Comparative Investigation on Corrosion Resistance of Stainless Steels Coated with Titanium Nitride, Nitrogen Titanium Carbide and Titanium-Diamond-like Carbon Films
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
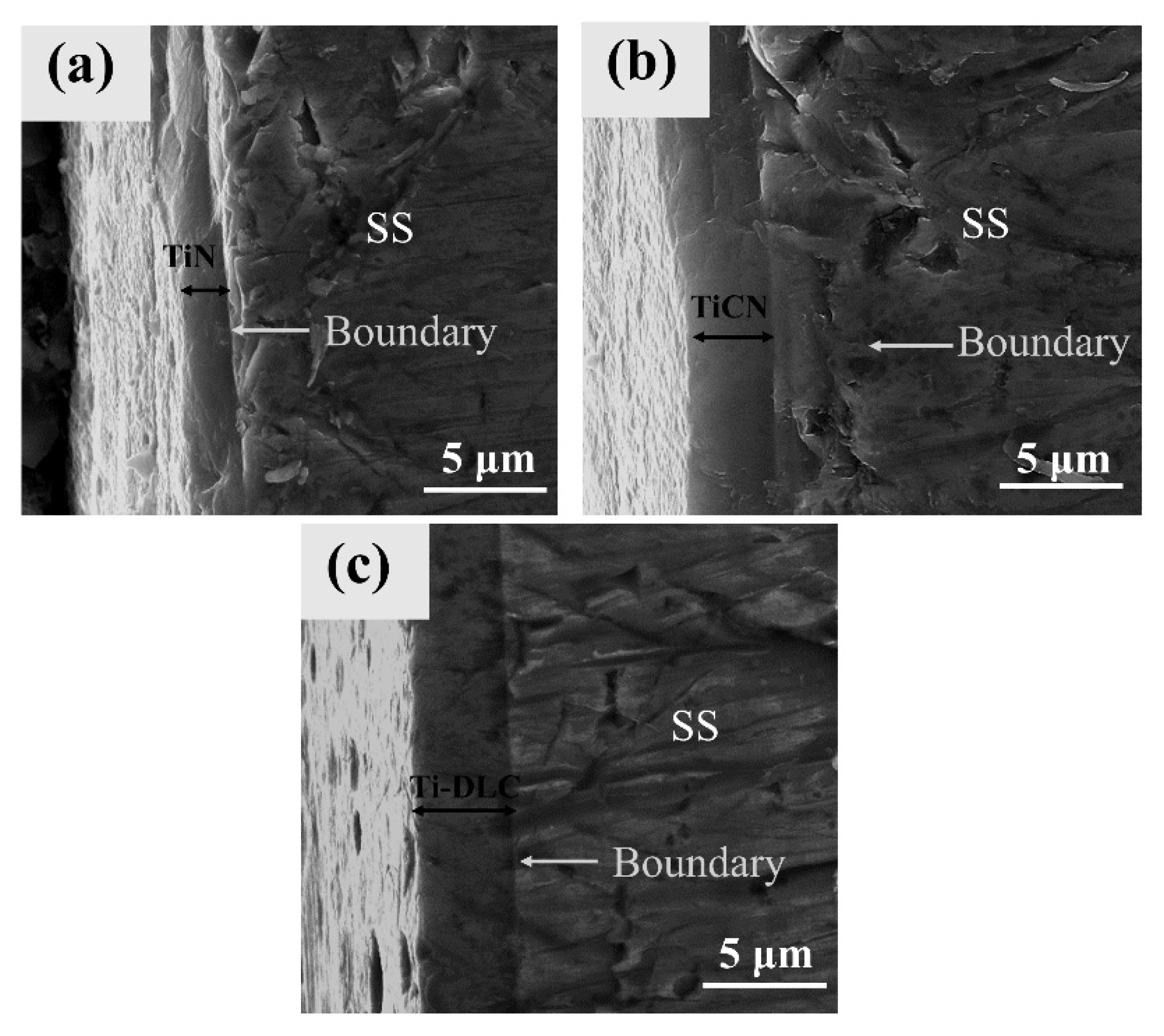
3.1. Surface Morphologies and Composition Analysis
3.2. OCP Test
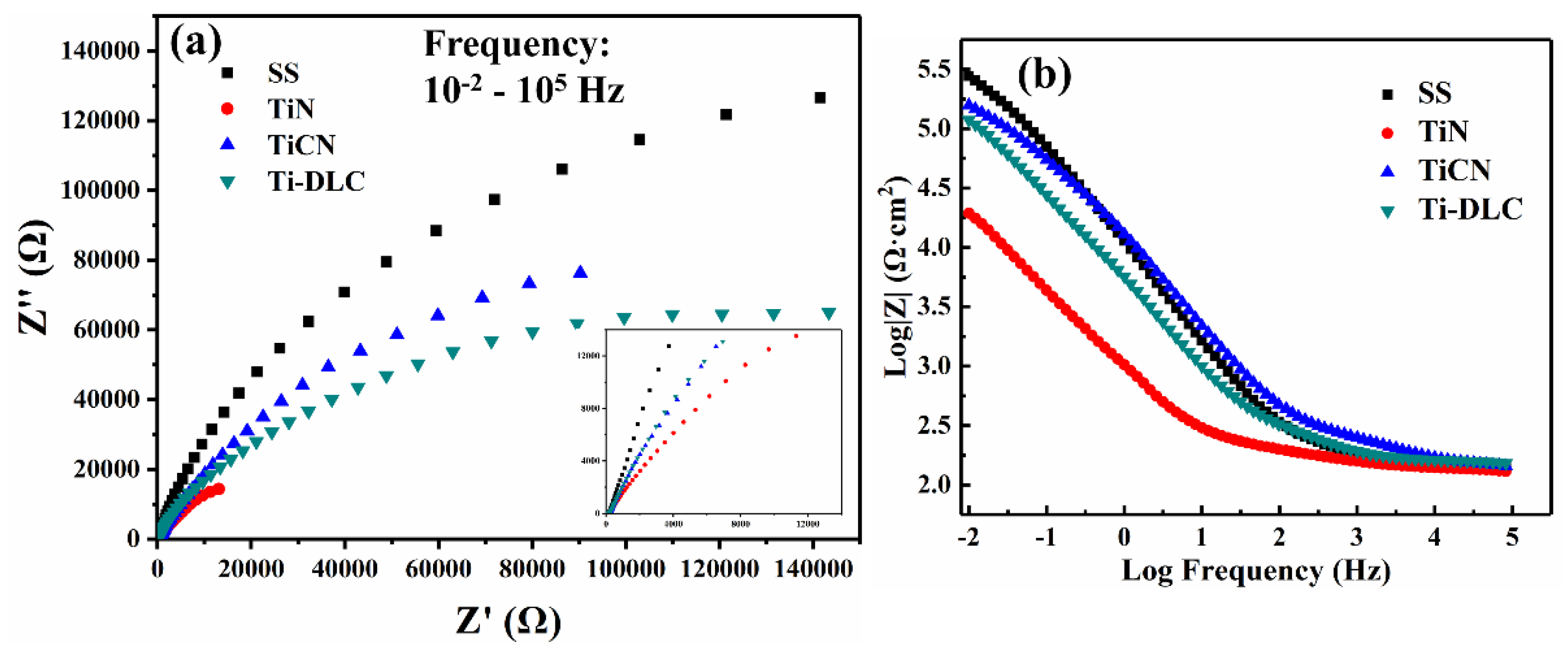
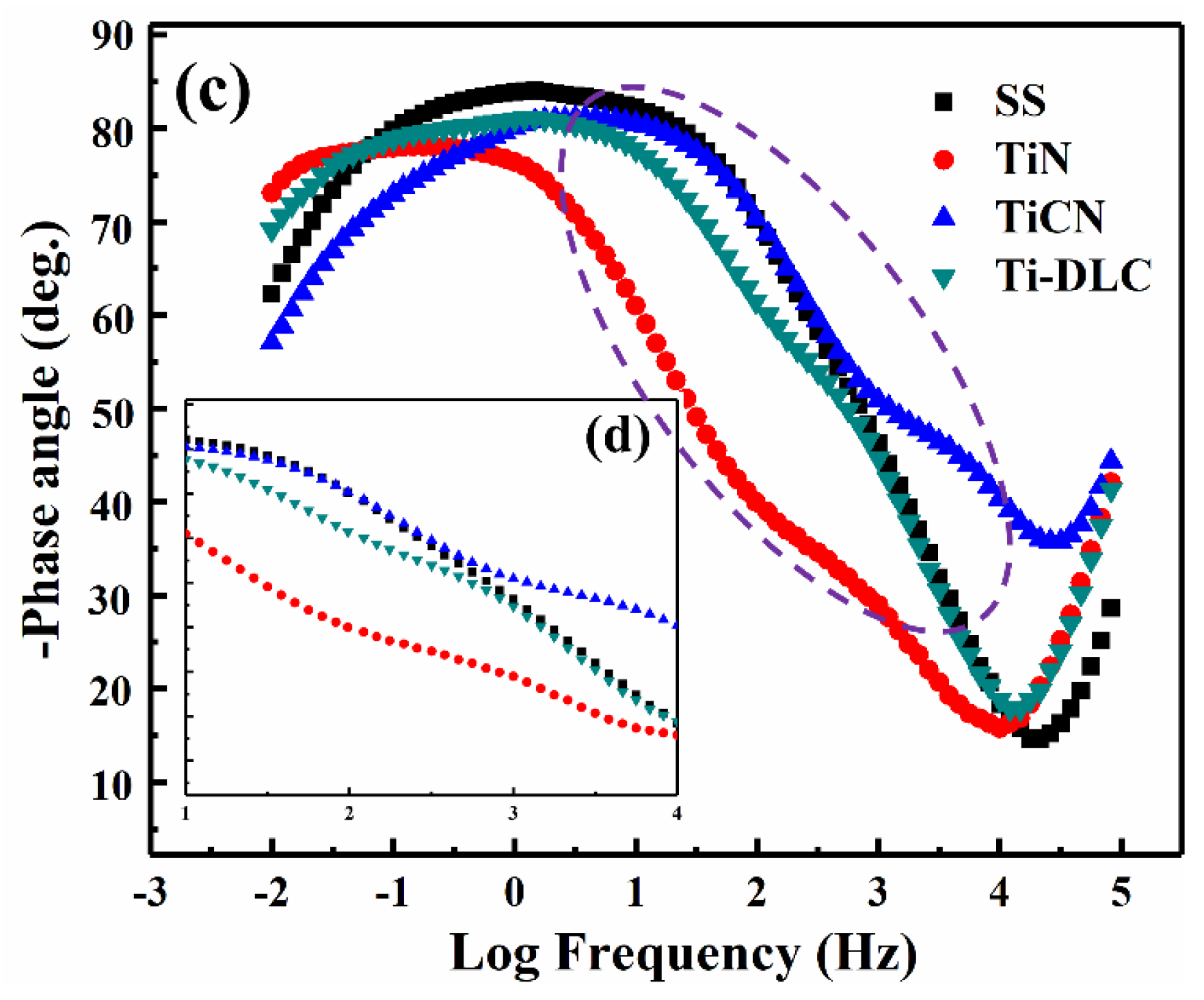
3.3. EIS Results
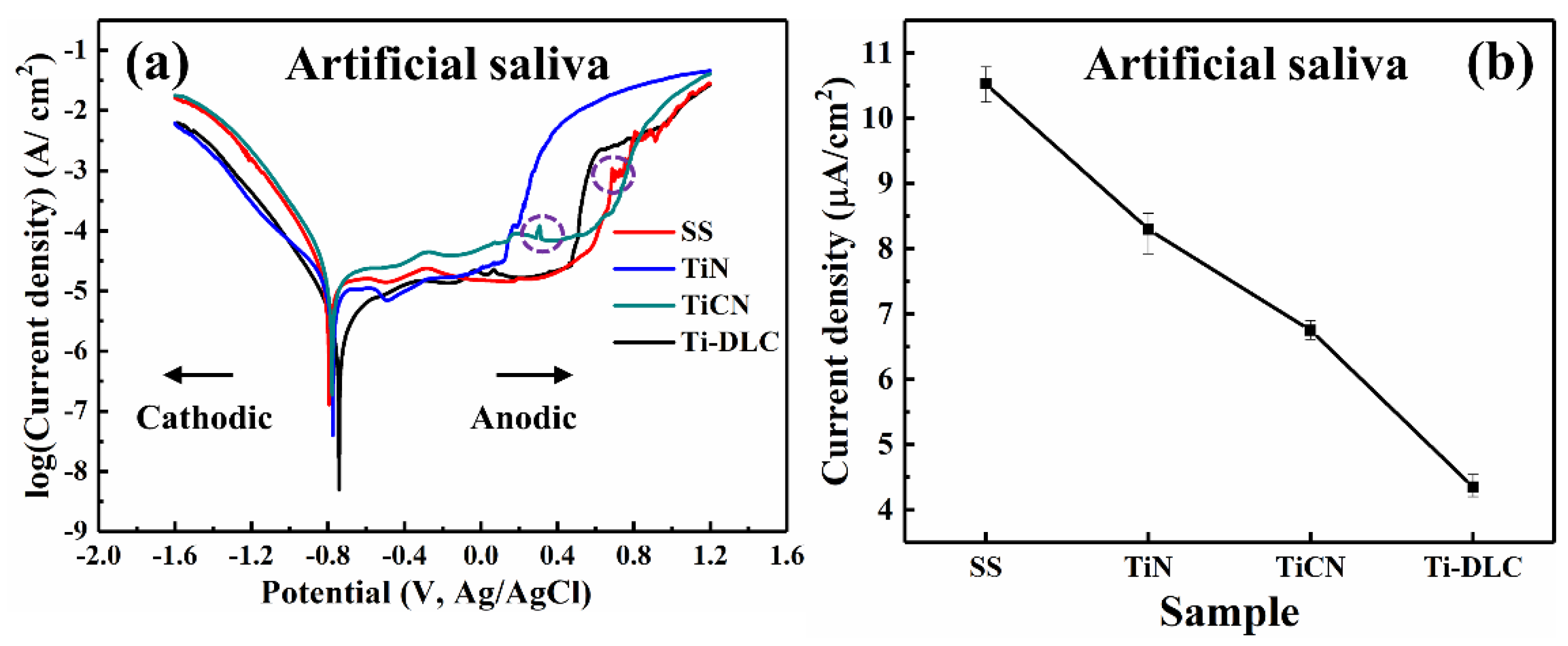
3.4. Potentiometric Test
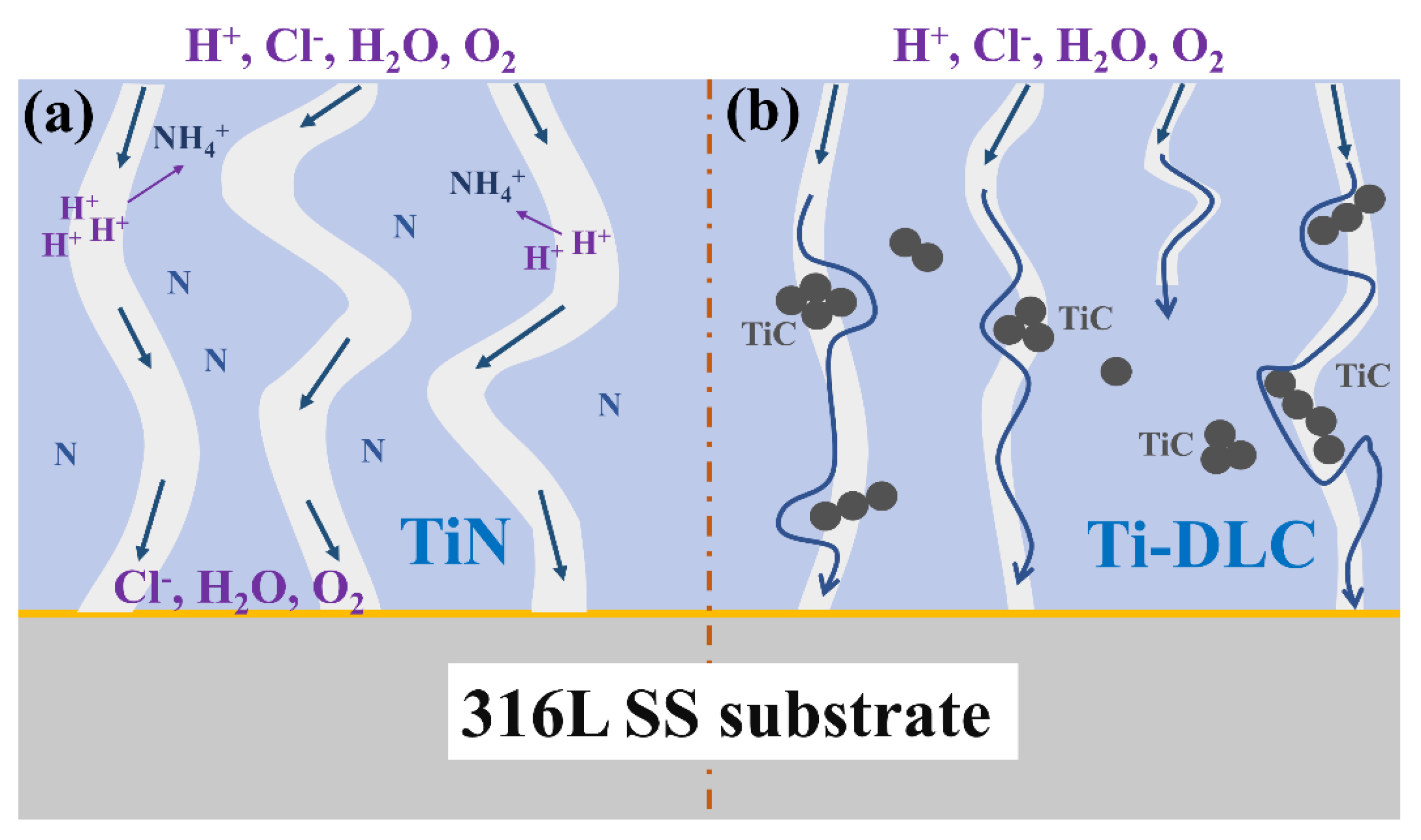
3.5. Surface Morphologies and Thickness of Sample after Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nattrass, C.; Sandy, J. Adult orthodontics—A review. Br. J. Orthod. 1995, 22, 331–337. [Google Scholar] [CrossRef]
- Hu, H.; Li, C.; Li, F.; Chen, J.; Sun, J.; Zou, S.; Sandham, A.; Xu, Q.; Riley, P.; Ye, Q. Enamel etching for bonding fixed orthodontic braces. Cochrane Database Syst. Rev. 2013, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Littlewood, S.J.; Millett, D.T.; Doubleday, B.; Bearn, D.R.; Worthington, H.V. Retention procedures for stabilising tooth position after treatment with orthodontic braces. Cochrane Database Syst. Rev. 2016, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rezaee, S.; Arman, A.; Jurečka, S.; Korpi, A.G.; Mwema, F.; Luna, C.; Sobola, D.; Kulesza, S.; Shakoury, R.; Bramowicz, M. Effect of annealing on the micromorphology and corrosion properties of Ti/SS thin films. Superlattices Microstruct. 2020, 146, 106681. [Google Scholar] [CrossRef]
- Khelfaoui, Y.; Kerkar, M.; Bali, A.; Dalard, F. Electrochemical characterisation of a PVD film of titanium on AISI 316L stainless steel. Surf. Coat. Technol. 2006, 200, 4523–4529. [Google Scholar] [CrossRef]
