Deuterium Retention and Release Behavior from Beryllium Co-Deposited Layers at Distinct Ar/D Ratio
Abstract
1. Introduction
2. Materials and Methods
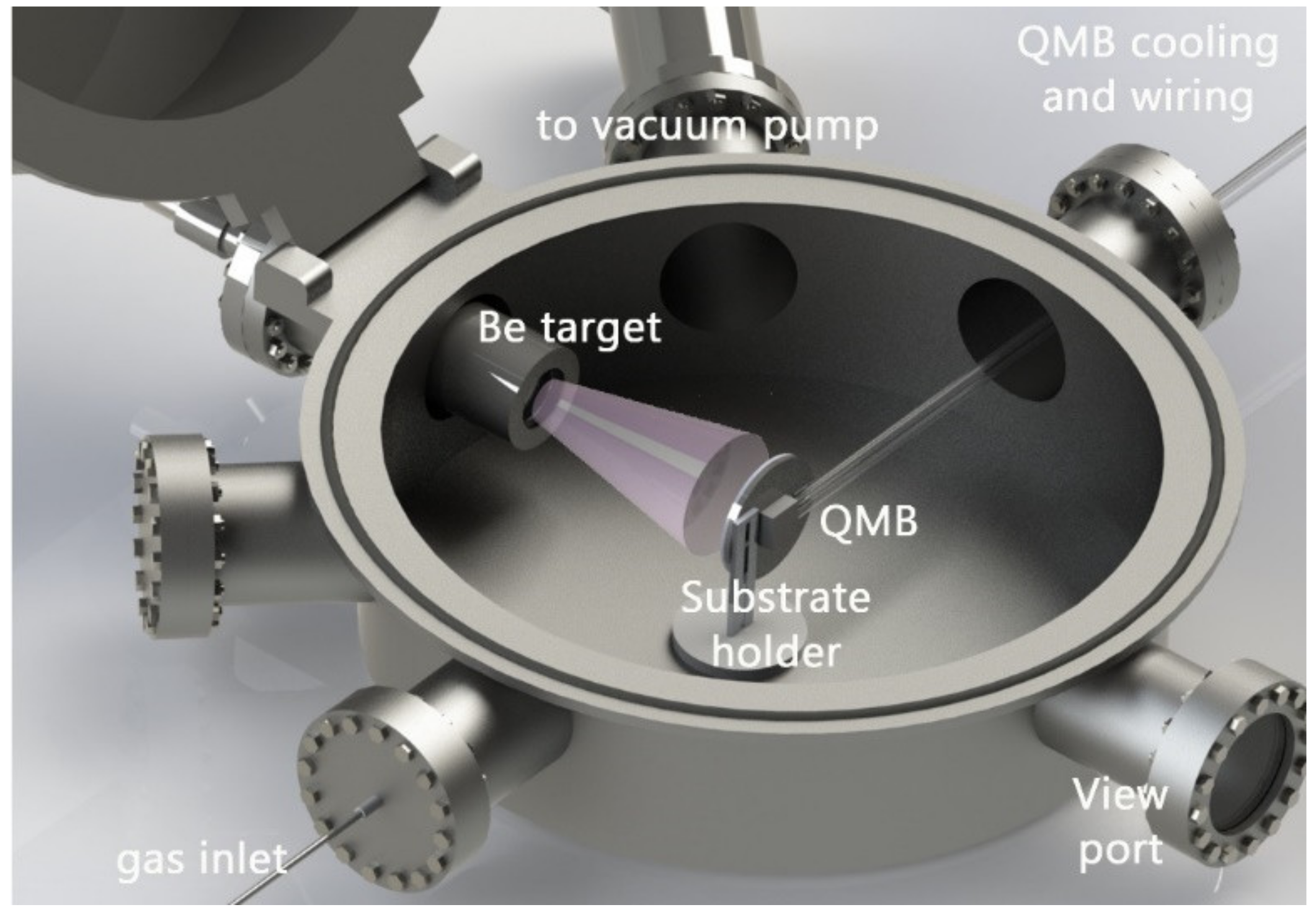
2.1. Layer Deposition
2.2. Layer Analysis
3. Results and Discussion
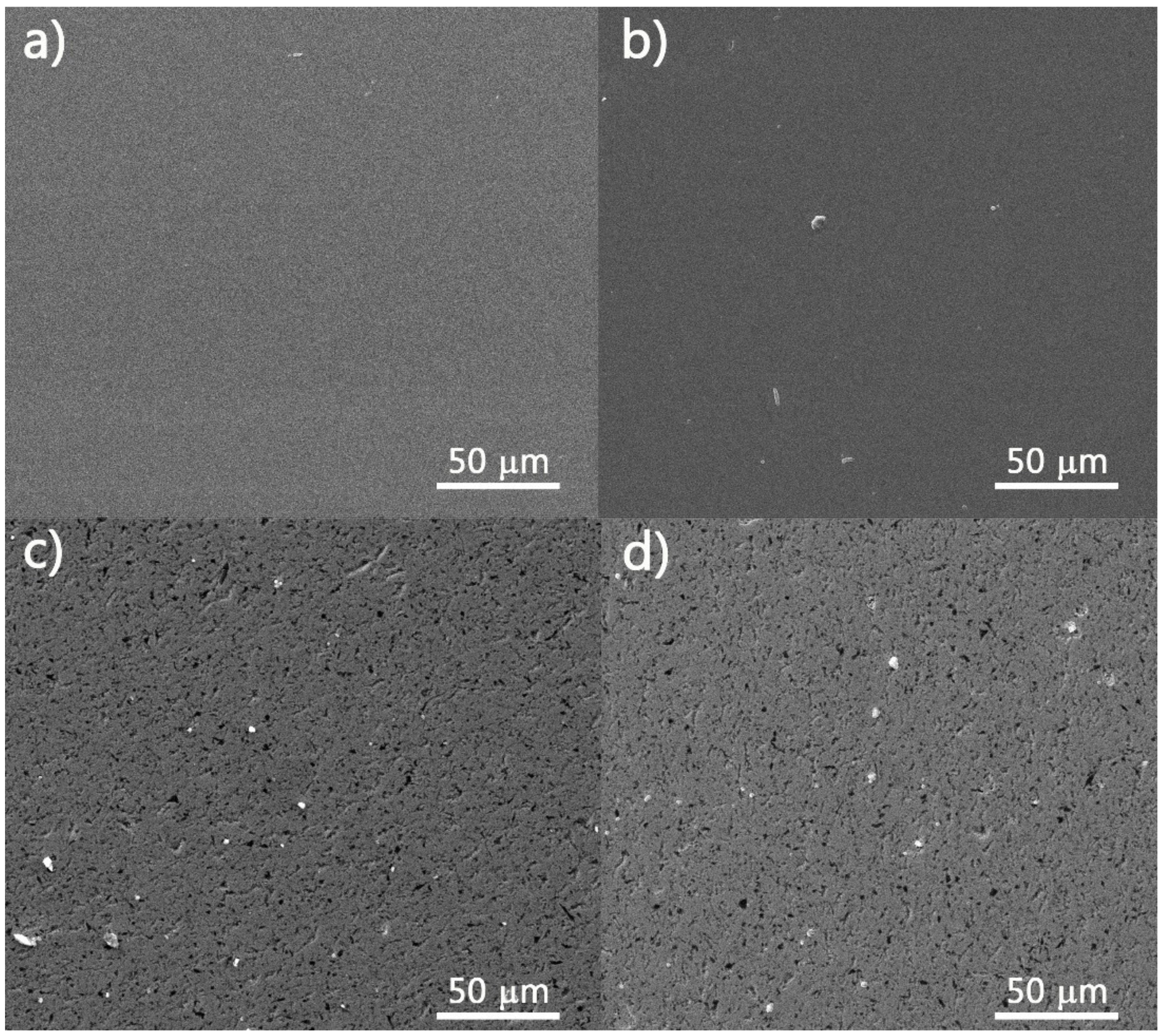
3.1. Layers Morphology
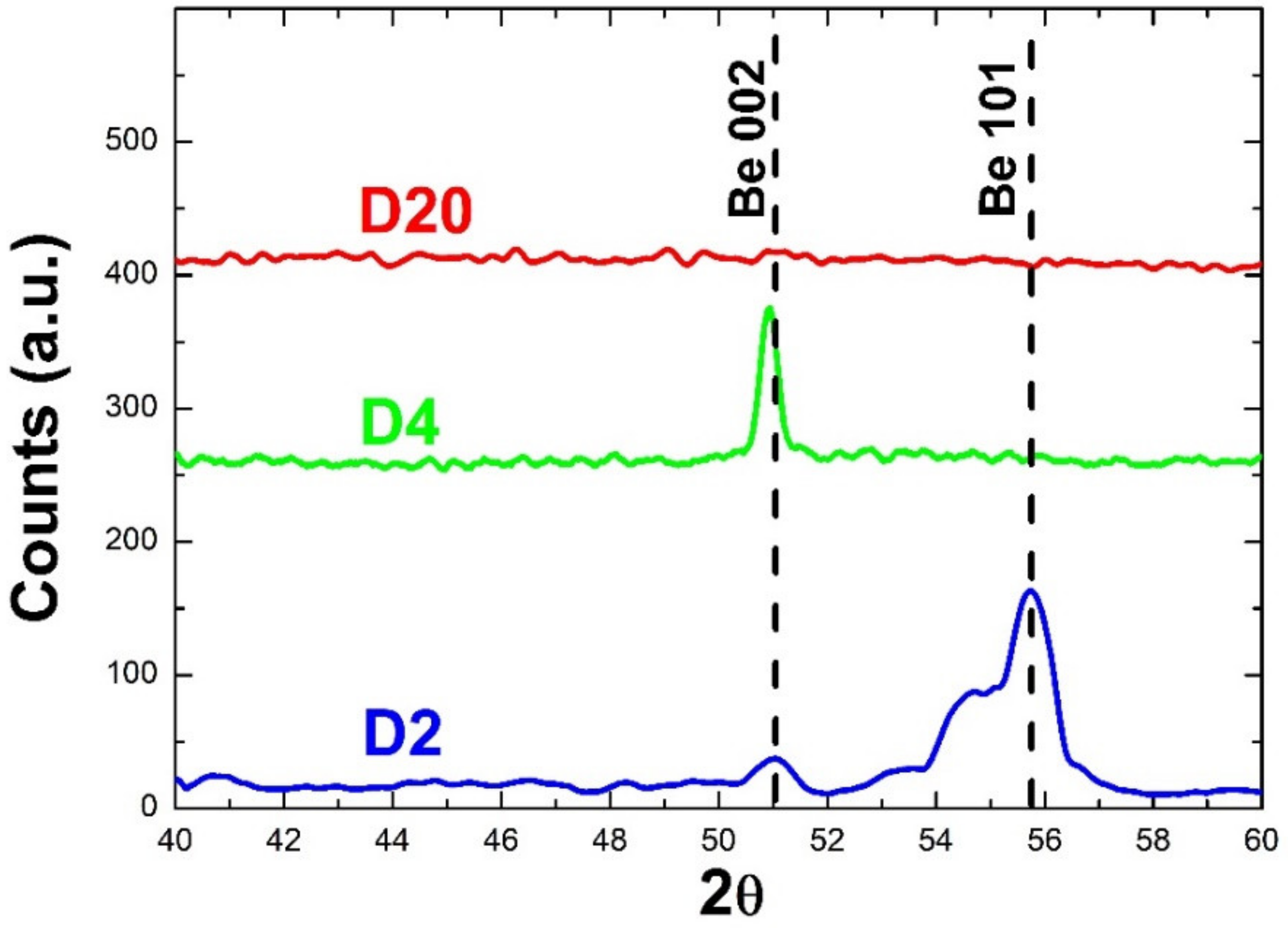
3.2. Layers Crystalline Structure
3.3. Layers Composition
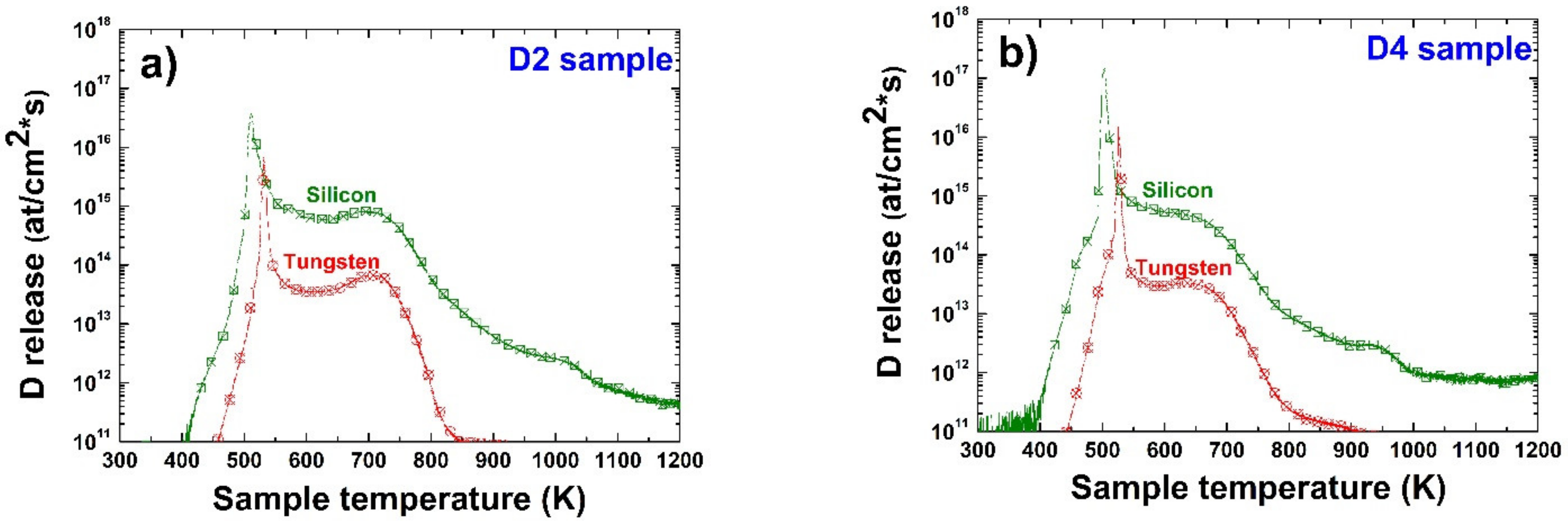
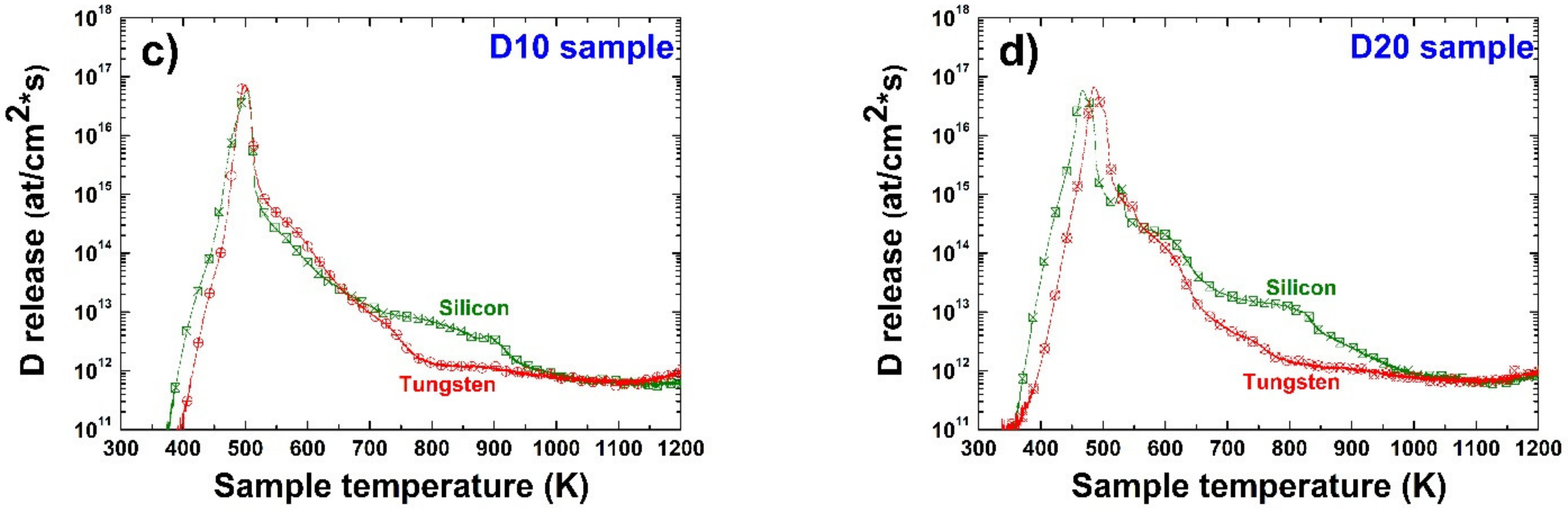
3.4. Deuterium Desorption and Thermal Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rubel, M.; Philipps, V.; Marot, L.; Petersson, P.; Pospieszczyk, A.; Schweer, B. Nitrogen and neon retention in plasma-facing materials. Proc. J. Nucl. Mater. 2011, 415, S223–S226. [Google Scholar] [CrossRef][Green Version]
- Undp, A. Human Development Report 2016: Human Development for Everyone; United Nations Development Programme: New York, NY, USA, 2016. [Google Scholar]
- Mayer, M.; Krat, S.; Baron-Wiechec, A.; Gasparyan, Y.; Heinola, K.; Koivuranta, S.; Likonen, J.; Ruset, C.; De Saint-Aubin, G.; Widdowson, A. Erosion and deposition in the JET divertor during the second ITER-like wall campaign. Proc. Phys. Scr. 2017, 2017, 014058. [Google Scholar] [CrossRef]
