Guided Insertion of Temporary Anchorage Device in Form of Orthodontic Titanium Miniscrews with Customized 3D Templates—A Systematic Review with Meta-Analysis of Clinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- (1)
- Type of study: Quantitative randomized controlled clinical trials and quantitative nonrandomized clinical studies.
- (2)
- Results of the study: Accuracy of 3D guided orthodontic miniscrew insertion in comparison to other methods.
- (3)
- Objective of the study: Comparison of the efficacy and accuracy of guided orthodontic-miniscrew insertion procedure to standard procedure.
- (4)
- Subject of the study: human subjects.
2.3. Data Extraction
2.4. Quality Assessment
2.5. Meta-Analysis
3. Results
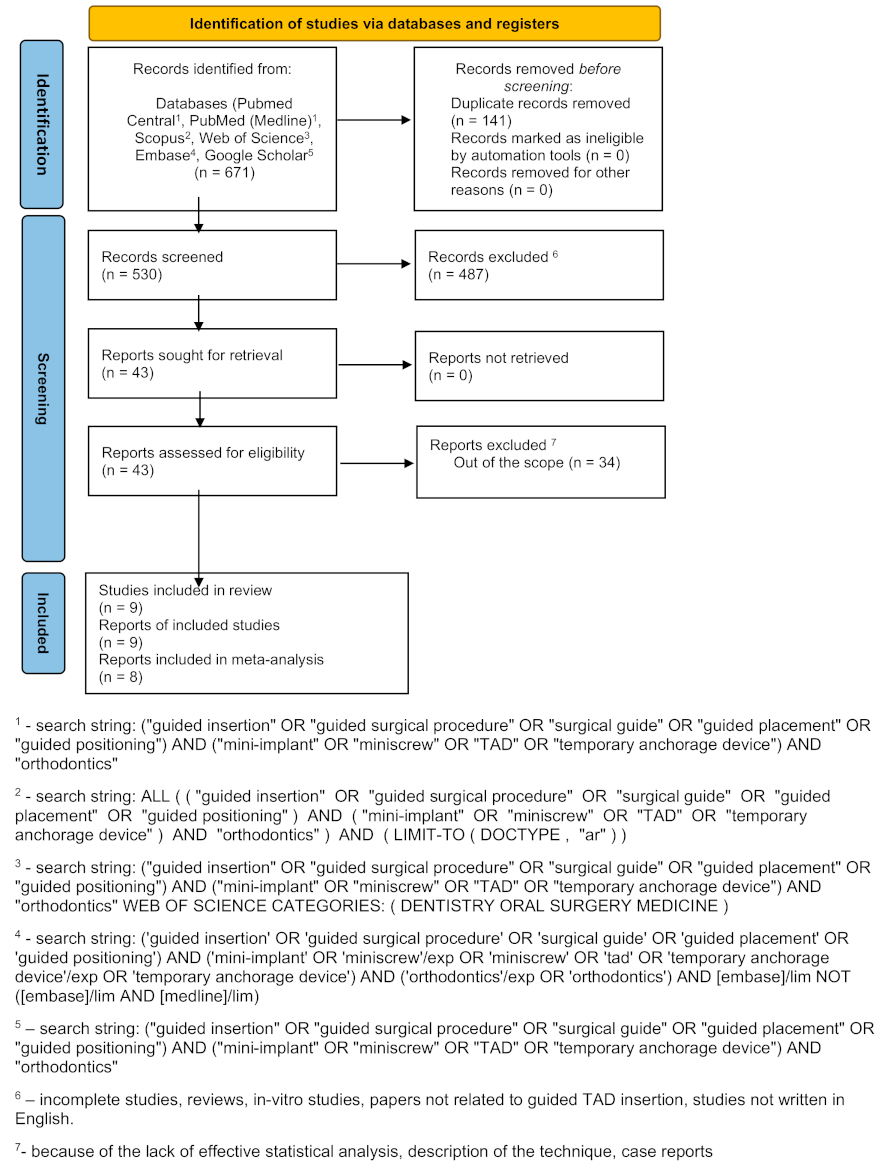
3.1. Search Results
