1. Introduction
In recent years, with the development of aviation, aerospace, electronics, and transportation industries, higher requirements are placed on the strength, hardness, and wear resistance of engineering materials. For example, engine cylinders operate at high-temperatures and in high-pressure environments for a long time, often resulting in wear failure due to insufficient lubrication, which affects the overall service life of the engine [
1,
2]. An insufficient surface protection performance of metal parts decreases the overall service life of mechanical products. Electrodeposition is a common way to prepare composite coatings that can protect material surfaces and help repair worn surfaces; this method is cost effective and has an excellent performance [
3,
4,
5].
In the process of preparing composite coatings, Ni, Cu, and Zn have been used as common composite coating substrates, playing a decisive role in the performance of composite coatings [
6,
7,
8]. Among them, a pure Ni substrate has been widely studied and applied owing to its good mechanical properties and chemical stability. However, in some complex environments, a pure Ni substrate cannot meet the requirements [
9]. Therefore, combining two or more metallic or nonmetallic materials into a new composite material through a certain process can help improve the performance [
10,
11,
12]. For example, adding transition metal elements to pure Ni coatings, such as Mn, can help form Ni–Mn alloy coatings and improve the surface morphology and mechanical and chemical properties of coatings [
13,
14,
15]. The excellent properties of Ni–Mn alloys have attracted wide attention from researchers in the field of materials. Wang and Zhang [
16] found that electrodeposited Ni–Mn alloys have good high-temperature stabilities, with elongations 4–6 times that of pure Ni. Pan [
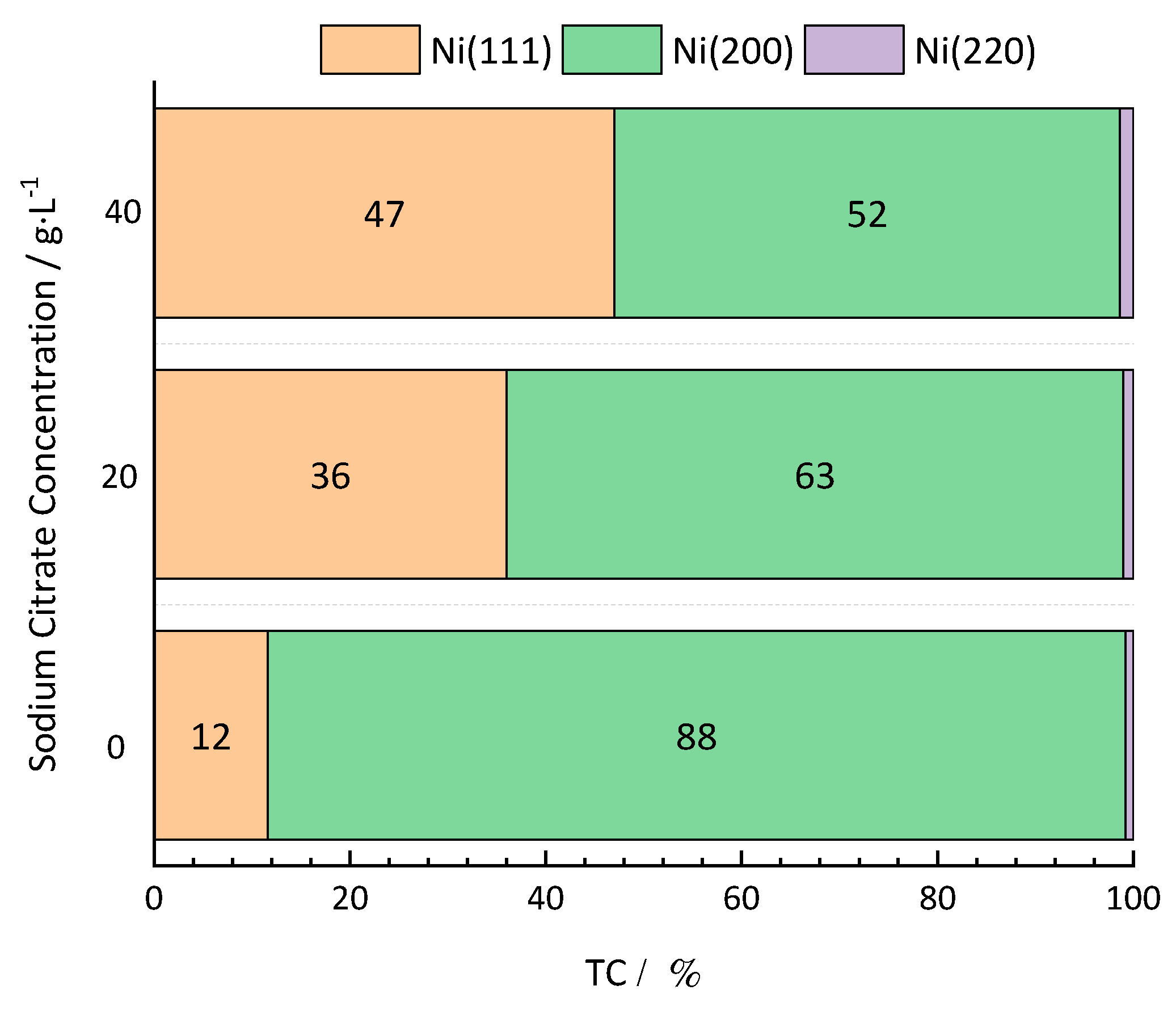
17] studied Ni–Mn alloy electrodeposition and showed that the composition and morphology of alloys deposited under different electrodeposition conditions differ, as well as their electrocrystallization, follows a 3D continuous nucleation mechanism. The addition of Mn increases the nucleation rate constant of electrocrystallization, and the growth rate and diffusion coefficient of nickel ions decrease. During electrodeposition, monometallic Mn, as an induced codeposition metal, precipitates with iron transition metals such as Fe, Co, and Ni. However, the precipitation potentials of Ni and Mn are quite different. In an actual production process, complexing agents, such as citric acid, sodium citrate, and disodium oxalate, are typically used to narrow the precipitation potential of Ni