- Widdowson, A.; Coad, J.P.; Alves, E.; Baron-Wiechec, A.; Catarino, N.; Corregidor, V.; Heinola, K.; Krat, S.; Makepeace, C.; Matthews, G.F.; et al. Deposition of impurity metals during campaigns with the JET ITER-like Wall. Nucl. Mater. Energy 2019, 19, 218–224. [Google Scholar] [CrossRef]
- Taylor, N.; Alejaldre, C.; Cortes, P. Progress in the safety and licensing of ITER. Fusion Sci. Technol. 2013, 64, 111–117. [Google Scholar] [CrossRef]
- Federici, G.; Anderl, R.A.; Andrew, P.; Brooks, J.N.; Causey, R.A.; Coad, J.P.; Cowgill, D.; Doerner, R.P.; Haasz, A.A.; Janeschitz, G.; et al. In-vessel tritium retention and removal in ITER. J. Nucl. Mater. 1999, 266, 14–29. [Google Scholar] [CrossRef]
- Federici, G.; Andrew, P.; Barabaschi, P.; Brooks, J.; Doerner, R.; Geier, A.; Herrmann, A.; Janeschitz, G.; Krieger, K.; Kukushkin, A.; et al. Key ITER plasma edge and plasma-material interaction issues. Proc. J. Nucl. Mater. 2003, 313, 11–22. [Google Scholar] [CrossRef]
- Parker, R.R. ITER in-vessel system design and performance. Nucl. Fusion 2000, 40, 473. [Google Scholar] [CrossRef]
- Pitts, R.A.; Carpentier, S.; Escourbiac, F.; Hirai, T.; Komarov, V.; Lisgo, S.; Kukushkin, A.S.; Loarte, A.; Merola, M.; Sashala Naik, A.; et al. A full tungsten divertor for ITER: Physics issues and design status. J. Nucl. Mater. 2013, 438, S48–S56. [Google Scholar] [CrossRef]