3.2. Quality Assessment
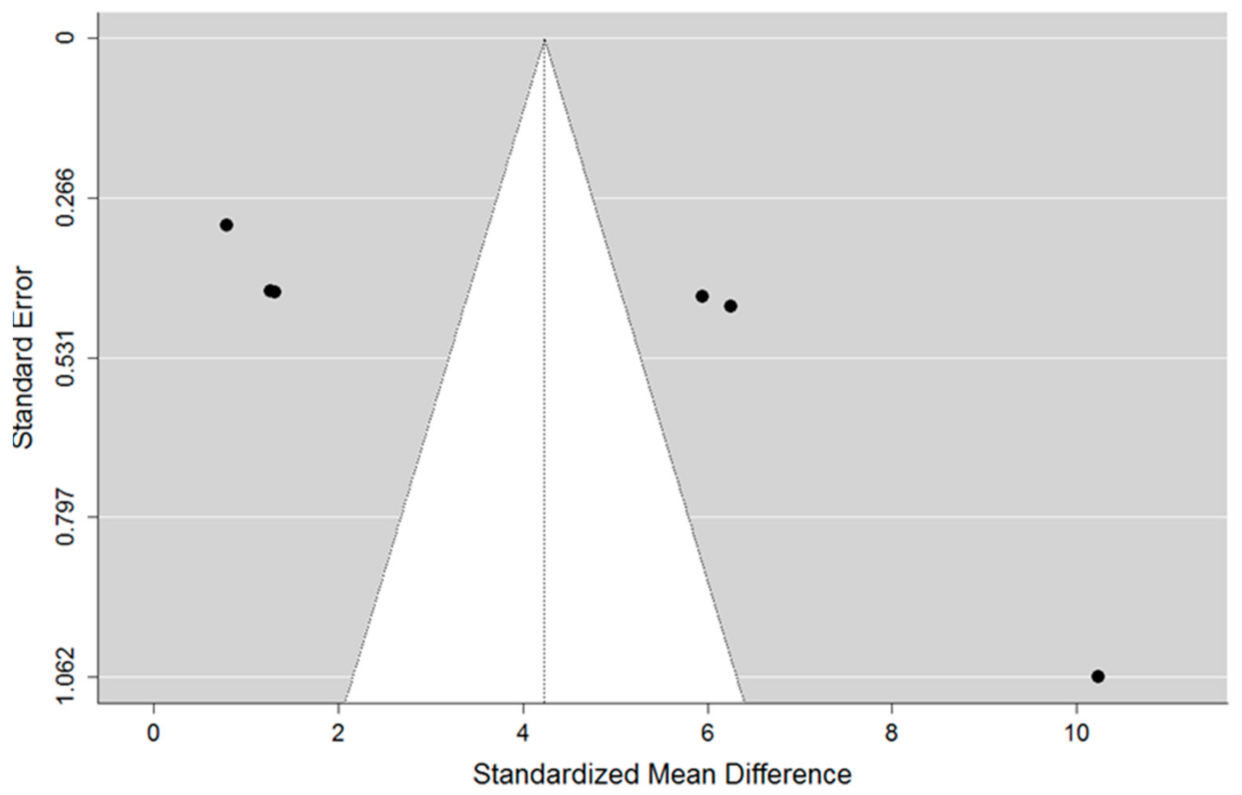
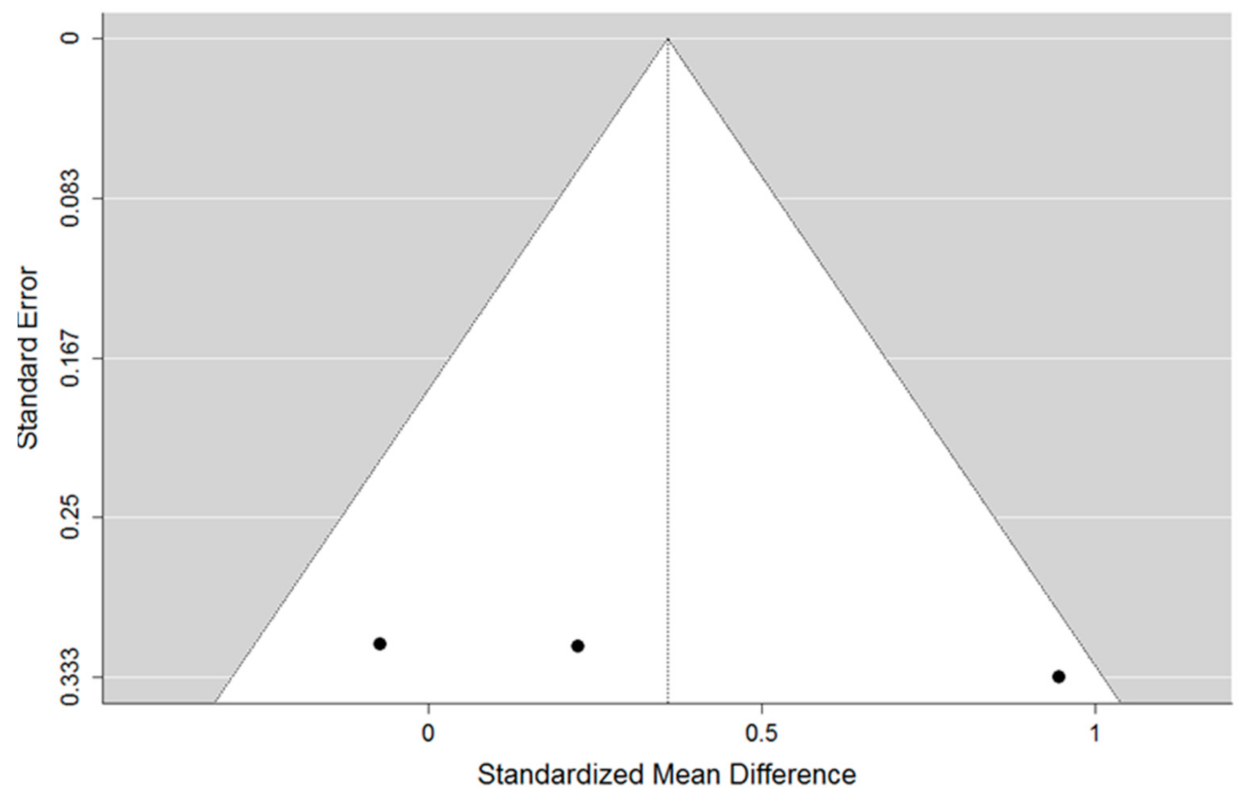
3.3. Meta-Analysis
- (a)
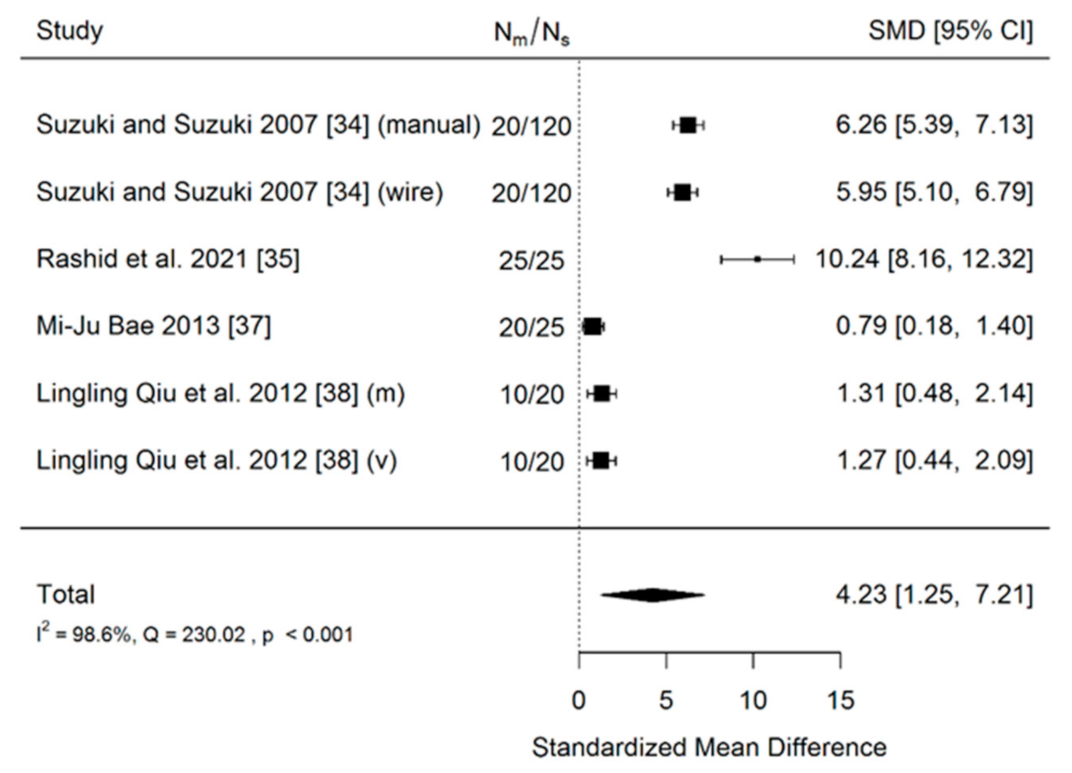
- Accuracy of insertion of mini-implants using a 3D surgical guide to these inserted manually (no-guide). Three studies were included in the meta-analysis. The total sample size of all included studies was 220 implants.
- (b)
- Accuracy of insertion of mini-implants using a 3D surgical guide in comparison to those inserted using a less-advanced method (manually and wire guides combined). There were four included studies in meta-analysis. The total sample size of all included studies was 285 implants.
- (c)
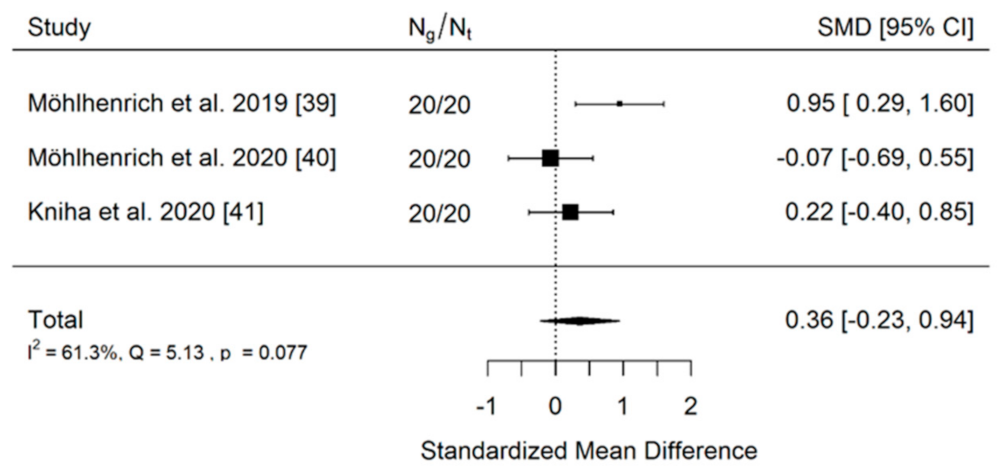
- Accuracy of insertion of mini-implants using a tooth-borne 3D surgical guide to these inserted using mucosa-borne ones. Three studies were included in the meta-analysis. The total sample size of all included studies was 120 implants.
- (a)
- The first comparison
- (b)
- The second comparison
- (c)
- The third comparison
4. Discussion
5. Conclusions
- (1)
- The current literature concerning guided MI insertion consists primarily of articles presenting a low methodological level, mainly technical papers or studies carried out without a control group. High-quality clinical trials, which exclude software-bias and operator-bias in their methodological flow, are in a minority.
- (2)
- The use of surgical guides increases mini-implant insertion accuracy and stability and reduces the failure rate of orthodontic miniscrews.
