.; Wu, S.W.; Li, Q.Y.; Lv, J.Y.; Wang, J.J.; Jiang, L. Bioinspired solid organogel materials with a regenerable sacrificial alkane surface layer. Adv. Mater. 2017, 29, 1700865. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Q.; Zeng, X.R.; Li, H.Q.; Lai, X.J. Fabrication and characterization of stable superhydrophobic fluorinated-polyacrylate/silica hybrid coating. Appl. Surf. Sci. 2014, 298, 214–220. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.F.; Hao, L.F.; Huang, L.X. Synthesis, film morphology, and performance of cationic fluorinated polyacrylate emulsion with core-shell structure. J. Appl. Polym. Sci. 2012, 125, 2376–2383. [Google Scholar] [CrossRef]
- Wang, H.; He, G.G.; Tian, Q.Q. Effects of nano-fluorocarbon coating on icing. Appl. Surf. Sci. 2012, 258, 7219–7224. [Google Scholar] [CrossRef]
- Rico, V.; Mora, J.; García, P.; Agüero, A.; Borrás, A.; González-Elipe, A.R.; López-Santos, C. Robust anti-icing superhydrophobic aluminum alloy surfaces by grafting fluorocarbon molecular chains. Appl. Mater. Today 2020, 21, 100815. [Google Scholar] [CrossRef]
- Jia, L.; Sun, J.; Li, X.X.; Zhang, X.; Chen, L.; Tian, X.Y. Preparation and anti-frost performance of PDMS-SiO2/SS superhydrophobic coating. Coatings 2020, 10, 1051. [Google Scholar] [CrossRef]
- Bai, C.Y.; Zhang, X.Y.; Dai, J.B.; Wang, J.H. Synthesis of UV crosslinkable waterborne siloxane-polyurethane dispersion PDMS-PEDA-PU and the properties of the films. J. Coat. Technol. Res. 2008, 5, 251–257. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, J.F.; Zhou, X.P.; Lu, C.; Tong, X.T.; He, G.X.; Wang, X.Q. Synthesis of core-shell fluoroacrylate copolymer latex via emulsion polymerization and its application in ink-jet ink. J. Appl. Polym. Sci. 2012, 126, 110–115. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.R.; Deng, M.; Yuan, D.X.; Ni, H.G.; Zhang, W.; Wang, X.P. Surface properties and chain structure of fluorinated acrylate copolymers prepared by emulsion polymerization. Polym. Bull. 2010, 64, 81–97. [Google Scholar] [CrossRef]
- Wu, Z.F.; Wang, H.; Tian, X.Y.; Xue, M.; Ding, X.; Ye, X.Z.; Cui, Z.Y. Surface and mechanical properties of hydrophobic silica contained hybrid films of waterborne polyurethane and fluorinated polymethacrylate. Polymer 2014, 55, 187–194. [Google Scholar] [CrossRef]
- Zhang, P.; Lv, F.Y. A review of the recent advances in superhydrophobic surfaces and the emerging energy-related applications. Energy 2015, 82, 1068–1087. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.M.; Cui, D.P.; Zhang, Q.L.; Zhang, Y.F.; Xu, F.J.; Zhou, X.; Wang, J.J.; Song, Y.L.; Jiang, L. Robust prototypical anti-icing coatings with a self-lubricating liquid water layer between ice and substrate. ACS Appl. Mater. Interfaces 2013, 5, 4026–4030. [Google Scholar] [CrossRef]
- Na, B.; Webb, R.L. A fundamental understanding of factors affecting frost nucleation. Int. J. Heat Mass Transfer. 2003, 46, 3797–3808. [Google Scholar] [CrossRef]
- Miljkovic, N.; Wang, E.N. Condensation heat transfer on superhydrophobic surfaces. MRS Bull. 2013, 38, 397–406. [Google Scholar] [CrossRef]
- Murphy, D.M.; Koop, T. Review of the vapour pressures of ice and supercooled water for atmospheric applications. Q. J. R. Meteorol. Soc. 2005, 131, 1539–1565. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus operandi of protective and anti-icing mechanisms underlying the design of longstanding outdoor icephobic coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Sun, J.; Li, X.; Zhang, X.; Chen, L.; Tian, X. Synthesis and Frost Suppression Performance of PDMS-SiO2/PFA Hybrid Coating. Coatings 2021, 11, 256. https://doi.org/10.3390/coatings11020256
Jia L, Sun J, Li X, Zhang X, Chen L, Tian X. Synthesis and Frost Suppression Performance of PDMS-SiO2/PFA Hybrid Coating. Coatings. 2021; 11(2):256. https://doi.org/10.3390/coatings11020256
Chicago/Turabian StyleJia, Li, Jun Sun, Xiaoxiao Li, Xian Zhang, Lin Chen, and Xinyou Tian. 2021. "Synthesis and Frost Suppression Performance of PDMS-SiO2/PFA Hybrid Coating" Coatings 11, no. 2: 256. https://doi.org/10.3390/coatings11020256
APA StyleJia, L., Sun, J., Li, X., Zhang, X., Chen, L., & Tian, X. (2021). Synthesis and Frost Suppression Performance of PDMS-SiO2/PFA Hybrid Coating. Coatings, 11(2), 256. https://doi.org/10.3390/coatings11020256