- Causey, R.A. Hydrogen isotope retention and recycling in fusion reactor plasma-facing components. J. Nucl. Mater. 2002, 300, 91–117. [Google Scholar] [CrossRef]
- Hirai, T.; Linke, J.; Sundelin, P.; Rubel, M.; Kühnlein, W.; Wessel, E.; Coad, J.P.; Lungu, C.P.; Matthews, G.F.; Pedrick, L.; et al. Characterization and heat flux testing of beryllium coatings on Inconel for JET ITER-like wall project. Proc. Phys. Scr. 2007, T128, 166. [Google Scholar] [CrossRef]
- Thomser, C.; Bailescu, V.; Brezinsek, S.; Coenen, J.W.; Greuner, H.; Hirai, T.; Linke, J.; Lungu, C.P.; Maier, H.; Matthews, G.; et al. Plasma facing materials for the jet iter-like wall. Proc. Fusion Sci. Technol. 2012, 62, 1–8. [Google Scholar] [CrossRef]
- Raffray, A.R.; Merola, M. Overview of the design and R&D of the ITER blanket system. Proc. Fusion Eng. Des. 2012, 87, 769–776. [Google Scholar]
- Skinner, C.H.; Haasz, A.A.; Almov, V.K.H.; Bekris, N.; Causey, R.A.; Clark, R.E.H.; Coad, J.P.; Davis, J.W.; Doerner, R.P.; Mayer, M.; et al. Recent advances on hydrogen retention in iter’s plasma-facing materials: Beryllium, carbon. And tungsten. Fusion Sci. Technol. 2008, 54, 891–945. [Google Scholar] [CrossRef]
- Roth, J.; Tsitrone, E.; Loarte, A.; Loarer, T.; Counsell, G.; Neu, R.; Philipps, V.; Brezinsek, S.; Lehnen, M.; Coad, P.; et al. Recent analysis of key plasma wall interactions issues for ITER. J. Nucl. Mater. 2009, 390, 1–9. [Google Scholar] [CrossRef]