2+ and Mn
2+ and increase the element contents in the coatings [
18,
19].
By adding reinforcing-phase particles to an alloy substrate, a composite plating layer can be prepared with excellent properties. The properties and concentration of the reinforcing-phase particles also significantly influence the main properties such as the strength and corrosion resistance of coatings. Guo [
20] prepared Ni–ultra-high molecular weight polyethylene (UHMWPE) composite coatings using a two-step method and explored the strengthening mechanism of its strength and tribological properties. The results showed that, on the one hand, the addition of UHMWPE particles reduced the microhardness of the coating, which easily deforms during friction and shear. On the other hand, the large number of UHMWPE particles on the contact surface can make the coating exhibit excellent self-lubricity, resistance and stickiness. The general trend of adding UHMWPE particles is to strengthen the wear resistance of the coating. The main reason is that the latter has a more significant effect. Zhang [
21] added BN(h) and Al
2O
3 nanoparticles to Ni–Co–P composite coatings to prepare binary nanocomposite coatings. The study showed that BN(h) nanoparticles have self-lubricating properties and can reduce the friction coefficient and improve the wear resistance of the coating. Al
2O
3 nanoparticles are hard ceramic particles which can significantly improve the mechanical strength and hardness of coatings, thereby improving the ability of the material to resist plastic deformation and improving the wear resistance of the coating. Some of the common reinforcing-phase particles used in composite electrodeposition are SiC, BN and TiN [
22]. Among them, SiC is a carbide ceramic material formed by the polymerization of fine crystals. Its main advantages are high hardness, good high-temperature resistance and chemical stability; as a reinforcing phase added to nickel-based alloy plating layers, SiC can effectively improve the microhardness of alloy coatings [
23].
