Self-Healing Properties of Cerium-Modified Molybdate Conversion Coating on Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electrochemical Measurements
2.3. Morphology and Composition
3. Results and Discussion
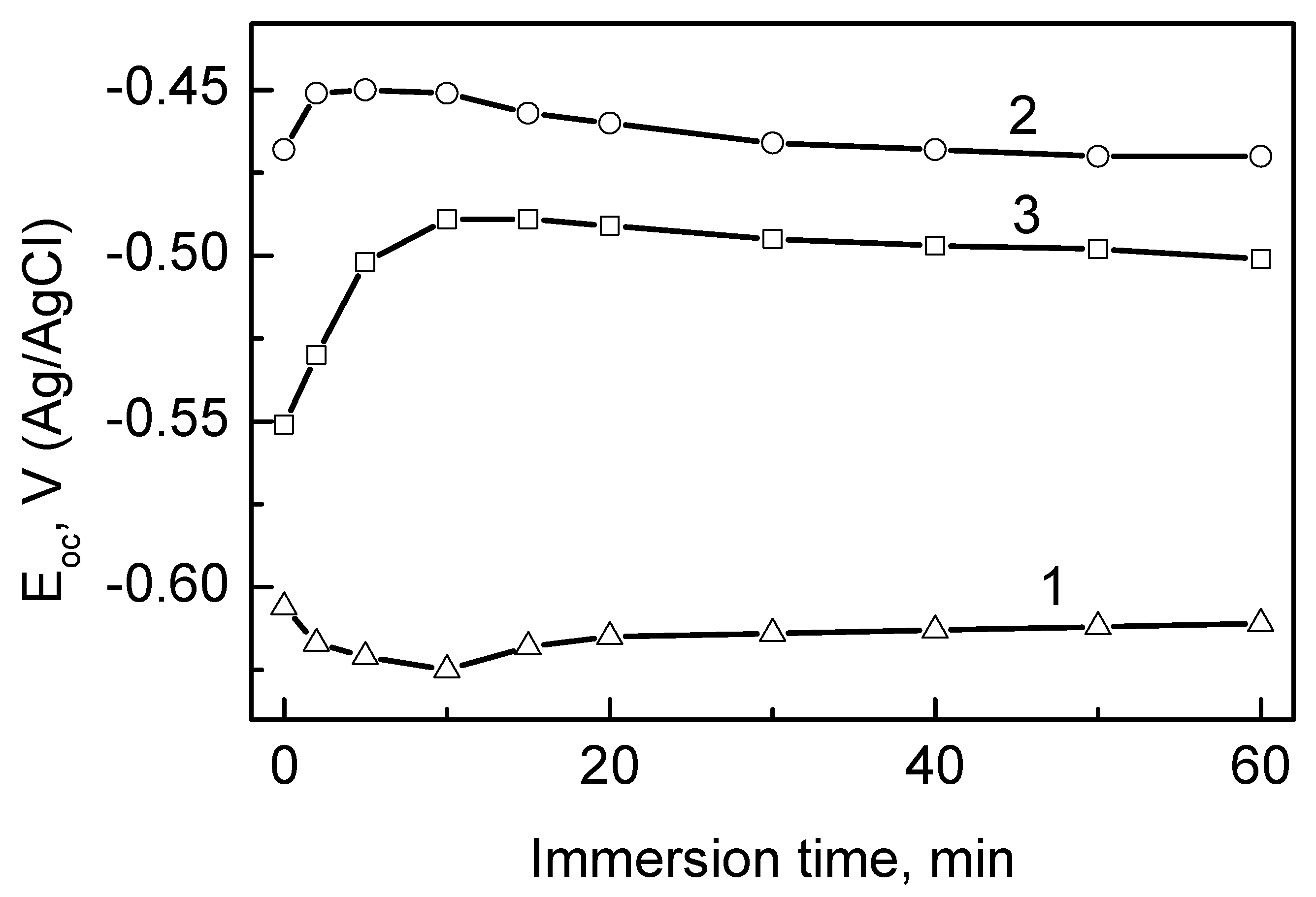
3.1. Formation of Conversion Coatings
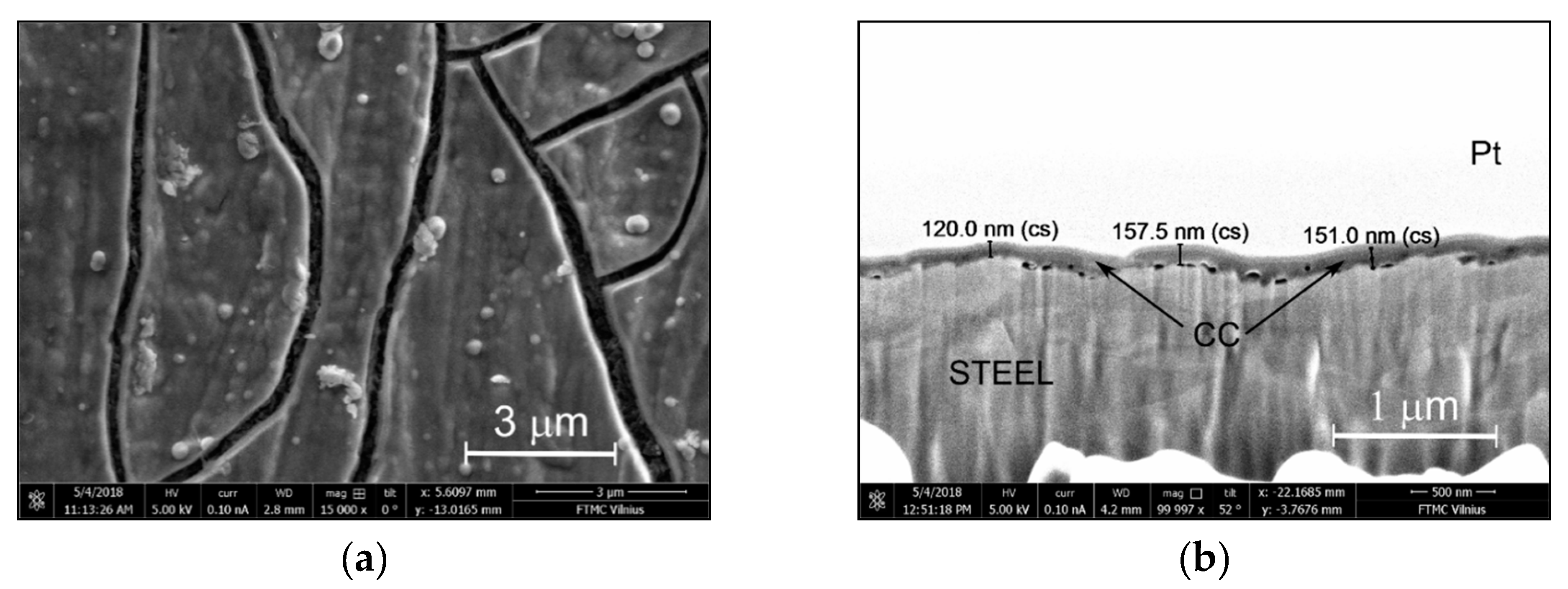
3.2. Surface Morphology and Inner Structure
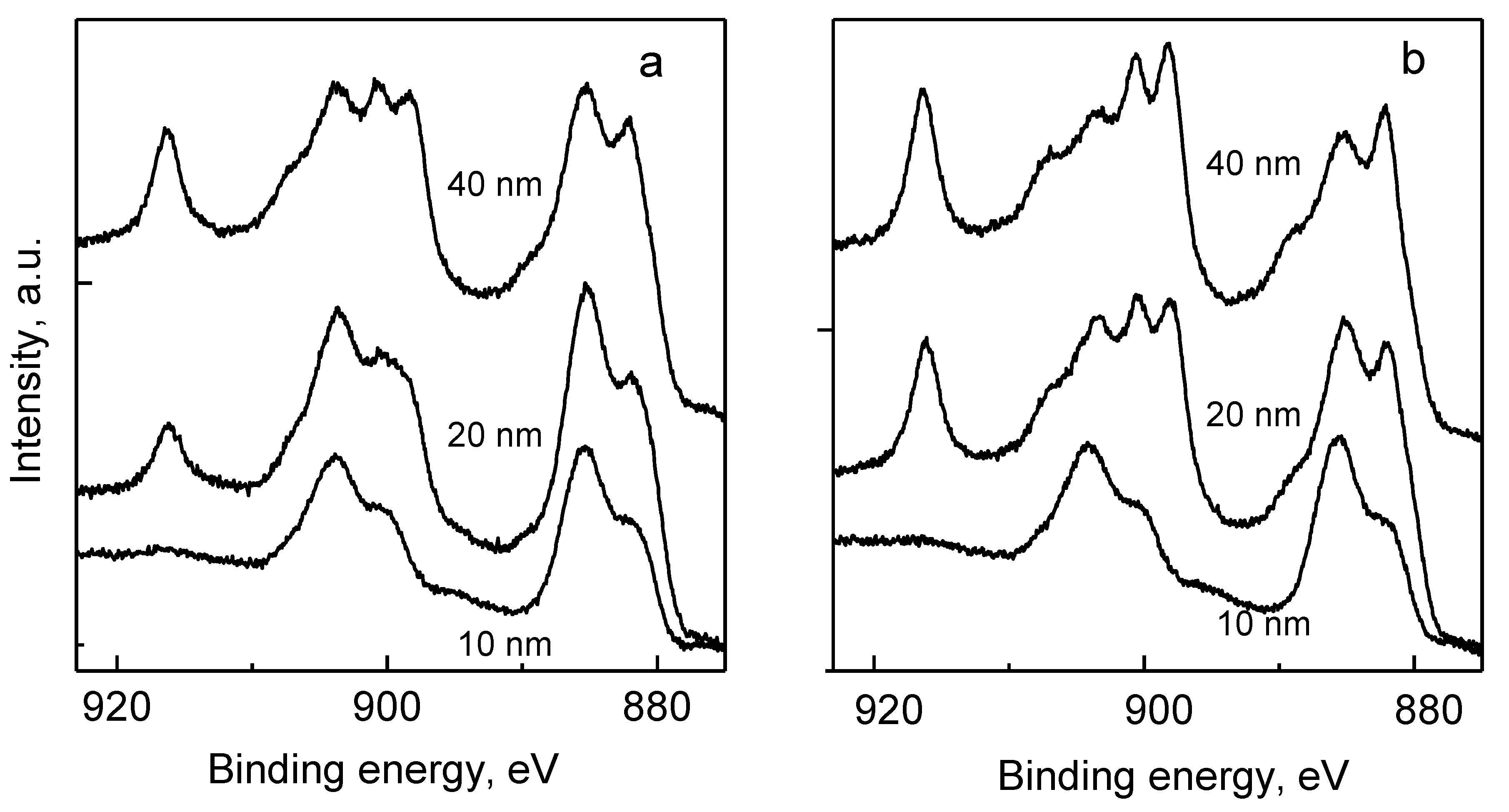
3.3. Chemical Composition
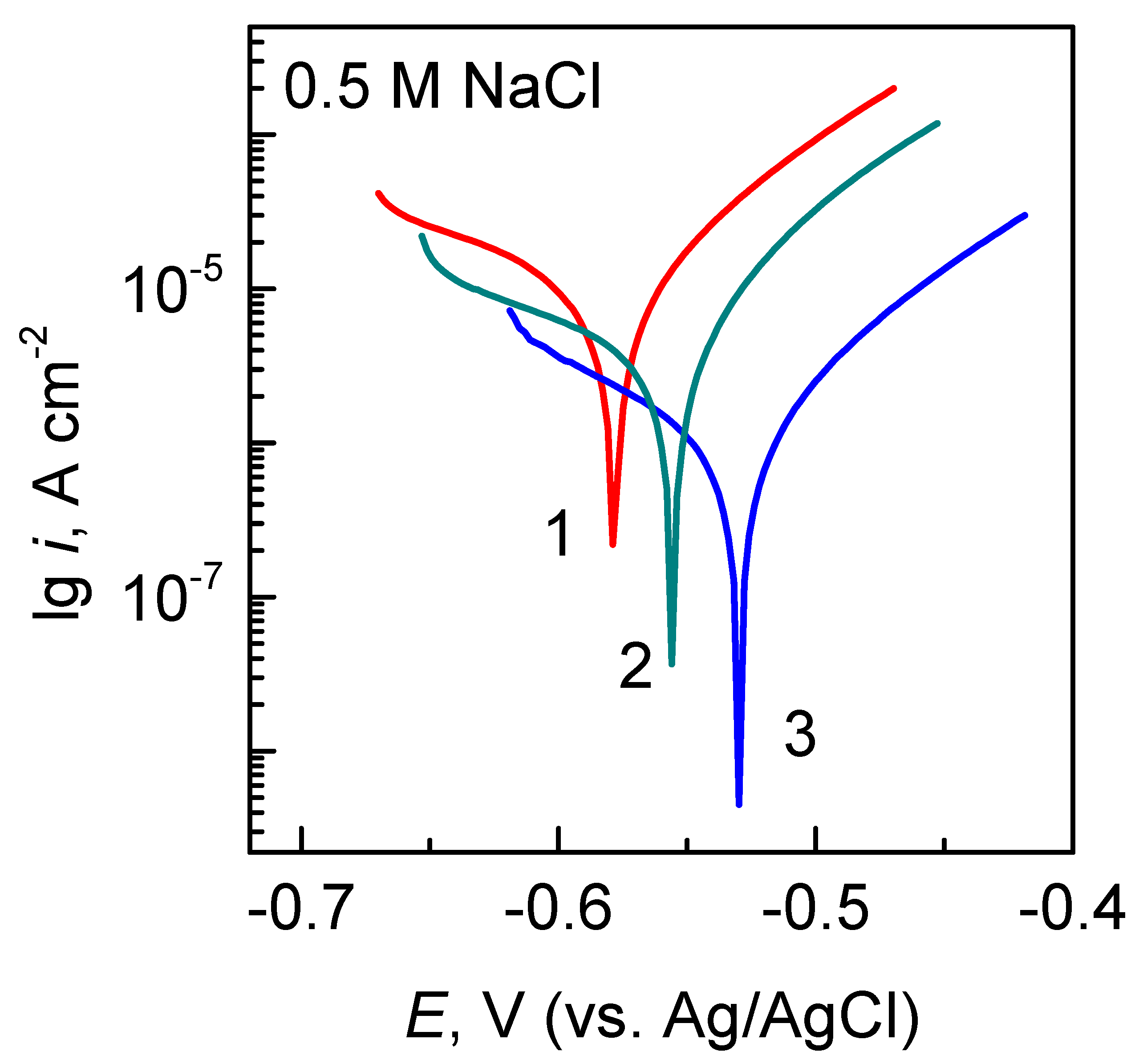
3.4. Protective Ability
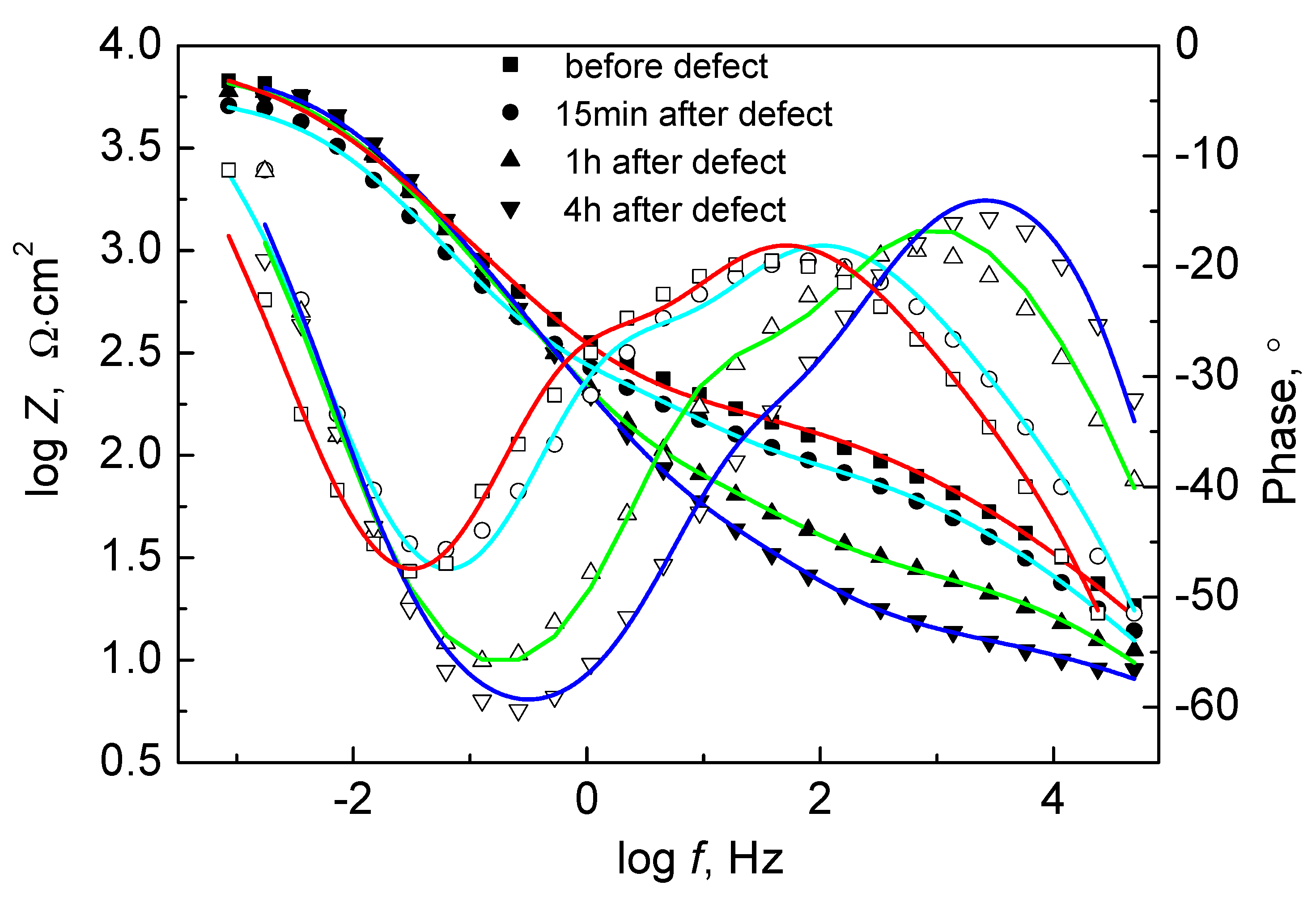
3.5. Self-Healing Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weng, D.; Jokiel, P.; Uebleis, A.; Boehni, H. Corrosion and protection characteristics of zinc and manganese phosphate coatings. Surf. Coat. Technol. 1997, 88, 147–156. [Google Scholar] [CrossRef]
- Ogle, K.; Tomandl, A.; Meddahi, N.; Wolpers, M. The alkaline stability of phosphate coatings I: ICP atomic emission spectroelectrochemistry. Corros. Sci. 2004, 46, 979–995. [Google Scholar] [CrossRef]