- Federici, G.; Doerner, R.; Lorenzetto, P.; Barabash, V. Beryllium as a plasma-facing material for near-term fusion devices. Compr. Nucl. Mater. 2012, 4, 110–119. [Google Scholar]
- Widdowson, A.; Coad, J.P.; Alves, E.; Baron-Wiechec, A.; Barradas, N.P.; Brezinsek, S.; Catarino, N.; Corregidor, V.; Heinola, K.; Koivuranta, S.; et al. Overview of fuel inventory in JET with the ITER-like wall. Nucl. Fusion 2017, 57, 086045. [Google Scholar] [CrossRef]
- Khan, A.; De Temmerman, G.; Lisgo, S.W.; Bonnin, X.; Anand, H.; Miller, M.A.; Pitts, R.A.; Schmid, K.; Kukushkin, A.S. WallDYN simulations of material migration and fuel retention in ITER low power H plasmas and high power neon-seeded DT plasmas. Nucl. Mater. Energy 2019, 20, 100674. [Google Scholar] [CrossRef]
- Counsell, G.; Coad, P.; Grisola, C.; Hopf, C.; Jacob, W.; Kirschner, A.; Kreter, A.; Krieger, K.; Likonen, J.; Philipps, V.; et al. Tritium retention in next step devices and the requirements for mitigation and removal techniques. Plasma Phys. Control Fusion 2006, 48, B189. [Google Scholar] [CrossRef]
- De Temmerman, G.; Baldwin, M.J.; Doerner, R.P.; Nishijima, D.; Schmid, K. An empirical scaling for deuterium retention in co-deposited beryllium layers. Nucl. Fusion 2008, 48, 075008. [Google Scholar] [CrossRef]