- Mirjalili, M.; Momeni, M.; Ebrahimi, N.; Moayed, M.H. Comparative study on corrosion behaviour of Nitinol and stainless steel orthodontic wires in simulated saliva solution in presence of fluoride ions. Mater. Sci. Eng. C 2013, 33, 2084–2093. [Google Scholar] [CrossRef]
- Vaughan, J.L.; Duncanson, M.G., Jr.; Nanda, R.S.; Currier, G.F. Relative kinetic frictional forces between sintered stainless steel brackets and orthodontic wires. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 20–27. [Google Scholar] [CrossRef]
- Zhang, J.; Ju, P.; Wang, C.; Dun, Y.; Zhao, X.; Zuo, Y.; Tang, Y. Corrosion Behaviour of 316L Stainless Steel in Hot Dilute Sulphuric Acid Solution with Sulphate and NaCl. Prot. Met. Phys. Chem. 2019, 55, 148–156. [Google Scholar] [CrossRef]
- Paetyangkul, A.; Türk, T.; Elekdağ-Türk, S.; Jones, A.S.; Petocz, P.; Cheng, L.L.; Darendeliler, M.A. Physical properties of root cementum: Part 16. Comparisons of root resorption and resorption craters after the application of light and heavy continuous and controlled orthodontic forces for 4, 8, and 12 weeks. Am. J. Orthod. Dentofac. Orthop. 2011, 139, e279–e284. [Google Scholar] [CrossRef]
- Alfonso, M.; Espinar, E.; Llamas, J.M.; Rupérez, E.; Manero, J.; Barrera, J.; Solano, E.; Gil, F. Friction coefficients and wear rates of different orthodontic archwires in artificial saliva. J. Mater. Sci. Mater. Med. 2013, 24, 1327–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, C.S.; Lisby, S.; Baadsgaard, O.; Byrialsen, K.; Menné, T. Release of nickel ions from stainless steel alloys used in dental braces and their patch test reactivity in nickel-sensitive individuals. Contact Dermat. 2003, 48, 300–304. [Google Scholar] [CrossRef]
