Fabrication of a High Water Flux Conductive MWCNTs/PVC Composite Membrane with Effective Electrically Enhanced Antifouling Behavior
Abstract
:1. Introduction
2. Experimental
2.1. Materials
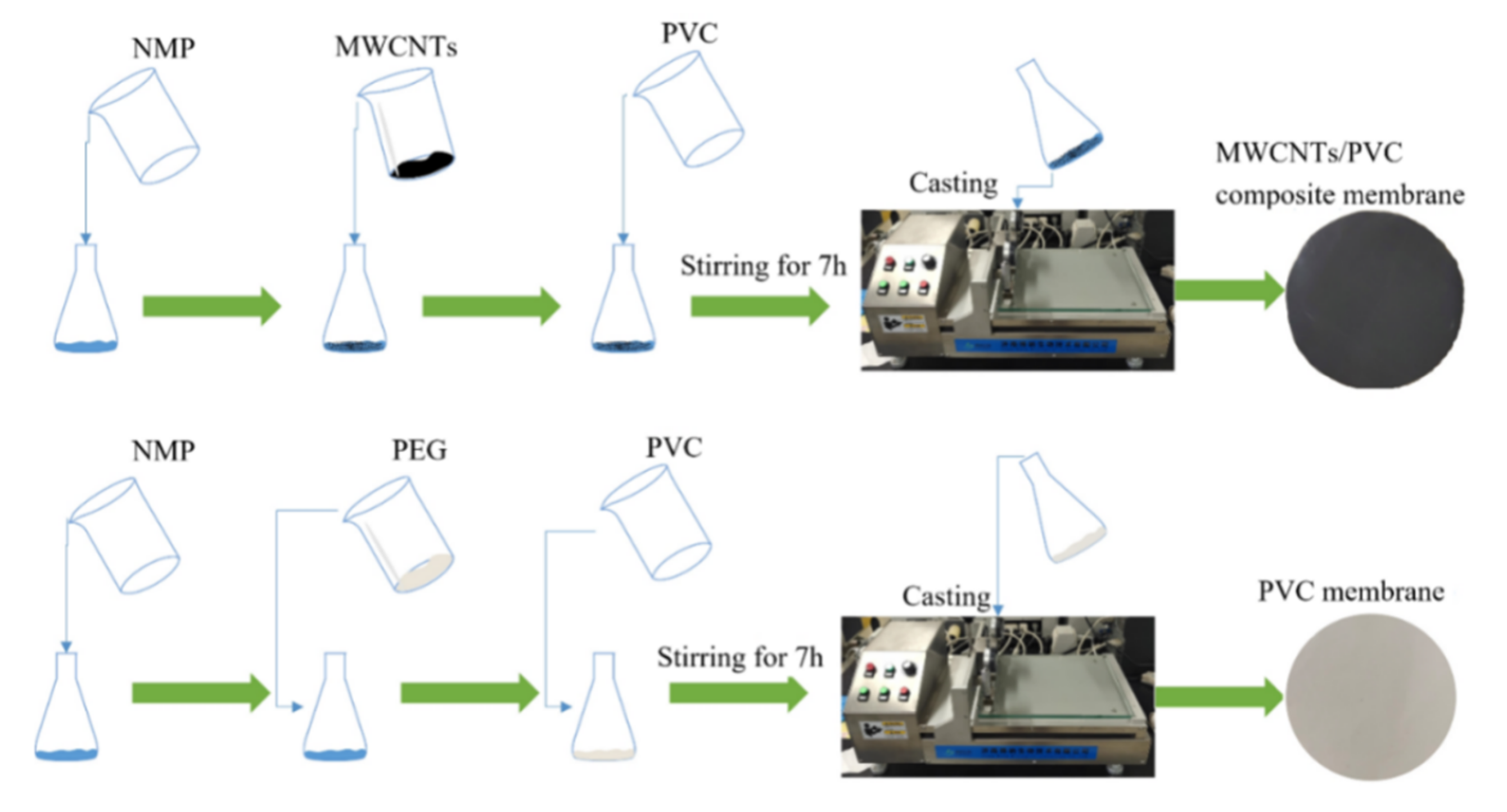
2.2. Fabrication of MWCNTs-PVC Composite Conductive Membranes
2.3. Water Flux, Rejection, and Antifouling Performance
2.4. Membrane Characterizations
3. Results and Discussion
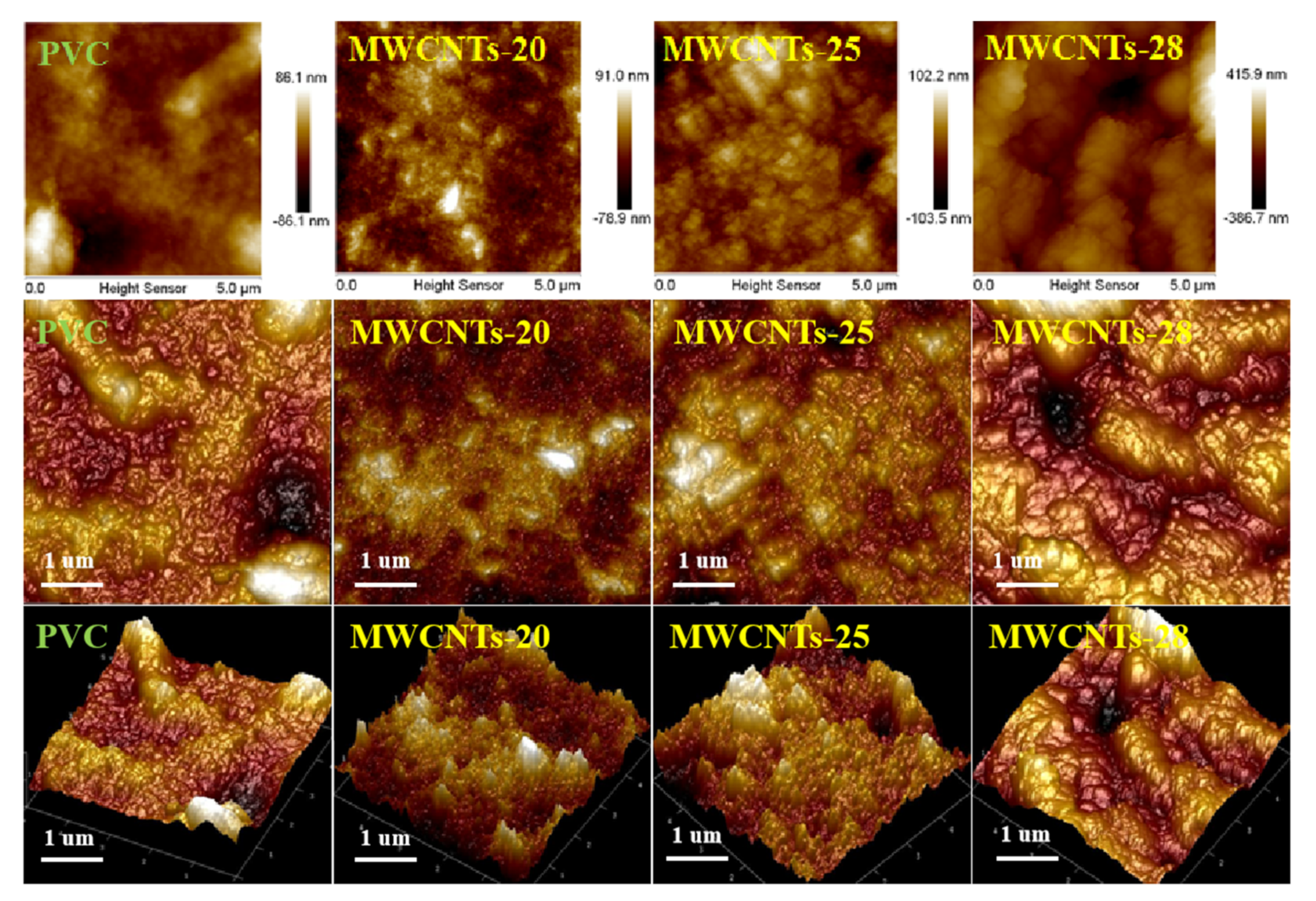
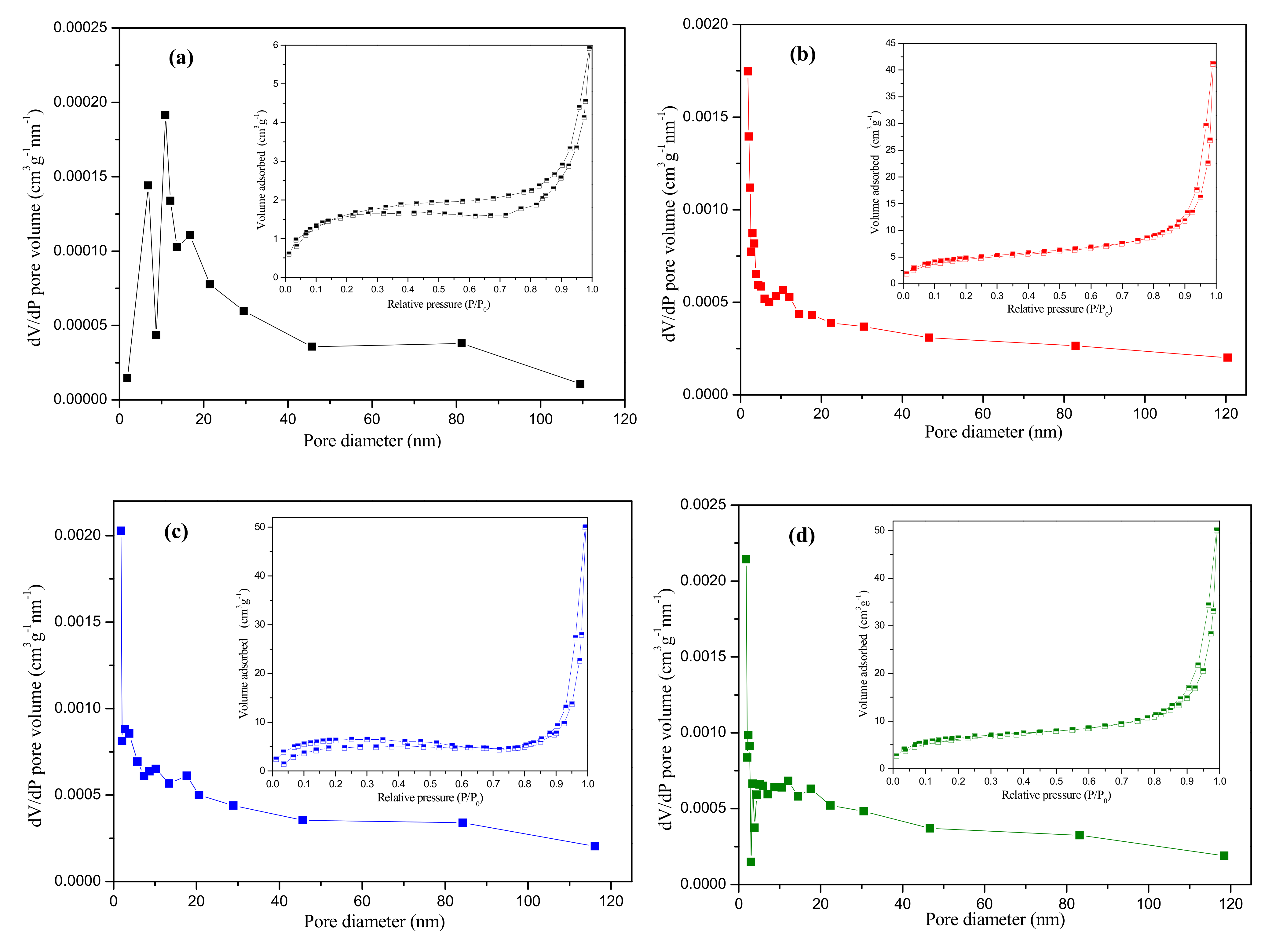
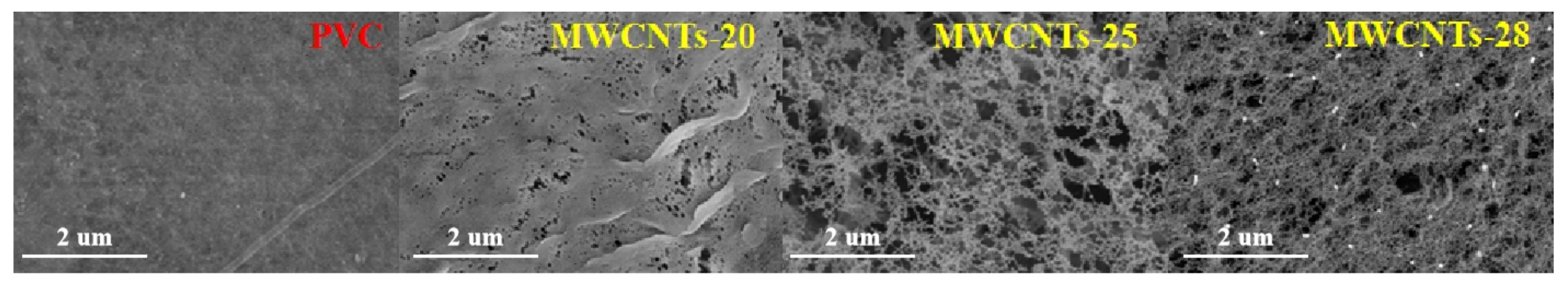
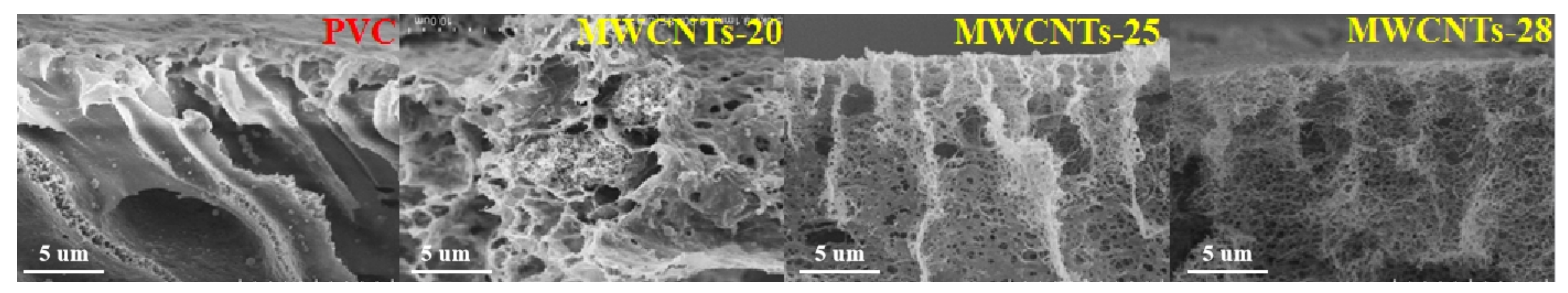
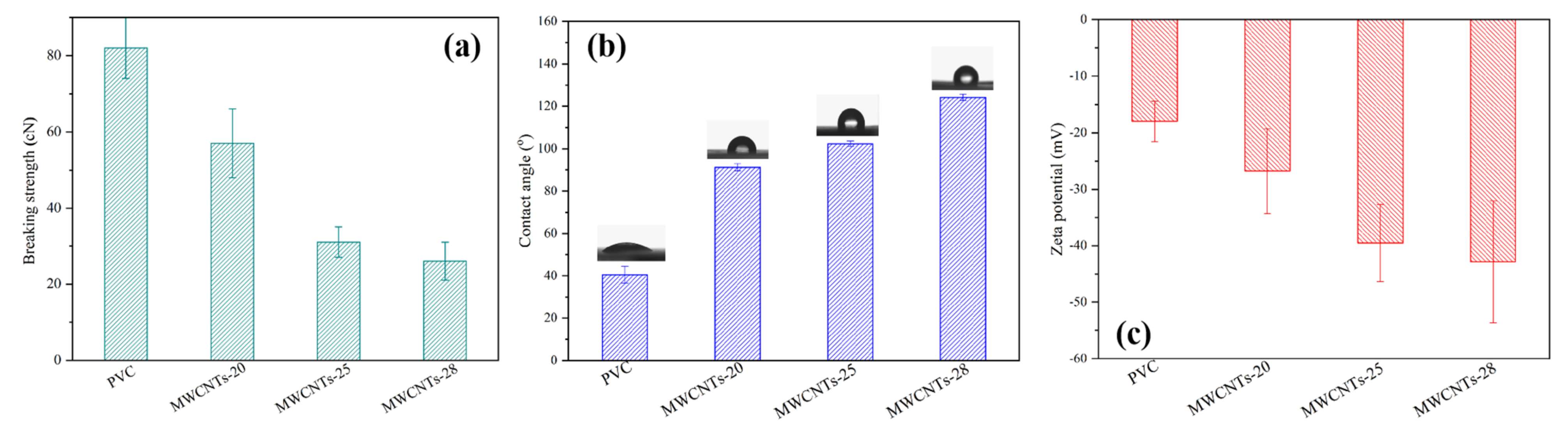
3.1. Membrane Characterization
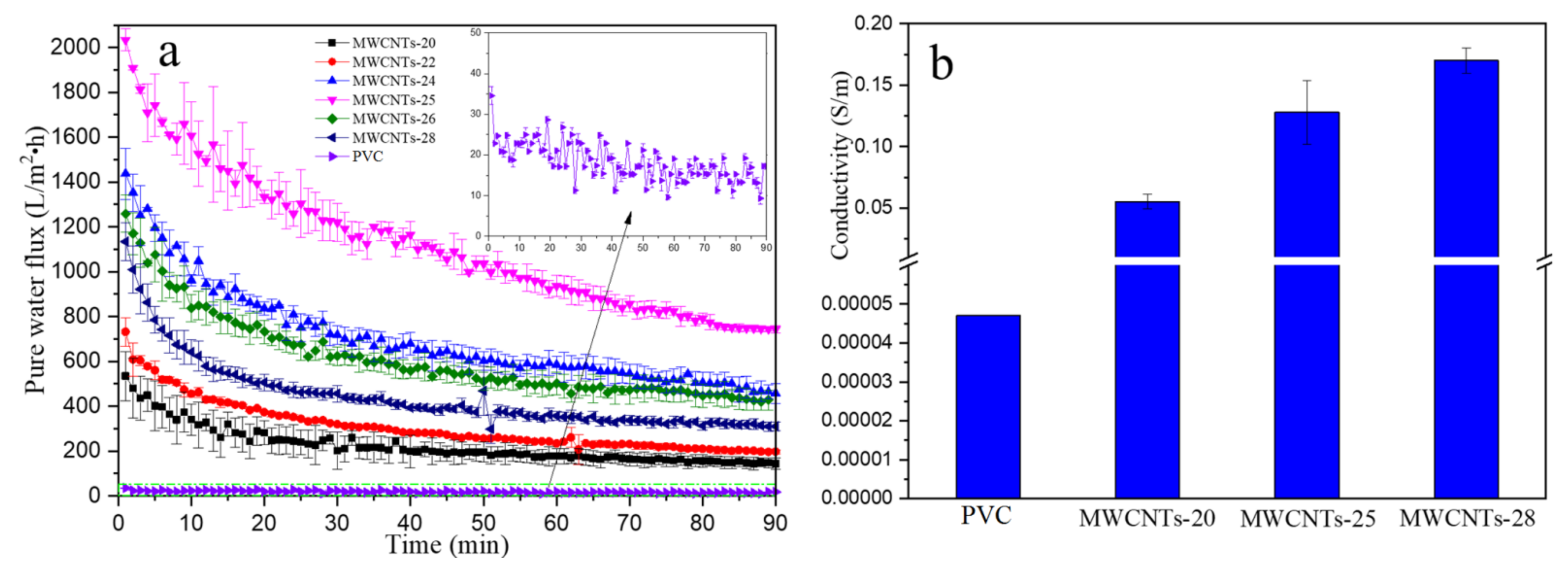
3.2. Effect of MWCNTs Proportion on the Pure Water Flux and Electrical Conductivity of the Composite Membrane
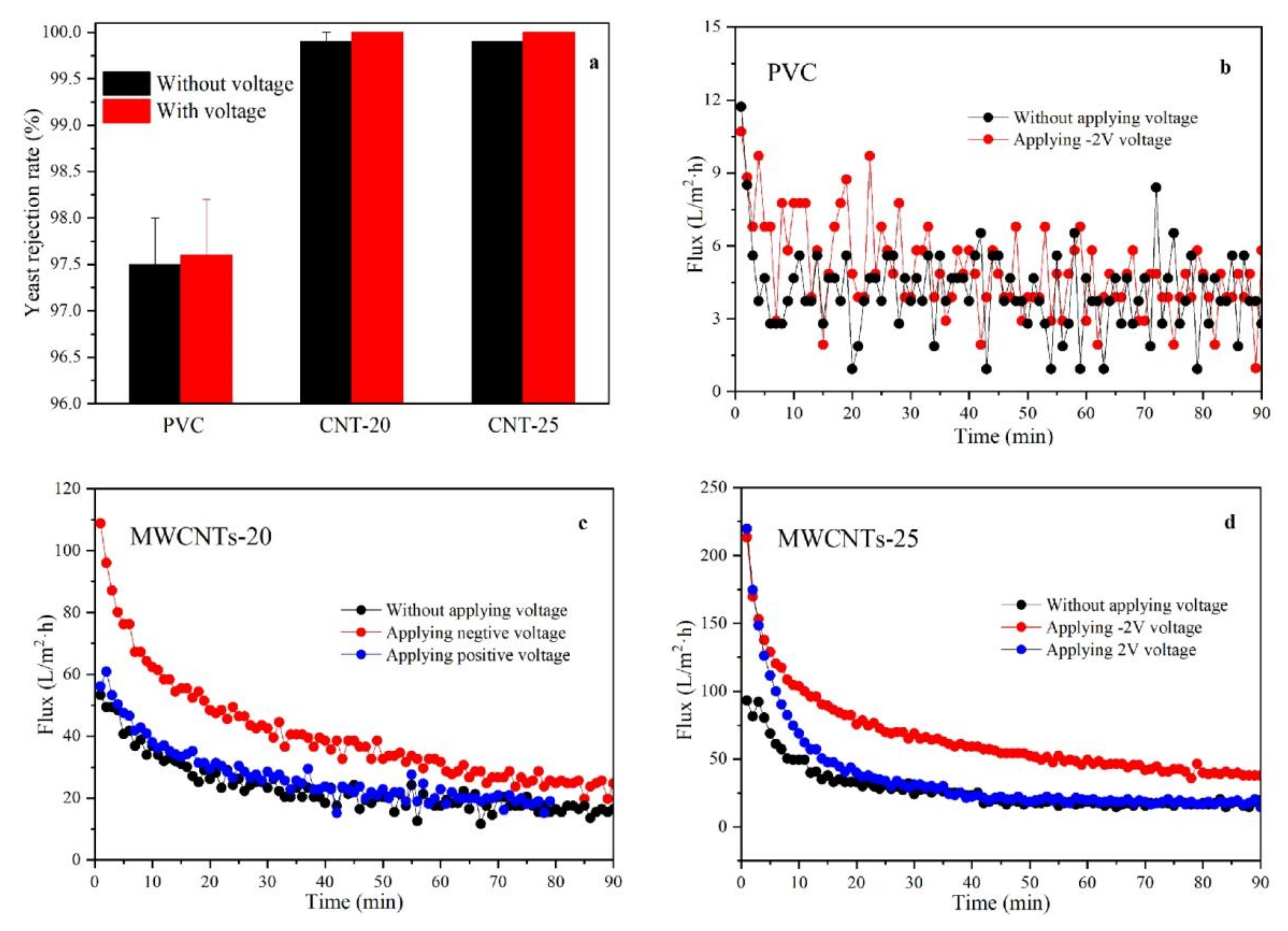
3.3. Rejection and Electrically Enhanced Antifouling Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Hilal, N.; Leo, C.P. A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 2015, 363, 2–18. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Li, M.; Yu, K.; Hu, J.; Zhang, X.; Wang, L. Sulfonated multiwall carbon nanotubes assisted thin-film nanocomposite membrane with enhanced filtrate flux and anti-fouling property. J. Membr. Sci. 2017, 524, 344–353. [Google Scholar] [CrossRef]