- Tamilselvi, M.; Kamaraj, P.; Arthanareeswari, M.; Devikala, S. Nano zinc phosphate coatings for enhanced corrosion resistance of mild steel. Appl. Surf. Sci. 2015, 327, 218–225. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Wu, Z.; Ji, S.; Wu, H.; Chen, P.; Ma, Z.; Wu, Z.; Fu, R.K.Y.; Lin, H.; et al. Corrosion-resistant plasma electrolytic oxidation coating modified by Zinc phosphate and self-healing mechanism in the salt-spray environment. Surf. Coat. Technol. 2020, 384, 125321. [Google Scholar] [CrossRef]
- Kurosawa, K.; Fukushima, T. Effects of pH of an Na2MoO4-H3PO4 type aqueous solution on the formation of chemical conversion coatings on steels. Corros. Sci. 1989, 29, 1103–1114. [Google Scholar] [CrossRef]
- Konno, H.; Narumi, K.; Habazaki, H. Molybdate/Al(III) composite films on steel and zinc-plated steel by chemical conversion. Corros. Sci. 2002, 44, 1889–1900. [Google Scholar] [CrossRef]
- Song, Y.K.; Mansfeld, F. Development of a Molybdate–Phosphate–Silane–Silicate (MPSS) coating process for electrogalvanized steel. Corros. Sci. 2006, 48, 154–164. [Google Scholar] [CrossRef]
- Walker, D.E.; Wilcox, G.D. Molybdate based conversion coatings for zinc and zinc alloy surfaces: A review. Trans. IMF 2008, 86, 251–259. [Google Scholar] [CrossRef]