- De Temmerman, G.; Doerner, R.P. Revised scaling equation for the prediction of tritium retention in beryllium co-deposited layers. Nucl. Fusion 2009, 49, 042002. [Google Scholar] [CrossRef]
- Reinelt, M.; Linsmeier, C. Temperature programmed desorption of 1 keV deuterium implanted into clean beryllium. Proc. Phys. Scr. T 2007, T128, 111. [Google Scholar] [CrossRef]
- Mayer, M. SIMNRA User’s Guide; Technical Report IPP, 9/113; Max-Planck Institut fur Plasmaphysik: Garching, Germany, 1997. [Google Scholar]
- Dinca, P.; Butoi, B.; Porosnicu, C.; Pompilian, O.G.; Staicu, C.; Lungu, C.P.; Burducea, I. Structure, morphology and deuterium retention and release properties of pure and mixed Be and W layers. J. Phys. D Appl. Phys. 2020, 53, 325304. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Xu, K. Effect of substrate on surface morphology evolution of Cu thin films deposited by magnetron sputtering. Surf. Coat. Technol. 2007, 201, 5574–5577. [Google Scholar] [CrossRef]
- Zibrov, M.S.; Baldwin, M.J.; Mayer, M.; Nguyen, H.Q.; Brezinsek, S.; Doerner, R.P. Deuterium retention in mixed Be-W-D codeposited layers. Nucl. Fusion 2020, 60, 126005. [Google Scholar] [CrossRef]
- Markin, A.V.; Chernikov, V.N.; Rybakov, S.Y.; Zakharov, A.P. Thermal desorption of deuterium implanted into beryllium. J. Nucl. Mater. 1996, 233, 865–869. [Google Scholar] [CrossRef]