- Ma, J.; Guo, X.; Ying, Y.; Liu, D.; Zhong, C. Composite ultrafiltration membrane tailored by MOF@ GO with highly improved water purification performance. Chem. Eng. J. 2017, 313, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.-L.; Han, Z.-S.; Li, G.-B. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Katsoufidou, K.; Yiantsios, S.; Karabelas, A. A study of ultrafiltration membrane fouling by humic acids and flux recovery by backwashing: Experiments and modeling. J. Membr. Sci. 2005, 266, 40–50. [Google Scholar] [CrossRef]
- Asatekin, A.; Kang, S.; Elimelech, M.; Mayes, A.M. Anti-fouling ultrafiltration membranes containing polyacrylonitrile-graft-poly (ethylene oxide) comb copolymer additives. J. Membr. Sci. 2007, 298, 136–146. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Bo, G.; Yang, F.; Crittenden, J.; Chen, Y. Conductive and hydrophilic polypyrrole modified membrane cathodes and fouling reduction in MBR. J. Membr. Sci. 2013, 429, 252–258. [Google Scholar] [CrossRef]
- Liu, J.; Tian, C.; Xiong, J.; Wang, L. Polypyrrole blending modification for PVDF conductive membrane preparing and fouling mitigation. J. Colloid Interface Sci. 2017, 494, 124–129. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, H.; Liu, Y.; Quan, X.; Yu, H.; Chen, S. Enhanced Permeability, Selectivity, and Antifouling Ability of CNTs/Al2O3 Membrane under Electrochemical Assistance. Environ. Sci. Technol. 2015, 49, 2293–2300. [Google Scholar] [CrossRef]
- Wang, K.; Xu, L.; Li, K.; Liu, L.; Zhang, Y.; Wang, J. Development of polyaniline conductive membrane for electrically enhanced membrane fouling mitigation. J. Membr. Sci. 2019, 570, 371–379. [Google Scholar] [CrossRef]
- Tiwari, P.; Janas, D.; Chandra, R. Self-standing MoS2/CNT and MnO2/CNT one dimensional core shell heterostructures for asymmetric supercapacitor application. Carbon 2021, 177, 291–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Li, Y.; Chen, X.; Yao, Y.; Ren, S.; Li, M.; Wu, Y.; An, C. Electrically conductive inorganic membranes: A review on principles, characteristics and applications. Chem. Eng. J. 2022, 427, 131987. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Gao, B.; Yang, F. Cathode membrane fouling reduction and sludge property in membrane bioreactor integrating electrocoagulation and electrostatic repulsion. Sep. Purif. Technol. 2012, 100, 44–50. [Google Scholar] [CrossRef]
- Li, N.; Liu, L.; Yang, F. Highly conductive graphene/PANi-phytic acid modified cathodic filter membrane and its antifouling property in EMBR in neutral conditions. Desalination 2014, 338, 10–16. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Gao, B.; Yang, F.; Chellam, S. Fouling reductions in a membrane bioreactor using an intermittent electric field and cathodic membrane modified by vapor phase polymerized pyrrole. J. Membr. Sci. 2012, 394, 202–208. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Zhao, C.; Liu, J.; Yang, F. Hydraulic power and electric field combined antifouling effect of a novel conductive poly (aminoanthraquinone)/reduced graphene oxide nanohybrid blended PVDF ultrafiltration membrane. J. Mater. Chem. A 2015, 3, 20277–20287. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; Walker, S.; Jassby, D. Polyaniline-coated carbon nanotube ultrafiltration membranes: Enhanced anodic stability for in situ cleaning and electro-oxidation processes. ACS Appl. Mater. Interface 2016, 8, 22574–22584. [Google Scholar] [CrossRef]