- Jindal, P.; Santhanam, A.; Schleinkofer, U.; Shuster, A. Performance of PVD TiN, TiCN, and TiAlN coated cemented carbide tools in turning. Int. J. Refract. Met. Hard Mater. 1999, 17, 163–170. [Google Scholar] [CrossRef]
- Datta, S.; Das, M.; Balla, V.K.; Bodhak, S.; Murugesan, V. Mechanical, wear, corrosion and biological properties of arc deposited titanium nitride coatings. Surf. Coat. Technol. 2018, 344, 214–222. [Google Scholar] [CrossRef]
- Liu, C.; Chu, P.K.; Lin, G.; Qi, M. Anti-corrosion characteristics of nitride-coated AISI 316L stainless steel coronary stents. Surf. Coat. Technol. 2006, 201, 2802–2806. [Google Scholar] [CrossRef]
- Kao, C.T.; Ding, S.J.; Chen, Y.C.; Huang, T.H. The anticorrosion ability of titanium nitride (TiN) plating on an orthodontic metal bracket and its biocompatibility. J. Biomed. Mater. Res. 2002, 63, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Takadoum, J.; Houmid-Bennani, H.; Mairey, D.; Zsiga, Z. Adhesion and wear resistance of thin hard coatings. Eur. Ceram. Soc. 1997, 17, 1929–1932. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, R.; Wang, W.; Song, X.; Zhang, Y.; Zhang, C. Corrosion resistance of TiCN films prepared with combining multi-arc ion plating and magnetron sputtering technique. Rare Met. Mater. Eng. 2018, 47, 2028–2036. [Google Scholar] [CrossRef]
- Antunes, R.; Rodas, A.; Lima, N.; Higa, O.; Costa, I. Study of the corrosion resistance and in vitro biocompatibility of PVD TiCN-coated AISI 316 L austenitic stainless steel for orthopedic applications. Surf. Coat. Technol. 2010, 205, 2074–2081. [Google Scholar] [CrossRef]
- Cheng, Y.; Browne, T.; Heckerman, B.; Meletis, E. Influence of the C content on the mechanical and tribological properties of the TiCN coatings deposited by LAFAD technique. Surf. Coat. Technol. 2011, 205, 4024–4029. [Google Scholar] [CrossRef]