- Reinelt, M.; Allouche, A.; Oberkofler, M.; Linsmeier, C. Retention mechanisms and binding states of deuterium implanted into beryllium. New J. Phys. 2009, 11, 043023. [Google Scholar] [CrossRef]
- Doerner, R.P.; Baldwin, M.J.; Buchenauer, D.; De Temmerman, G.; Nishijima, D. The role of beryllium deuteride in plasma-beryllium interactions. J. Nucl. Mater. 2009, 390, 681–684. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Doerner, R.P. Effect of layer thickness on the thermal release from Be-D co-deposited layers. Nucl. Fusion 2014, 54, 083032. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, H.W.; Xu, J.; Chai, L.Q.; Hu, M.; Wang, P. Deuterium retention and release behaviours of tungsten and deuterium co-deposited layers. J. Nucl. Mater. 2018, 502, 247–254. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Založnik, A.; Smirnov, R.D.; Doerner, R.P. Experimental measurements and modeling of the deuterium release from tungsten co-deposited layers. Nucl. Mater. Energy 2020, 23, 100743. [Google Scholar] [CrossRef]


| Sample Index | Input Power (W) | Ar Flow (sccm) | D Flow (sccm) | Deposition Rate (nm/s) |
|---|---|---|---|---|
| D2 | 175 | 20 | 2 | 0.06 |
| D4 | 175 | 20 | 4 | 0.06 |
| D10 | 175 | 20 | 10 | 0.06 |
| D20 | 175 | 20 | 20 | 0.06 |
| Sample Index | Be (at%) | O (at%) | D (at%) | Thickness (nm) |
|---|---|---|---|---|