- da Silva, C.G.; Margarit-Mattos, I.C.P.; Mattos, O.R.; Perrot, H.; Tribollet, B.; Vivier, V. The molybdate–zinc conversion process. Corros. Sci. 2009, 51, 151–158. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.M.; Carmezim, M.J. Characterization of rare-earth conversion films formed on the AZ31 magnesium alloy and its relation with corrosion protection. Appl. Surf. Sci. 2007, 253, 6922–6931. [Google Scholar] [CrossRef]
- Arenas, M.A.; de Damborenea, J.J. Surface characterisation of cerium layers on galvanised steel. Surf. Coat. Technol. 2004, 187, 320–325. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fujiwara, Y. Effect of SO42− on the corrosion behavior of cerium-based conversion coatings on galvanized steel. Electrochim. Acta 2006, 51, 4236–4242. [Google Scholar] [CrossRef]
- Fedel, M.; Ahniyaz, A.; Ecco, L.G.; Deflorian, F. Electrochemical investigation of the inhibition effect of CeO2 nanoparticles on the corrosion of mild steel. Electrochim. Acta 2014, 131, 71–78. [Google Scholar] [CrossRef]
- Mahidashti, Z.; Shahrabi, T.; Ramezanzadeh, B. The role of post-treatment of an ecofriendly cerium nanostructure conversion coating by green corrosion inhibitor on the adhesion and corrosion protection properties of the epoxy coating. Prog. Org. Coat. 2018, 114, 19–32. [Google Scholar] [CrossRef]
- Lin, B.-L.; Lu, J.-T.; Kong, G. Effect of molybdate post-sealing on the corrosion resistance of zinc phosphate coatings on hot-dip galvanized steel. Corros. Sci. 2008, 50, 962–967. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, F.; Wang, F. The characterization and corrosion resistance of cerium chemical conversion coatings for 304 stainless steel. Corros. Sci. 2004, 46, 75–89. [Google Scholar] [CrossRef]
- Heller, D.K.; Fahrenholtz, W.G.; O’Keefe, M.J. The effect of post-treatment time and temperature on cerium-based conversion coatings on Al 2024-T3. Corros. Sci. 2010, 52, 360–368. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.M.; Ferreira, M.G.S.; Carmezim, M.J. Composition and corrosion resistance of cerium conversion films on the AZ31 magnesium alloy and its relation to the salt anion. Appl. Surf. Sci. 2008, 254, 1806–1814. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Vakili, H.; Amini, R. Improved performance of cerium conversion coatings on steel with zinc phosphate post-treatment. J. Ind. Eng. Chem. 2015, 30, 225–233. [Google Scholar] [CrossRef]
- Chambers, B.D.; Taylor, S.R. The high throughput assessment of aluminium alloy corrosion using fluorometric methods. Part II—A combinatorial study of corrosion inhibitors and synergistic combinations. Corros. Sci. 2007, 49, 1597–1609. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S. Active corrosion protection of AA2024 by sol–gel coatings with cerium molybdate nanowires. Electrochim. Acta 2013, 112, 236–246. [Google Scholar] [CrossRef]
- Mu, S.; Du, J.; Jiang, H.; Li, W. Composition analysis and corrosion performance of a Mo–Ce conversion coating on AZ91 magnesium alloy. Surf. Coat. Technol. 2014, 254, 364–370. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Tedim, J.; Zheludkevich, M.L.; Drumm, R.; Shem, M.; Wittmar, M.; Veith, M.; Ferreira, M.G.S. Cerium molybdate nanowires for active corrosion protection of aluminium alloys. Corros. Sci. 2012, 58, 41–51. [Google Scholar] [CrossRef]
- Fachikov, L.; Ivanova, D. Surface treatment of zinc coatings by molybdate solutions. Appl. Surf. Sci. 2012, 258, 10160–10167. [Google Scholar] [CrossRef]
- Girčienė, O.; Gudavičiute, L.; Selskis, A.; Jasulaitiene, V.; Sakirzanovas, S.; Ramanauskas, R. The self-healing ability of cerium oxide films on carbon steel. Chemija 2015, 26, 175–183. [Google Scholar]
- Guergova, D.; Stoyanova, E.; Stoychev, D.; Avramova, I.; Stefanov, P. Self-healing effect of ceria electrodeposited thin films on stainless steel in aggressive 0.5 mol/L NaCl aqueous solution. J. Rare Earths 2015, 33, 1212–1227. [Google Scholar] [CrossRef]
- Liu, D.-L.; Yang, Z.-G.; Wang, Z.-Q.; Zhang, C. Synthesis and evaluation of corrosion resistance of molybdate-based conversion coatings on electroplated zinc. Surf. Coat. Technol. 2010, 205, 2328–2334. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Arrabal, R.; Viejo, F.; Muñoz, J.A. Ce conversion and electrolysis surface treatments applied to A3xx.x alloys and A3xx.x/SiCp composites. Appl. Surf. Sci. 2007, 253, 3334–3344. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Mamidipally, S.B.; Schmutz, P.; Guan, H. Active corrosion protection in Ce-modified hydrotalcite conversion coatings. Corrosion 2002, 58, 3–14. [Google Scholar] [CrossRef]