- de Lannoy, C.; Jassby, D.; Davis, D.; Wiesner, M. A highly electrically conductive polymer–multiwalled carbon nanotube nanocomposite membrane. J. Membr. Sci. 2012, 415, 718–724. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; de Leon, J.V.; Dudchenko, A.; Yao, S.; Corbala-Delgado, J.; Yan, A.; Matsumoto, M.; Jassby, D. Treating anaerobic sequencing batch reactor effluent with electrically conducting ultrafiltration and nanofiltration membranes for fouling control. J. Membr. Sci. 2016, 504, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Vecitis, C.D. Conductive CNT-PVDF membrane for capacitive organic fouling reduction. J. Membr. Sci. 2014, 459, 143–156. [Google Scholar] [CrossRef]
- Ho, K.; Teow, Y.; Mohammad, A.W.; Ang, W.; Lee, P. Development of graphene oxide (GO)/multi-walled carbon nanotubes (MWCNTs) nanocomposite conductive membranes for electrically enhanced fouling mitigation. J. Membr. Sci. 2018, 552, 189–201. [Google Scholar] [CrossRef]
- Yuan, X.-S.; Guo, Z.-Y.; Geng, H.-Z.; Rhen, D.S.; Wang, L.; Yuan, X.-T.; Li, J. Enhanced performance of conductive polysulfone/MWCNT/PANI ultrafiltration membrane in an online fouling monitoring application. J. Membr. Sci. 2019, 575, 160–169. [Google Scholar] [CrossRef]
- Masoumi, S.; Miroliaeia, A.R.; Jafarzadeh, Y. Preparation and characterization of MWCNT-COOH/PVC ultrafiltration membranes to use in water treatment. Adv. Environ. Technol. 2018, 2, 95–105. [Google Scholar]
- Demirel, E.; Zhang, B.; Papakyriakou, M.; Xia, S.; Chen, Y. Fe2O3 nanocomposite PVC membrane with enhanced properties and separation performance. J. Membr. Sci. 2017, 529, 170–184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tong, X.; Zhang, B.; Zhang, C.; Zhang, H.; Chen, Y. Enhanced permeation and antifouling performance of polyvinyl chloride (PVC) blend Pluronic F127 ultrafiltration membrane by using salt coagulation bath (SCB). J. Membr. Sci. 2018, 548, 32–41. [Google Scholar] [CrossRef]
- Coasne, B.; Gubbins, K.E.; Pellenq, R.J.M. A grand canonical Monte Carlo study of adsorption and capillary phenomena in nanopores of various morphologies and topologies: Testing the BET and BJH characterization methods. Part. Part. Syst. Charact. 2004, 21, 149–160. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Khalili, S.; Mollahosseini, A.; Zirepour, A.; Rajaeian, B. Novel functionalized carbon nanotubes for improving the surface properties and performance of polyethersulfone (PES) membrane. Desalination 2012, 286, 99–107. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Vatanpour, V. A comprehensive study on the performance and antifouling enhancement of the PVDF mixed matrix membranes by embedding different nanoparticulates: Clay, functionalized carbon nanotube, SiO2 and TiO2. SEP Purif. Technol. 2018, 197, 372–381. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Wang, H.; Song, X.; Wang, T.; He, B.; Liang, X.; Ngo, H.H. An electrocatalytic membrane reactor with self-cleaning function for industrial wastewater treatment. Angew. Chem. Int. Ed. 2011, 50, 2148–2150. [Google Scholar] [CrossRef]


| Membrane Samples | NMP (mL) | PVC (g) | PEG (g) | MWCNTs (g) | MWCNTs Proportion (%) |
|---|---|---|---|---|---|
| PVC | 40 | 4 | 1.6 | 0 | 0 |
| MWCNTs-20 | 40 | 3 | 0 | 0.75 | 20% |
| MWCNTs-22 | 40 | 2.66 | 0 | 0.75 | 22% |
| MWCNTs-24 | 40 | 2.38 | 0 | 0.75 | 24% |
| MWCNTs-25 | 40 | 2.25 | 0 | 0.75 | 25% |
| MWCNTs-26 | 40 | 2.14 | 0 | 0.75 | 26% |