- Huang, S.; Ng, M.; Samandi, M.; Brandt, M. Tribological behaviour and microstructure of TiCxN (1− x) coatings deposited by filtered arc. Wear 2002, 252, 566–579. [Google Scholar] [CrossRef]
- Senna, L.; Achete, C.; Hirsch, T.; Freire, F., Jr. Structural, chemical, mechanical and corrosion resistance characterization of TiCN coatings prepared by magnetron sputtering. Surf. Coat. Technol. 1997, 94, 390–397. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Zhou, Z.; Li, L.K.-Y.; Yan, J. Electrochemical performance of TiCN coatings with low carbon concentration in simulated body fluid. Surf. Coat. Technol. 2014, 253, 199–204. [Google Scholar] [CrossRef]
- Jo, Y.J.; Zhang, T.F.; Son, M.J.; Kim, K.H. Synthesis and electrochemical properties of Ti-doped DLC films by a hybrid PVD/PECVD process. Appl. Surf. Sci. 2018, 433, 1184–1191. [Google Scholar] [CrossRef]
- Zhao, F.; Li, H.; Ji, L.; Wang, Y.; Zhou, H.; Chen, J. Ti-DLC films with superior friction performance. Diam. Relat. Mater. 2010, 19, 342–349. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, B.; Zhou, Y.; Zhang, J. Improving the internal stress and wear resistance of DLC film by low content Ti doping. Solid State Sci. 2013, 20, 17–22. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Liu, L.; Guo, P.; Li, X.; Lee, K.-R.; Cui, P.; Wang, A. Corrosion behavior of diamond-like carbon film induced by Al/Ti co-doping. Appl. Surf. Sci. 2020, 509, 144877. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, J.; He, H.; Xie, Y. Comparative investigation on the tribological performances of TiN, TiCN, and Ti-DLC film-coated stainless steel. JOM 2019, 71, 4872–4879. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, M.; Yang, Y.; Zhang, Y.; Yan, F.; Li, H. Excellent mechanical, tribological and anti-corrosive performance of novel Ti-DLC nanocomposite thin films prepared via magnetron sputtering method. Carbon 2019, 151, 136–147. [Google Scholar] [CrossRef]