Type 45 steel is widely used in mechanical products owing to its excellent cutting performance and low cost. However, the increasingly complex working environment places more stringent requirements on the surface protection of mechanical products, which can be effectively improved in terms of the performance and longevity by coating and plating. An Ni–Mn–SiC composite coating has good strength and wear resistance, which can significantly increase the service life of mechanical parts. Currently, there are few studies on the process parameters and performance of Ni–Mn–SiC composite coatings. Therefore, it is of great significance to discuss the surface and cross-sectional morphology, composition and phase structure, microhardness, surface roughness and wear resistance of Ni–Mn–SiC composite coatings. This study aims to improve the wear resistance of type 45 steel surface. Ni–Mn alloy coatings and Ni–Mn–SiC composite coatings were prepared on type 45 steel surface by electrodeposition. This paper explored the effects of sodium citrate concentration on the surface and cross-sectional morphology, composition, phase structure and wear resistance of the Ni–Mn alloy coatings, based on which different concentrations of SiC particles were added to the electrolyte to study the effects of SiC particle concentration on the surface and cross-sectional morphology, composition, phase structure, and wear resistance of the Ni–Mn–SiC composite coatings. This study is expected to aid the research and application of Ni-based composite coatings.
2. Materials and Methods
2.1. Coating Preparation
In the electrodeposition experiment, a graphite electrode plate with dimensions of 200 mm × 45 mm × 5 mm was selected for the anode and placed parallel to the cathode. Type 45 steel was used as the base metal with a sample size of 30 mm × 8 mm × 7 mm, and was ground using no. 320, no. 800 and no. 1500 wet-and-dry sandpapers successively. The sharp edges and corners in the sample were removed to prevent tip discharge from affecting the coating quality during the experiment. The sample was preprocessed before electrodeposition: degreasing → pickling → activation.
First, an experiment was conducted to explore the effect of sodium citrate concentration on the Ni–Mn alloy coatings and to determine the optimal sodium citrate concentration through a comprehensive performance comparison. Based on the results, we explored the effect of SiC particle concentration on the Ni–Mn–SiC composite coatings.
Table 1 lists the electrolyte composition, where the reagents used were all analytically pure and prepared with deionized water. The average particle size of the SiC particles was 20 nm. Before disposing the electrolyte, SiC particles were added to an appropriate amount of deionized water, stirred with ultrasonic waves for 30 min and left to stand for 24 h to make the particles fully wet. After the particles were fully wetted, they were placed in a prepared basic electrolyte and stirred again under ultrasonic waves for 30 min to make the particles evenly dispersed. The process parameters for preparing the coatings were as follows: The current density was 1.35 A/dm
2 for the Ni–Mn alloy coatings and 0.9 A/dm
2 for the Ni–Mn–SiC composite coatings. The electrolyte temperature was 60 °C, and the electrodeposition time was 60 min. Electrodeposition was carried out in a 500 mL beaker, which required a constant-temperature magnetic stirrer to make the electrolyte flow at a constant speed and ensure the temperature. Finally, the samples were cleaned using an ultrasonic machine, dried, and finally preserved for testing.