- (3)
- Tooth-borne insertion guides, supported on the edges of teeth, ensure a higher insertion precision compared to mucosa-borne guides.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAD | Temporary anchorage device |
| MI | Mini-implant |
| CBCT | Cone Beam Computed Tomography |
| MMAT | Mixed Methods Appraisal Tool |
References
- Antoszewska-Smith, J.; Sarul, M.; Łyczek, J.; Konopka, T.; Kawala, B. Effectiveness of orthodontic miniscrew implants in anchorage reinforcement during en-masse retraction: A systematic review and meta-analysis. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Baumgaertel, S. Temporary skeletal anchorage devices: The case for miniscrews. Am. J. Orthod. Dentofac. Orthop. 2009, 145, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Gainsforth, B.; Higley, L. A study of orthodontic anchorage possibilities in basal bone. Am. J. Orthod. Oral Surg. 1945, 31, 406–417. [Google Scholar] [CrossRef]
- Giudice, A.L.; Rustico, L.; Longo, M.; Oteri, G.; Papadopoulos, M.A.; Nucera, R. Complications reported with the use of orthodontic miniscrews: A systematic review. Korean J. Orthod. 2021, 51, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Hourfar, J.; Bister, D.; Kanavakis, G.; Lisson, J.A.; Ludwig, B. Influence of interradicular and palatal placement of orthodontic mini-implants on the success (survival) rate. Head Face Med. 2017, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Valladares-Neto, J.; Evangelista, K. Infrazygomatic mini-implant penetration into the maxillary sinus. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 462–463. [Google Scholar] [CrossRef]
- Moslemzadeh, S.H.; Sohrabi, A.; Rafighi, A.; Kananizadeh, Y.; Nourizadeh, A. Evaluation of interdental spaces of the mandibular posterior area for orthodontic mini-implants with cone-beam computed tomography. J. Clin. Diagn. Res. 2017, 11, ZC09–ZC12. [Google Scholar] [CrossRef]
- Haddad, R.; Saadeh, M. Distance to alveolar crestal bone: A critical factor in the success of orthodontic mini-implants. Prog. Orthod. 2019, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.C. The glossary of prosthodontic terms. J. Prosthet. Dent. 2005, 94, 10–92. [Google Scholar] [CrossRef]
- Ramasamy, M.; Giri, R.R.; Subramonian, K.; Narendrakumar, R. Implant surgical guides: From the past to the present. J. Pharm. Bioallied Sci. 2013, 5 (Suppl. 1), S98–S102. [Google Scholar] [CrossRef]
- Kalra, S.; Tripathi, T.; Rai, P.; Kanase, A. Evaluation of orthodontic mini-implant placement: A CBCT study. Prog. Orthod. 2014, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Kapila, S.D.; Nervina, J.M. CBCT in orthodontics: Assessment of treatment outcomes and indications for its use. Dentomaxillofac. Radiol. 2015, 44, 20140282. [Google Scholar] [CrossRef]
- Bae, S.-M.; Park, H.-S.; Kyung, H.-M.; Kwon, O.-W.; Sung, J.-H. Clinical application of micro-implant anchorage. J. Clin. Orthod. 2002, 36, 298–302. [Google Scholar] [PubMed]
- Felicita, A.S. A simple three-dimensional stent for proper placement of mini-implant. Prog. Orthod. 2013, 14, 45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Souza, K.M.; Aras, M.A. Types of implant surgical guides in dentistry: A review. J. Oral Implantol. 2012, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-printing: Transferring art from the laboratories to the clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.; Heather, J.C.; Lianshan, L.; Scott, L.; Wook, -J.S. Effect of surgical guide design and surgeon’s experience on the accuracy of implant placement. J. Oral Implant. 2012, 38, 311–323. [Google Scholar]
- Ravidà, A.; Barootchi, S.; Tattan, M.; Saleh, M.H.A.; Gargallo-Albiol, J.; Wang, H.L. Clinical outcomes and cost effectiveness of computer-guided versus conventional implant-retained hybrid prostheses: A long-term retrospective analysis of treatment protocols. J. Periodontol. 2018, 89, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.; Altieri, F.; Di Giorgio, R.; Barbato, E. Palatal orthodontic miniscrew insertion using a CAD-CAM surgical guide: Description of a technique. Int. J. Oral Maxillofac. Surg. 2018, 47, 1195–1198. [Google Scholar] [CrossRef]
- Barros, S.E.C.; Janson, G.; Chiqueto, K.; De Freitas, M.R.; Henriques, J.F.C.; Pinzan, A. A three-dimensional radiographic-surgical guide for mini-implant placement. J. Clin. Orthod. 2006, 40, 548–554. [Google Scholar]
- Kim, S.-H.; Kang, J.-M.; Choi, B.; Nelson, G. Clinical application of a stereolithographic surgical guide for simple positioning of orthodontic mini-implants. World J. Orthod. 2008, 9, 371–382. [Google Scholar]
- Suzuki, E.Y.; Buranastidporn, B. An adjustable surgical guide for miniscrew placement. J. Clin. Orthod. 2005, 39, 588–590. [Google Scholar]