- Konkhunthot, N.; Photongkam, P.; Wongpanya, P. Improvement of thermal stability, adhesion strength and corrosion performance of diamond-like carbon films with titanium doping. Appl. Surf. Sci. 2019, 469, 471–486. [Google Scholar] [CrossRef]
- Wongpanya, P.; Pintitraratibodee, N.; Thumanu, K.; Euaruksakul, C. Improvement of corrosion resistance and biocompatibility of 316L stainless steel for joint replacement application by Ti-doped and Ti-interlayered DLC films. Surf. Coat. Technol. 2021, 425, 127734. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Hu, T.; Wang, Q.; Shao, W.; Rao, L.; Xing, X.; Yang, Q. Role of TiC nanocrystalline and interface of TiC and amorphous carbon on corrosion mechanism of titanium doped diamond-like carbon films: Exploration by experimental and first principle calculation. Appl. Surf. Sci. 2021, 542, 148740. [Google Scholar] [CrossRef]
- Pytko-Polonczyk, J.; Jakubik, A.; Przeklasa-Bierowiec, A.; Muszynska, B. Artificial saliva and its use in biological experiments. J. Physiol. Pharmacol. 2017, 68, 807–813. [Google Scholar]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of growth defects in thin films prepared by PVD techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Cheng, Y.; Zheng, Y. Characterization of TiN, TiC and TiCN coatings on Ti–50.6 at.% Ni alloy deposited by PIII and deposition technique. Surf. Coat. Technol. 2007, 201, 4909–4912. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; Shu, F.; Wang, G.; Liu, B.; Xu, B. Study on the corrosion behavior of steel Q315NS heat-affected zone in a HCl solution using electrochemical noise. RSC Adv. 2018, 8, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.; Hu, R.; Dong, S.; Zhang, X.; Hou, R.; Du, R.; Lin, C.; Pan, J. EIS analysis on chloride-induced corrosion behavior of reinforcement steel in simulated carbonated concrete pore solutions. J. Electroanal. Chem. 2013, 688, 275–281. [Google Scholar] [CrossRef]
- Alves, M.M.; Prošek, T.; Santos, C.F.; Montemor, M.F. Evolution of the in vitro degradation of Zn–Mg alloys under simulated physiological conditions. RSC Adv. 2017, 7, 28224–28233. [Google Scholar] [CrossRef] [Green Version]
- Bayón, R.; Igartua, A.; González, J.; De Gopegui, U.R. Influence of the carbon content on the corrosion and tribocorrosion performance of Ti-DLC coatings for biomedical alloys. Tribol. Int. 2015, 88, 115–125. [Google Scholar] [CrossRef]
- Carnot, A.; Frateur, I.; Zanna, S.; Tribollet, B.; Dubois-Brugger, I.; Marcus, P. Corrosion mechanisms of steel concrete moulds in contact with a demoulding agent studied by EIS and XPS. Corros. Sci. 2003, 45, 2513–2524. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Wang, M.; Yang, Z.; Pan, Y.; Liu, G. Inhibiting the corrosion-promotion activity of graphene. Chem. Mater. 2015, 27, 2367–2373. [Google Scholar] [CrossRef]
- Shih, C.; Shih, C.; Su, Y.; Su, L.H.; Chang, M.; Lin, S. Effect of surface oxide properties on corrosion resistance of 316L stainless steel for biomedical applications. Corros. Sci. 2004, 46, 427–441. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 115–221. [Google Scholar] [CrossRef]
- Liu, C.; Chu, P.K.; Lin, G.; Yang, D. Effects of Ti/TiN multilayer on corrosion resistance of nickel–titanium orthodontic brackets in artificial saliva. Corro. Sci. 2007, 49, 3783–3796. [Google Scholar] [CrossRef]