- Aramaki, K. Self-healing mechanism of a protective film prepared on a Ce(NO3)3-pretreated zinc electrode by modification with Zn(NO3)2 and Na3PO4. Corros. Sci. 2003, 45, 1085–1101. [Google Scholar] [CrossRef]



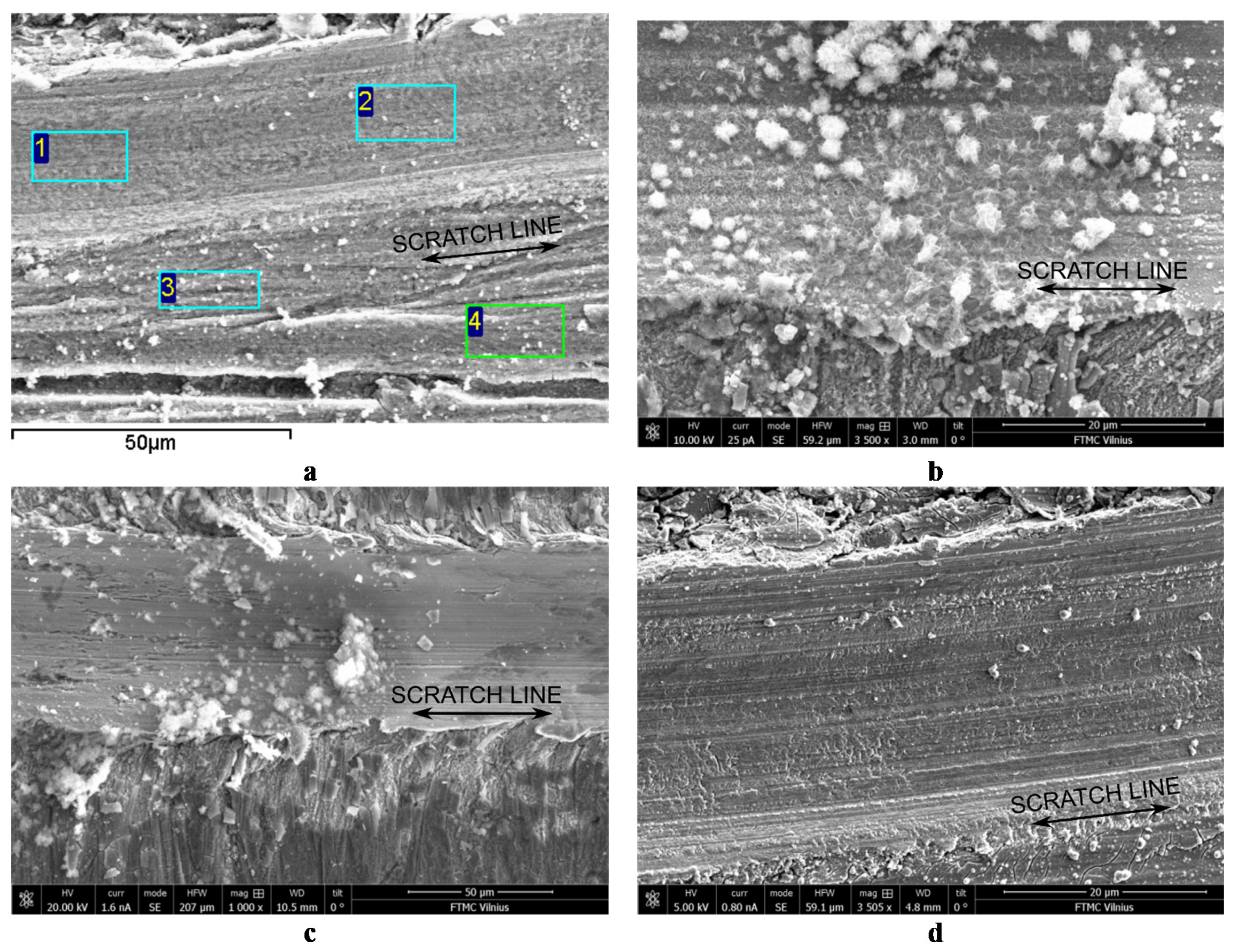

| Sample | Element Concentration (at.%) | |||||
|---|---|---|---|---|---|---|
| Fe | O | P | Mo | Ce | S | |
| Fe/P-Mo | 67.39 | 29.48 | 1.34 | 1.79 | - | - |
| Fe/P-Mo-Ce1 | 50.17 | 44.99 | 1.45 | 1.39 | 1.75 | - |
| Fe/P-Mo-Ce2 | 57.67 | 37.74 | 1.58 | 1.22 | 2.24 | 0.22 |
| Sample | Electrochemical Parameters | ||
|---|---|---|---|
| Ecorr, V (vs. Ag/AgCl) | icorr, A·cm−2 | P% by Equation (1) | |
| Fe/P-Mo | −0.569 | 2.2 × 10−6 | 59 |
| Fe/P-Mo-Ce1 | −0.515 | 1.7 × 10−6 | 68 |
| Fe/P-Mo-Ce2 | −0.490 | 2.7 ×1 0−7 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirdeikiene, A.; Girčiene, O.; Gudavičiūte, L.; Jasulaitiene, V.; Selskis, A.; Tutliene, S.; Skruodiene, M.; Pilipavičius, J.; Juodkazyte, J.; Ramanauskas, R. Self-Healing Properties of Cerium-Modified Molybdate Conversion Coating on Steel. Coatings 2021, 11, 194. https://doi.org/10.3390/coatings11020194