| D2 | 85.36 | 2.43 | 12.19 | 581 |
| D4 | 84.70 | 2.35 | 12.95 | 598 |
| D10 | 75.00 | 2.27 | 22.72 | 548 |
| D20 | 71.40 | 2.61 | 26.19 | 498 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinca, P.; Staicu, C.; Porosnicu, C.; Pompilian, O.G.; Banici, A.-M.; Butoi, B.; Lungu, C.P.; Burducea, I. Deuterium Retention and Release Behavior from Beryllium Co-Deposited Layers at Distinct Ar/D Ratio. Coatings 2021, 11, 1443. https://doi.org/10.3390/coatings11121443
Dinca P, Staicu C, Porosnicu C, Pompilian OG, Banici A-M, Butoi B, Lungu CP, Burducea I. Deuterium Retention and Release Behavior from Beryllium Co-Deposited Layers at Distinct Ar/D Ratio. Coatings. 2021; 11(12):1443. https://doi.org/10.3390/coatings11121443
Chicago/Turabian StyleDinca, Paul, Cornel Staicu, Corneliu Porosnicu, Oana G. Pompilian, Ana-Maria Banici, Bogdan Butoi, Cristian P. Lungu, and Ion Burducea. 2021. "Deuterium Retention and Release Behavior from Beryllium Co-Deposited Layers at Distinct Ar/D Ratio" Coatings 11, no. 12: 1443. https://doi.org/10.3390/coatings11121443
APA StyleDinca, P., Staicu, C., Porosnicu, C., Pompilian, O. G., Banici, A.-M., Butoi, B., Lungu, C. P., & Burducea, I. (2021). Deuterium Retention and Release Behavior from Beryllium Co-Deposited Layers at Distinct Ar/D Ratio. Coatings, 11(12), 1443. https://doi.org/10.3390/coatings11121443