- Dong, H.; Sun, Y.; Bell, T. Enhanced corrosion resistance of duplex coatings. Surf. Coat. Technol. 1997, 90, 91–101. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S.; Zhao, W.; Ouyang, L. Comparative corrosion resistance properties between (Cu, Ce)-DLC and Ti co-doped (Cu, Ce)/Ti-DLC films prepared via magnetron sputtering method. Chem. Phys. Lett. 2018, 705, 50–58. [Google Scholar] [CrossRef]





| Ingredient | NaCl | KCl | CaCl2 | K2PO4 | KSCN | CH4N2O |
|---|---|---|---|---|---|---|
| Content (mg/L) | 400 | 400 | 795 | 690 | 300 | 1000 |
| Concentration (g/L) | (0.9%, 6.6) | (0.9%, 3) | (3%, 6.6) | (3%, 3) |
|---|---|---|---|---|
| NaCl (mL) | 9 | 9 | 30 | 30 |
| 0.1 M H3Cit (mL) | 5.6 | 74.4 | 5.6 | 74.4 |
| 0.1 M K3Cit (mL) | 74.4 | 5.6 | 74.4 | 5.6 |
| Sample | Rs (Ω·cm2) | R1/Rf (Ω·cm2) | R2/Rct (Ω·cm2) | C1 (F/cm2) | C2/Cdl (F/cm2) | Chi-Squared (Χ2) |
|---|---|---|---|---|---|---|
| SS | 17.85 | 218.3 | 25,320 | 1.297 × 10−4 | 1.775 × 10−4 | 2.3 × 10−3 |
| TiN | 17.82 | 154.9 | 2230 | 9.884 × 10−4 | 3.251 × 10−3 | 7.2 × 10−4 |
| TiCN | 21.16 | 637.8 | 11,760 | 1.404 × 10−4 | 3.959 × 10−4 | 4.5 × 10−3 |
| Ti-DLC | 20.22 | 329.1 | 10,250 | 1.574 × 10−4 | 3.240 × 10−4 | 5.3 × 10−4 |
| Electrolyte | Sample | Ecorr (V) | Icorr (A) | βa (dec) | βa (dec) | η (%) |
|---|---|---|---|---|---|---|
| artificial | SS | −0.7936 | 1.05 × 10−5 | 690 | −134 | - |
| TiN | −0.7508 | 0.83 × 10−5 | 106 | −167 | 20.95 | |
| TiCN | −0.7342 | 0.68 × 10−5 | 225 | −108 | 35.24 | |
| Ti-DLC | −0.7292 | 0.44 × 10−5 | 750 | −225 | 58.09 | |
| 0.9%, 6.6 | SS | −0.7806 | 0.91 × 10−5 | 125 | −171 | - |
| TiN | −0.643 | 0.71 × 10−5 | 225 | 667 | 21.98 | |
| TiCN | −0.8038 | 0.55 × 10−5 | 208 | −113 | 39.56 | |
| Ti-DLC | −0.425 | 0.28 × 10−5 | 667 | −417 | 69.23 | |
| 0.9%, 3 | SS | −0.6544 | 2.61 × 10−5 | 125 | −225 | - |
| TiN | −0.4787 | 1.06 × 10−5 | 75 | −105 | 59.39 | |