Kirdeikiene A, Girčiene O, Gudavičiūte L, Jasulaitiene V, Selskis A, Tutliene S, Skruodiene M, Pilipavičius J, Juodkazyte J, Ramanauskas R. Self-Healing Properties of Cerium-Modified Molybdate Conversion Coating on Steel. Coatings. 2021; 11(2):194. https://doi.org/10.3390/coatings11020194
Chicago/Turabian StyleKirdeikiene, Aliona, Olga Girčiene, Laima Gudavičiūte, Vitalija Jasulaitiene, Algirdas Selskis, Skirmante Tutliene, Monika Skruodiene, Jurgis Pilipavičius, Jurga Juodkazyte, and Rimantas Ramanauskas. 2021. "Self-Healing Properties of Cerium-Modified Molybdate Conversion Coating on Steel" Coatings 11, no. 2: 194. https://doi.org/10.3390/coatings11020194
APA StyleKirdeikiene, A., Girčiene, O., Gudavičiūte, L., Jasulaitiene, V., Selskis, A., Tutliene, S., Skruodiene, M., Pilipavičius, J., Juodkazyte, J., & Ramanauskas, R. (2021). Self-Healing Properties of Cerium-Modified Molybdate Conversion Coating on Steel. Coatings, 11(2), 194. https://doi.org/10.3390/coatings11020194