- Miyazawa, K.; Kawaguchi, M.; Tabuchi, M.; Goto, S. Accurate pre-surgical determination for self-drilling miniscrew implant placement using surgical guides and cone-beam computed tomography. Eur. J. Orthod. 2010, 32, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Morea, C.; Hayek, J.E.; Oleskovicz, C.; Dominguez, G.C.; Chilvarquer, I. Precise insertion of orthodontic miniscrews with a stereolithographic surgical guide based on cone beam computed tomography data: A pilot study. Int. J. Oral Maxillofac. Implantol. 2011, 26, 860–865. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Beller, E.M.; Glasziou, P.P.; Altman, D.G.; Hopewell, S.; Bastian, H.; Chalmers, I.; Gøtzsche, P.C.; Lasserson, T.; Tovey, D. PRISMA for abstracts: Reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013, 10, e1001419. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the prisma statement for reporting literature searches in systematic reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Welch, V.A., Ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Sackett, D.L.; Strauss, S.E.; Richardson, W.S.; Rosenberg, W.; Haynes, B.R. Evidence-Based Medicine: How to Practice and Teach EBM, 2nd ed.; Elsevier Churchill Livingstone: Philadelphia, PA, USA, 2000. [Google Scholar]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Del Re, A.C. A practical tutorial on conducting meta-analysis in R. Quant. Methods Psychol. 2015, 11, 37–50. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.L. Discrete Structures, Logic, and Computability, 2nd ed.; Jones and Bartlett Publishers, Inc.: Sudbury, MA, USA, 2002. [Google Scholar]
- Suzuki, E.Y.; Suzuki, B. Accuracy of miniscrew implant placement with a 3-dimensional surgical guide. J. Oral Maxillofac. Surg. 2008, 66, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; El Feky, H.; Issa, N. Accuracy of miniscrew insertion using a customized printed three-dimensional surgical guide (a comparative split mouth study). Egypt. Dent. J. 2021, 67, 109–118. [Google Scholar] [CrossRef]
- Kim, D. An Evaluation of Clinical Stability of Miniscrew with Surgical Guide Using Intraoral Scan Model and CBCT: Randomized Clinical Trial. Bachelor’s Dissertation, Yonsei University, Seoul, Korea, 2019. [Google Scholar]
- Bae, M.-J.; Kim, J.-Y.; Park, J.-T.; Cha, J.-Y.; Kim, H.-J.; Yu, H.-S.; Hwang, C.-J. Accuracy of miniscrew surgical guides assessed from cone-beam computed tomography and digital models. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 893–901. [Google Scholar] [CrossRef]
- Qiu, L.; Haruyama, N.; Suzuki, S.; Yamada, D.; Obayashi, N.; Kurabayashi, T.; Moriyama, K. Accuracy of orthodontic miniscrew implantation guided by stereolithographic surgical stent based on cone-beam CT–derived 3D images. Angle Orthod. 2012, 82, 284–293. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Brandt, M.; Kniha, K.; Bock, A.; Prescher, A.; Hölzle, F.; Modabber, A.; Danesh, G. Suitability of virtual plaster models superimposed with the lateral cephalogram for guided paramedian orthodontic mini-implant placement with regard to the bone support. Eignung virtuell überlagerter Situationsmodelle und korrespondierender Fernröntgenseitenaufnahmen zur schablonengeführten Mini-Implantat-Insertion unter Berücksichtigung des Knochenangebots. J. Orofac. Orthop. 2020, 81, 340–349. [Google Scholar]
- Möhlhenrich, S.C.; Brandt, M.; Kniha, K.; Prescher, A.; Hölzle, F.; Modabber, A.; Wolf, M.; Peters, F. Accuracy of orthodontic mini-implants placed at the anterior palate by tooth-borne or gingiva-borne guide support: A cadaveric study. Clin. Oral Investig. 2019, 23, 4425–4431. [Google Scholar] [CrossRef]
- Kniha, K.; Brandt, M.; Bock, A.; Modabber, A.; Prescher, A.; Hölzle, F.; Danesh, G.; Möhlhenrich, S.C. Accuracy of fully guided orthodontic mini-implant placement evaluated by cone-beam computed tomography: A study involving human cadaver heads. Clin. Oral Investig. 2021, 25, 1299–1306. [Google Scholar] [CrossRef]
- Altieri, F.; Cassetta, M. The impact of tooth-borne vs computer-guided bone-borne rapid maxillary expansion on pain and oral health–related quality of life: A parallel cohort study. Am. J. Orthod. Dentofac. Orthop. 2020, 158, e83–e90. [Google Scholar] [CrossRef]
- Wilmes, B.; Vasudavan, S.; Drescher, D. CAD-CAM–fabricated mini-implant insertion guides for the delivery of a distalization appliance in a single appointment. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 148–156. [Google Scholar] [CrossRef]
- Malhotra, S.; Nanda, P.; Sidhu, M.S. A guide for simple mini-implant placement. Orthodontics 2012, 13, 166–167. [Google Scholar]
- Cousley, R.R.J. A stent-guided mini-implant system. J. Clin. Orthod. 2009, 43, 403–407. [Google Scholar]
- Kim, S.-H.; Choi, Y.-S.; Hwang, E.-H.; Chung, K.-R.; Kook, Y.-A.; Nelson, G. Surgical positioning of orthodontic mini-implants with guides fabricated on models replicated with cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2007, 131 (Suppl. 4), S82–S89. [Google Scholar] [CrossRef]