| TiCN | −0.4528 | 0.98 × 10−5 | 334 | −149 | 62.45 | |
| Ti-DLC | −0.4088 | 0.46 × 10−5 | 183 | −417 | 82.24 | |
| 3%, 3 | SS | −0.7711 | 2.92 × 10−5 | 746 | 291 | - |
| TiN | −0.727 | 3.41 × 10−5 | 91 | −646 | - | |
| TiCN | −0.5396 | 2.06 × 10−5 | 159 | −158 | 29.45 | |
| Ti-DLC | −0.4571 | 0.61 × 10−5 | 747 | −118 | 79.11 | |
| 3%, 6.6 | SS | −0.86 | 1.96 × 10−5 | 167 | −91 | - |
| TiN | −0.8815 | 3.76 × 10−5 | 375 | 201 | - | |
| TiCN | −0.5373 | 1.54 × 10−5 | 146 | −267 | 21.43 | |
| Ti-DLC | −0.4118 | 0.46 × 10−5 | 167 | −133 | 76.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, J.; Gao, Z.; Zhang, J.; He, H.; Wang, X. Comparative Investigation on Corrosion Resistance of Stainless Steels Coated with Titanium Nitride, Nitrogen Titanium Carbide and Titanium-Diamond-like Carbon Films. Coatings 2021, 11, 1543. https://doi.org/10.3390/coatings11121543
Lou J, Gao Z, Zhang J, He H, Wang X. Comparative Investigation on Corrosion Resistance of Stainless Steels Coated with Titanium Nitride, Nitrogen Titanium Carbide and Titanium-Diamond-like Carbon Films. Coatings. 2021; 11(12):1543. https://doi.org/10.3390/coatings11121543
Chicago/Turabian StyleLou, Jia, Zonglong Gao, Jie Zhang, Hao He, and Xinming Wang. 2021. "Comparative Investigation on Corrosion Resistance of Stainless Steels Coated with Titanium Nitride, Nitrogen Titanium Carbide and Titanium-Diamond-like Carbon Films" Coatings 11, no. 12: 1543. https://doi.org/10.3390/coatings11121543
APA StyleLou, J., Gao, Z., Zhang, J., He, H., & Wang, X. (2021). Comparative Investigation on Corrosion Resistance of Stainless Steels Coated with Titanium Nitride, Nitrogen Titanium Carbide and Titanium-Diamond-like Carbon Films. Coatings, 11(12), 1543. https://doi.org/10.3390/coatings11121543