| MWCNTs-28 | 40 | 1.93 | 0 | 0.75 | 28% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Gao, J.; Song, Y.; Gong, Y.; Qi, M.; Hao, R. Fabrication of a High Water Flux Conductive MWCNTs/PVC Composite Membrane with Effective Electrically Enhanced Antifouling Behavior. Coatings 2021, 11, 1548. https://doi.org/10.3390/coatings11121548
Chen X, Gao J, Song Y, Gong Y, Qi M, Hao R. Fabrication of a High Water Flux Conductive MWCNTs/PVC Composite Membrane with Effective Electrically Enhanced Antifouling Behavior. Coatings. 2021; 11(12):1548. https://doi.org/10.3390/coatings11121548
Chicago/Turabian StyleChen, Xi, Jiabin Gao, Yunchang Song, Yaping Gong, Meng Qi, and Runlong Hao. 2021. "Fabrication of a High Water Flux Conductive MWCNTs/PVC Composite Membrane with Effective Electrically Enhanced Antifouling Behavior" Coatings 11, no. 12: 1548. https://doi.org/10.3390/coatings11121548
APA StyleChen, X., Gao, J., Song, Y., Gong, Y., Qi, M., & Hao, R. (2021). Fabrication of a High Water Flux Conductive MWCNTs/PVC Composite Membrane with Effective Electrically Enhanced Antifouling Behavior. Coatings, 11(12), 1548. https://doi.org/10.3390/coatings11121548