- D’Haese, J.; Van De Velde, T.; Elaut, L.; De Bruyn, H. A prospective study on the accuracy of mucosally supported stereolithographic surgical guides in fully edentulous maxillae. Clin. Implantol. Dent. Relat. Res. 2012, 14, 293–303. [Google Scholar] [CrossRef]
- Lee, J.-A.; Ahn, H.-W.; Oh, S.H.; Park, K.-H.; Kim, S.-H.; Nelson, G. Evaluation of interradicular space, soft tissue, and hard tissue of the posterior palatal alveolar process for orthodontic mini-implant, using cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 460–469. [Google Scholar] [CrossRef]
- Mallick, S.; Murali, P.S.; Kuttappa, M.N.; Shetty, P.; Nair, A. Optimal sites for mini-implant insertion in the lingual or palatal alveolar cortical bone as assessed by cone beam computed tomography in South Indian population. Orthod. Craniofac. Res. 2021, 24, 121–129. [Google Scholar] [CrossRef]
- Möhlhenrich, S.; Heussen, N.; Modabber, A.; Bock, A.; Hölzle, F.; Wilmes, B.; Danesh, G.; Szalma, J. Influence of bone density, screw size and surgical procedure on orthodontic mini-implant placement—Part B: Implant stability. Int. J. Oral Maxillofac. Surg. 2021, 50, 565–572. [Google Scholar] [CrossRef]
- Jedliński, M.; Mazur, M.; Grocholewicz, K.; Janiszewska-Olszowska, J. 3D scanners in orthodontics—Current knowledge and future perspectives—A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 1121. [Google Scholar] [CrossRef]
- Hong, S.-B.; Kusnoto, B.; Kim, E.-J.; BeGole, E.A.; Hwang, H.-S.; Lim, H.-J. Prognostic factors associated with the success rates of posterior orthodontic miniscrew implants: A subgroup meta-analysis. Korean J. Orthod. 2016, 46, 111–126. [Google Scholar] [CrossRef]
- Gandhi, V.; Upadhyay, M.; Tadinada, A.; Yadav, S. Variability associated with mandibular buccal shelf area width and height in subjects with different growth pattern, sex, and growth status. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.Y.; Suzuki, B.; Aramrattana, A.; Harnsiriwattanakit, K.; Kowanich, N. Assessment of miniscrew implant stability by resonance frequency analysis: A study in human cadavers. J. Oral Maxillofac. Surg. 2010, 68, 2682–2689. [Google Scholar] [CrossRef]
- Kuroda, S.; Yamada, K.; Deguchi, T.; Hashimoto, T.; Kyung, H.-M.; Yamamoto, T.T. Root proximity is a major factor for screw failure in orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2007, 131, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, Y.H.; Lin, L.D.; Yao, C.C.J. Removal torque of miniscrews used for orthodontic anchorage—A preliminary report. Int. J. Oral Maxillofac. Implantol. 2006, 21, 283–289. [Google Scholar]
- Askar, H.; Krois, J.; Rohrer, C.; Mertens, S.; Elhennawy, K.; Ottolenghi, L.; Mazur, M.; Paris, S.; Schwendicke, F. Detecting white spot lesions on dental photography using deep learning: A pilot study. J. Dent. 2021, 107, 103615. [Google Scholar] [CrossRef]
- Ezhov, M.; Gusarev, M.; Golitsyna, M.; Yates, J.M.; Kushnerev, E.; Tamimi, D.; Aksoy, S.; Shumilov, E.; Sanders, A.; Orhan, K. Clinically applicable artificial intelligence system for dental diagnosis with CBCT. Sci. Rep. 2021, 11, 15006. [Google Scholar] [CrossRef] [PubMed]


| Author and Year | Type of Article | Material or Subjects | Control Sample or Group | Method | Outcome Measured | Results | |
|---|---|---|---|---|---|---|---|
| Suzuki and Suzuki, 2007 [34] | Case—control study | 180 implants inserted with the use of a 3D surgical guide | (a) 20 implants inserted using a conventional wire guide (b) 20 implants inserted without any guide | Measurements on periapical radiographs | Deviation from “gold standard” lines projected by specialized software | The mean coronal deviation was 0.4–0.6 mm for the 3D surgical guide method, 0.4–1.0 mm for the wire guide, and 1.4–3.6 mm for the no-guide method. The mean apical deviation was 0.4–2.0 mm for the 3D surgical guide, 1.1–5.3 mm for the wire guide, and 3.5–10.5 mm for the no-guide method. All the mini-implants were inserted into interradicular space. | |
| Rashid et al., 2021 [35] | Randomized split-mouth clinical trial | 25 implants inserted with the use of a 3D surgical guide | 25 implants inserted without any guide | Measurements on CBCT scans | Deviation from “gold standard” lines projected by specialized software | The mean values for apical deviation were 0.69 ± 0.02 mm for guided screws and 1.44 ± 0.10 for hand-drilled screws, and 0.60 ± 0.03 and 2.47 ± 0.27 for coronal deviation, respectively. The mean mesiodistal angle was 2.53 ± 0.10 for guided implants and 11.67 ± 0.75 for hand-drilled group. The mean bucco-lingual angle was 0.18 ± 0.09 and 10.25 ± 0.91, respectively. All the mini-implants were inserted into interradicular space. | |
| Dasomi Kim, 2019 [36] | Randomized clinical trial | 45 implants inserted with the use of a 3D surgical guide | 47 implants inserted manually without any guide | Measurements on periapical radiographs, CBCT, insertion torque and Periotest | Percentage of success rate, root contact, insertion torque and Periotest | In the manual insertion group the success rate was 80.9% and for the guide group it was 88.9%. The root contact rate was 31.9% in the manual group and 0.4% in the surgical guide group. The insertion torque was 6.37 ± 2.64 Ncm in the manual group and 6.54 ± 2.90 Ncm in the guide group, and the Periotest value was 0.19 ± 2.86 in the guide group and 1.58 ± 2.13 in the manual group. All the miniimplants were inserted into interradicular space. | |
| Mi-Ju Bae, 2013 [37] | Nonradmized clinical experimental study | 25 implants inserted with the use of a 3D surgical guide | 20 implants inserted using a wire guide and periapical radiographs | Measurements on CBCT scans | Deviation from “gold standard” lines projected by specialized software | Median long-axis angular deviations were 3.14° (range, 1.02°–10.9°) for the surgical guide group and 9.57° (range, 3.15°–35.60°) for the control group. The mean apical deviation 0.73 was for the surgical group and 1.28 for control group. The mean coronal deviation was 0.73 for the surgical group and 1.56 for the control group. All the mini-implants were inserted into interradicular space. | |
| Lingling Qiu et al., 2012 [38] | Nonradmized clinical experimental study | 20 implants inserted with the use of a 3D surgical guide | 10 implants inserted manually without any guide | Measurements on CBCT scans | Deviation from “gold standard” lines projected by specialized software | Surgical Guided screws The mean apical deviation was 0.28 ± 0.23 mm and 0.33 ± 0.25 mm in mesiodistal and apical directions. The mean coronal deviation was 0.15 ± 0.09 mm and 0.19 ± 0.19 mm in mesiodistal and apical direction. The angular deviations were 1.47° ± 0.56 and 2.13° ± 1.48, respectively. All the mini-implants were inserted into interradicular space. | Manually inserted screws The mean apical deviation was 0.81 ± 0.61 mm and 0.78 ± 0.49 mm in mesiodistal and apical directions. The mean coronal deviation was 0.48 ± 0.46 mm, and 0.94 ± 0.87 mm in mesiodistal and apical directions. The angular deviations were 7.49° ± 6.09 and 6.31° ± 3.82, respectively. All the miniimplants were inserted into interradicular space. |
| Möhlhenrich et al., 2019 [39] | Nonradmized clinical experimental study | 20 implants inserted with the use of a 3D tooth-borne surgical guide | 20 implants inserted with the use of a 3D mucosa-borne surgical guide | Measurements on cephalograms, plaster models and intraoral scans | Deviation from “gold standard” lines projected by specialized software | Statistical differences between tooth-borne and mucosa-borne guides were detected for lateral deviations: 0.88 mm ± 0.46 versus 1.65 mm ± 1.03 and sagittal angular deviations: 3.67° ± 2.25 versus 6.46° ± 5.5. All the MI were inserted into interradicular space. | |
| Möhlhenrich et al., 2020 [40] | Nonradmized clinical experimental study | 20 implants inserted with the use of a 3D tooth-borne surgical guide | 20 implants inserted with the use of 3D a mucosa-borne surgical guide | Measurements on cephalograms, CBCT, plaster models and intraoral scans | Deviation from “gold standard” lines projected by specialized software separately on cephalogram and CBCT | Significant differences between T0 and T1 were only noted in terms of lateral deviation using the tooth-borne guide (T0: 4.7 ± 2.3 mm, T1: 3.0 ± 2.3 mm;) and linear sagittal deviation using the mucosa-borne guide (T0: 3.1 ± 3.5 mm, T1: 2.3 ± 3.2 mm). All the mini-implants were inserted into palate. | |
| Kniha et al., 2020 [41] | Nonradmized clinical experimental study | 20 implants inserted with the use of a 3D tooth-borne surgical guide | 20 implants inserted with the use of a 3D mucosa-borne surgical guide | Measurements on CBCT scans | Deviation from “gold standard” lines projected by specialized software | The only statistically significantly different variable was implant axis angulation. In tooth-borne guides it was 2.81° ± 2.69. In mucosa-borne guides it was 6.22° ± 4.26. All the mini-implants were inserted into interradicular space. | |
| Federica Altieri and Michele Cassetta, 2020 [42] | Randomized clinical trial | 18 subjects with computer-aided designed skeletal RME appliance | 18 subjects with classic hyrax appliance | Pain scales and shortened Oral Health Impact Profile (OHIP-14) questionnaire | Level of pain and quality of life for 14 days after insertion | The only differences were noted on the day of screw activation. Patients with a computer-guided skeletal RME appliance felt less comfortable. All the mini-implants were inserted into interradicular space. | |
| Category of Study Designs | Methodological Quality Criteria | Suzuki and Suzuki, 2007 | Rashid et al., 2021 | Dasomi Kim, 2019 | Mi-Ju Bae, 2013 | Lingling Qiu et al., 2012 | Möhlhenrich et al., 2019 | Möhlhenrich et al., 2020 | Kniha et al., 2020 | Federica Altieri and Michele Cassetta, 2020 |
|---|---|---|---|---|---|---|---|---|---|---|
| Screening questions | S1. Are there clear research questions? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S2. Do the collected data allow to address the research questions? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Quantitative randomized controlled trials | 1.1. Is randomization appropriately performed? | N/A | Yes | Yes | N/A | N/A | N/A | N/A | N/A | Yes |
| 1.2. Are the groups comparable at baseline? | N/A | Yes | Yes | N/A | N/A | N/A | N/A | N/A | Yes | |
| 1.3. Are there complete outcome data? | N/A | Yes | Yes | N/A | N/A | N/A | N/A | N/A | Yes | |
| 1.4. Are outcome assessors blinded to the intervention provided? | N/A | Can’t tell | No * | N/A | N/A | N/A | N/A | N/A | No * | |
| 1.5 Did the participants adhere to the assigned intervention? | N/A | Yes | Yes | N/A | N/A | N/A | N/A | N/A | Yes | |
| Quantitative nonrandomized | 2.1. Are the participants representative of the target population? | Yes | N/A | N/A | Can’t tell | No | Yes | Yes | Yes | N/A |
| 2.2. Are measurements appropriate regarding both the outcome and intervention (or exposure)? | Yes | N/A | N/A | Can’t tell | Yes | Yes | Yes | Yes | N/A | |
| 2.3. Are there complete outcome data? | Yes | N/A | N/A | Yes | Yes | Yes | Yes | Yes | N/A | |
| 2.4. Are the confounders accounted for in the design and analysis? | Yes | N/A | N/A | No * | No * | No * | No * | No * | N/A | |
| 2.5. During the study period, is the intervention administered (or exposure occurred) as intended? | Yes | N/A | N/A | Yes | Yes | Yes | Yes | Yes | N/A |
| Author and Year | Deviation in the Group with the Use of Surgical Guide | Deviation in the Group Where Implant Was Inserted Manually | ||
|---|---|---|---|---|
| No. of Implants | Values in mm | No. of Implants | Values in mm | |
| Suzuki and Suzuki, 2007 [34] | 120 | 2.0 ± 0.4 mm | 20 | 10.5 ± 3.5 mm |
| Rashid et al., 2021 [35] | 25 | 0.69 ± 0.02 mm | 25 | 1.44 ± 0.10 mm |
| Lingling Qiu et al., 2012 [38] | 20 | 0.28 ± 0.23 mm (mesiodistal) 0.33 ± 0.25 mm (vertical) | 10 | 0.81 ± 0.61 mm (mesiodistal) 0.78 ± 0.49 mm (vertical) |
| Author and Year | Deviation in the Group with the Use of Surgical Guide | Deviation in the Group Where Implant Was Inserted Manually or with Wire Guide | ||
|---|---|---|---|---|
| No. of Implants | Values in mm/Root Contact Rate | No. of Implants | Values in mm/Root Contact Rate | |
| Suzuki and Suzuki, 2007 [34] | 120 | 2.0 ± 0.4 mm | 20 | 10.5 ± 3.5 mm |
| Suzuki and Suzuki, 2007 [34] | 120 | 2.0 ± 0.4 mm | 20 | 5.3 ± 1.1 mm |
| Rashid et al., 2021 [35] | 25 | 0.69 ± 0.02 mm | 25 | 1.44 ± 0.10 mm |
| Mi-Ju Bae, 2013 [37] | 25 | 0.73 mm (0.24–2.07) | 20 | 1.28 mm (0.26–3.81) |
| Lingling Qiu et al., 2012 [38] | 20 | 0.28 ± 0.23 mm (mesiodistal) 0.33 ± 0.25 mm (vertical) | 10 | 0.81 ± 0.61 mm (mesiodistal) 0.78 ± 0.49 mm (vertical) |
| Author and Year | Deviation in the Group with a Tooth-Borne Surgical Guide | Deviation in the Group with a Mucosa-Borne Surgical Guide | ||
|---|---|---|---|---|
| No. of Implants | Values in Linear Deviation in mm | No. of Implants | Values in Linear Deviation in mm | |
| Möhlhenrich et al. 2019 [39] | 20 | 0.88 ±0.46 mm | 20 | 1.65 ± 1.03 mm |
| Möhlhenrich et al. 2020 [40] | 20 | 1.7 ± 1.2 mm | 20 | 1.6 ± 1.5 mm |
| Kniha et al. 2020 [41] | 20 | 0.10 ± 0.46 mm | 20 | 0.22 ± 0.58 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedliński, M.; Janiszewska-Olszowska, J.; Mazur, M.; Ottolenghi, L.; Grocholewicz, K.; Galluccio, G. Guided Insertion of Temporary Anchorage Device in Form of Orthodontic Titanium Miniscrews with Customized 3D Templates—A Systematic Review with Meta-Analysis of Clinical Studies. Coatings 2021, 11, 1488. https://doi.org/10.3390/coatings11121488
Jedliński M, Janiszewska-Olszowska J, Mazur M, Ottolenghi L, Grocholewicz K, Galluccio G. Guided Insertion of Temporary Anchorage Device in Form of Orthodontic Titanium Miniscrews with Customized 3D Templates—A Systematic Review with Meta-Analysis of Clinical Studies. Coatings. 2021; 11(12):1488. https://doi.org/10.3390/coatings11121488
Chicago/Turabian StyleJedliński, Maciej, Joanna Janiszewska-Olszowska, Marta Mazur, Livia Ottolenghi, Katarzyna Grocholewicz, and Gabriella Galluccio. 2021. "Guided Insertion of Temporary Anchorage Device in Form of Orthodontic Titanium Miniscrews with Customized 3D Templates—A Systematic Review with Meta-Analysis of Clinical Studies" Coatings 11, no. 12: 1488. https://doi.org/10.3390/coatings11121488
APA StyleJedliński, M., Janiszewska-Olszowska, J., Mazur, M., Ottolenghi, L., Grocholewicz, K., & Galluccio, G. (2021). Guided Insertion of Temporary Anchorage Device in Form of Orthodontic Titanium Miniscrews with Customized 3D Templates—A Systematic Review with Meta-Analysis of Clinical Studies. Coatings, 11(12), 1488. https://doi.org/10.3390/coatings11121488