2.2. Characterization of Coating Performance
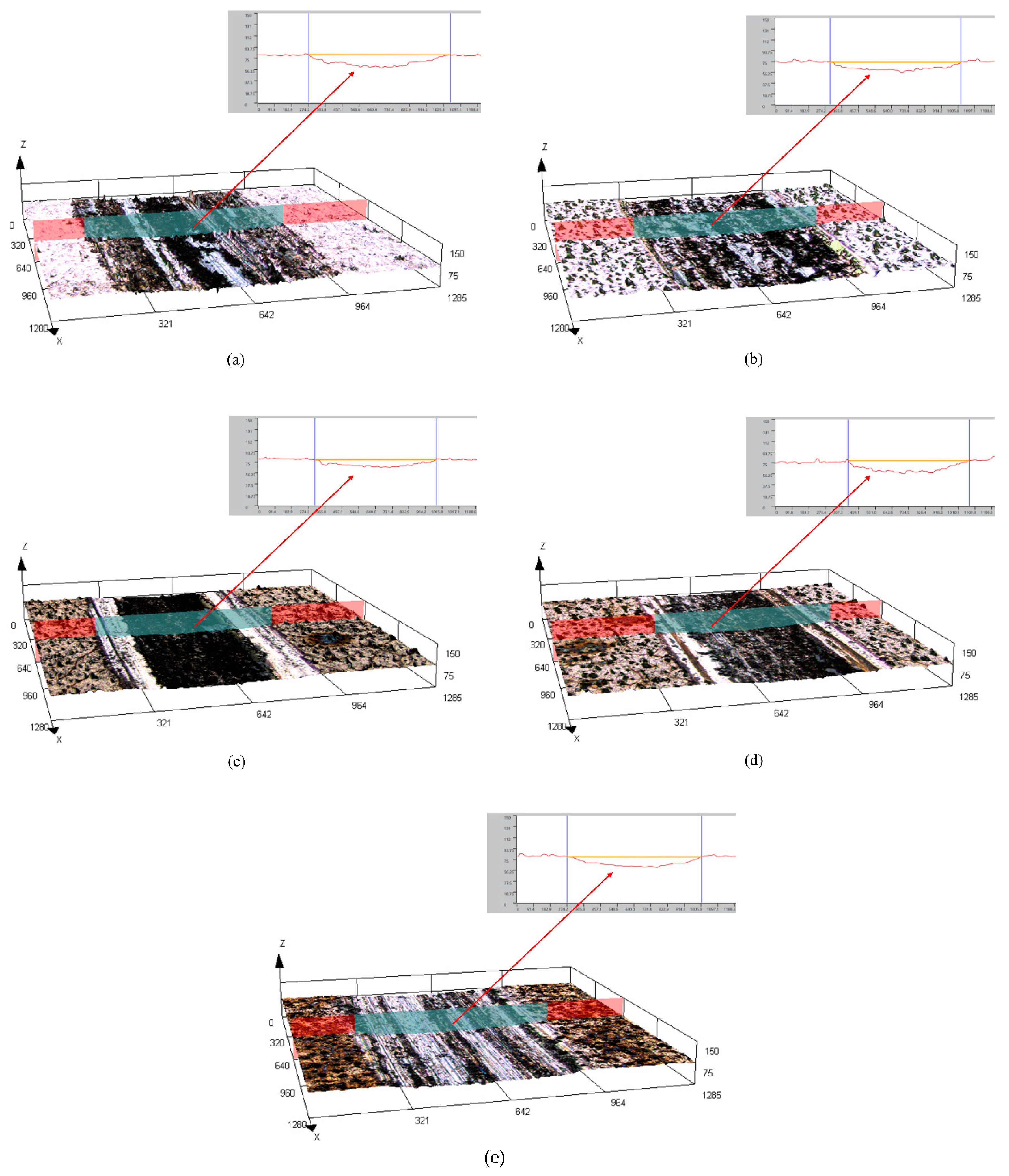
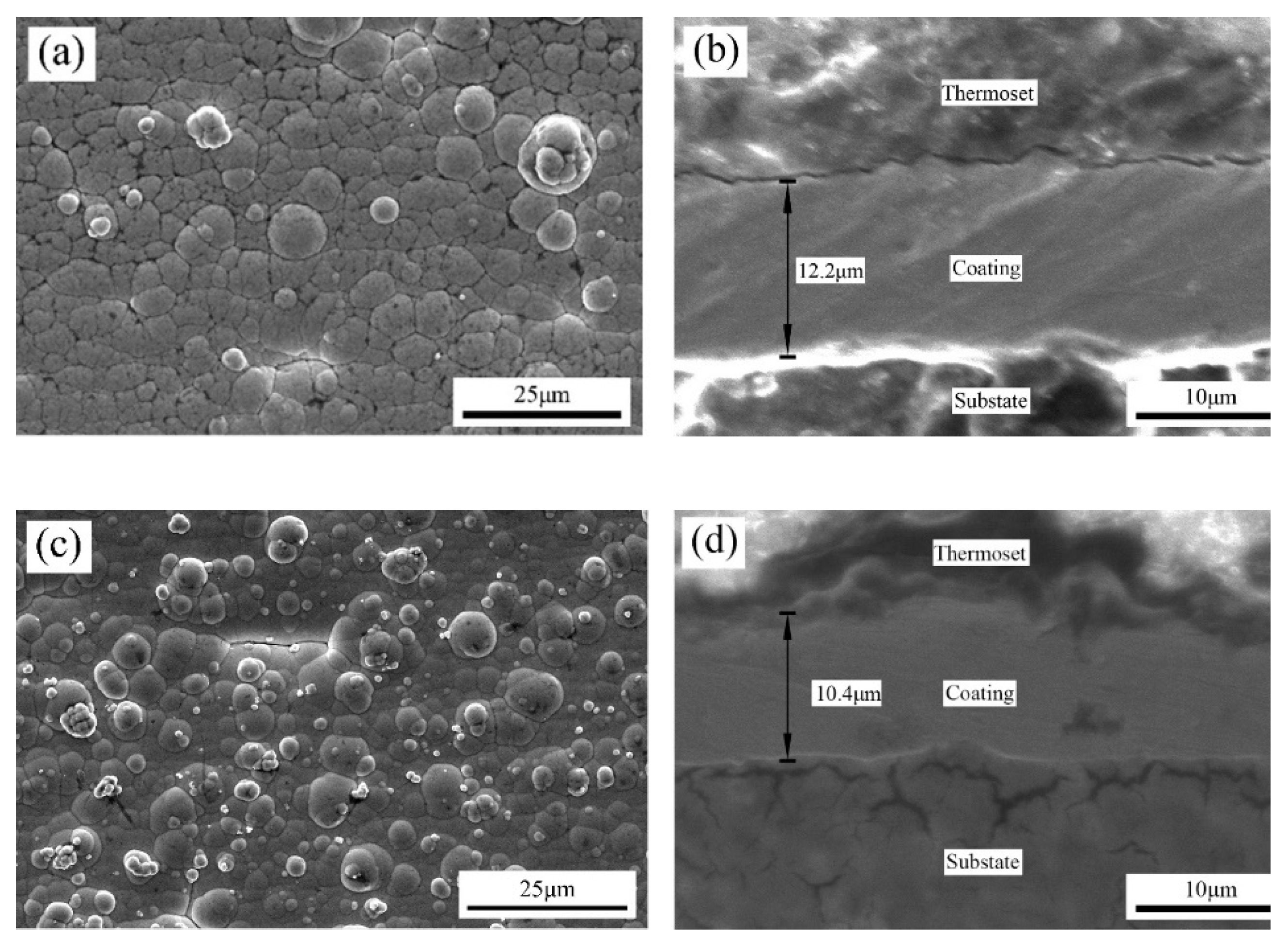
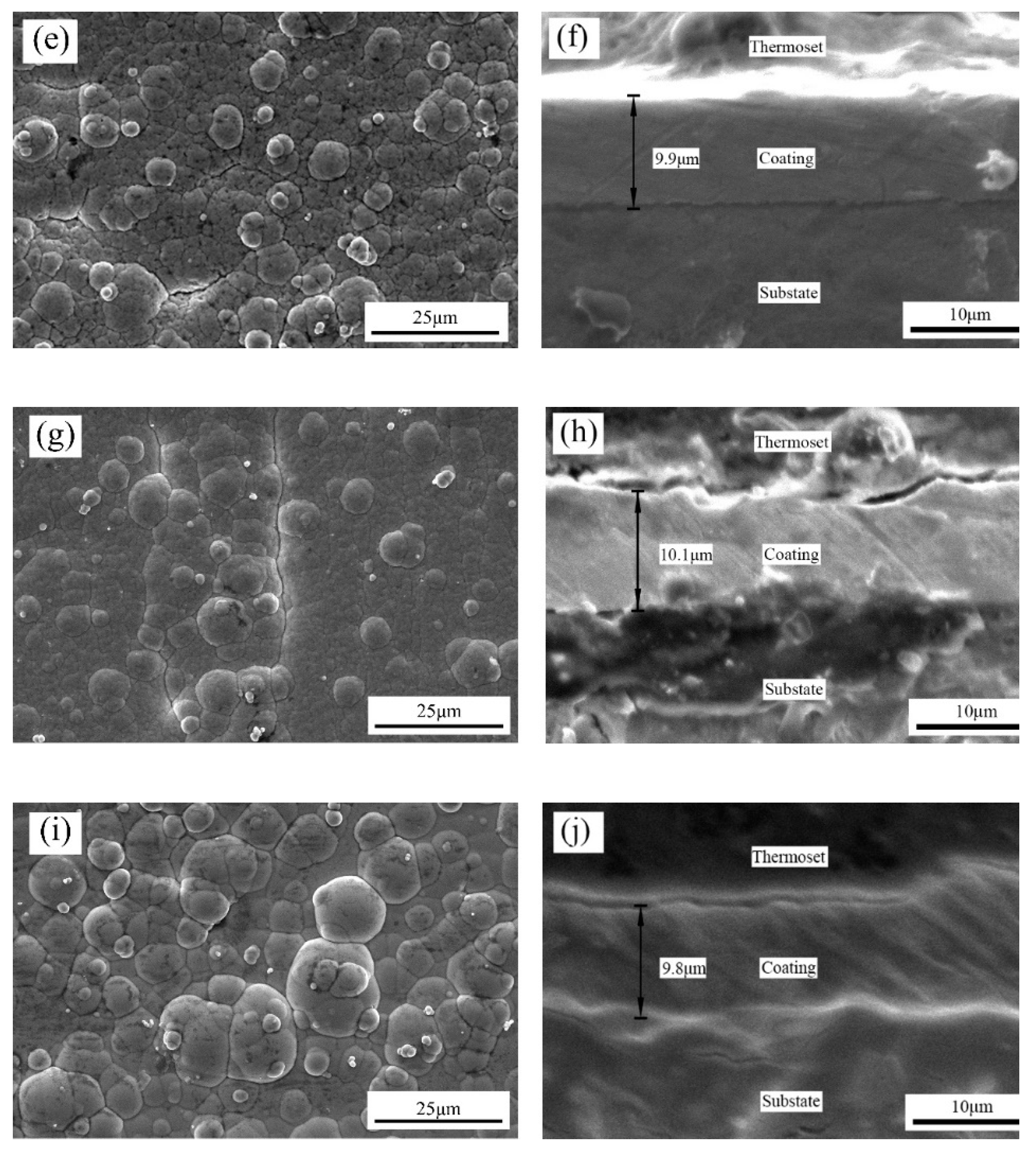
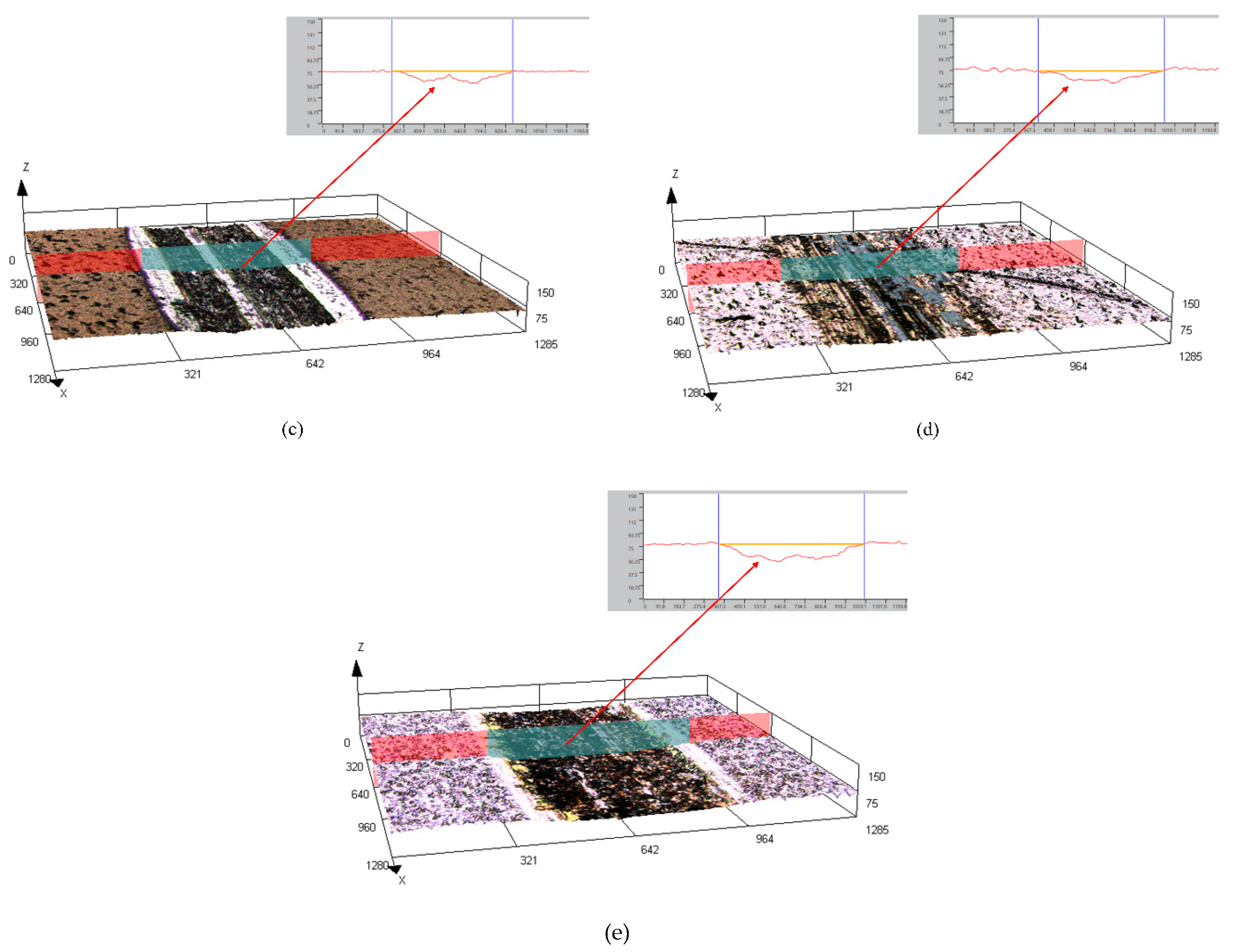
The surface and cross-sectional morphology, composition and phase structure of the coatings were characterized by scanning electron microscopy (SEM, FEI Instruments Inc., Hillsboro, OR, USA), energy dispersive spectroscopy (EDS, XFlash Detector 5030, Bruker AXS, Inc., Berlin, Germany) and X-ray diffraction (XRD, PANalytical X’pert, PANalytical, Inc., Almelo, Netherlands). The HighScore Plus software (version 3.0e) was used to analyze the XRD results. A microhardness tester (Duramin-40, Struers, Cleveland, OH, USA) was used to measure the microhardness of the coating surface, with a load of 50 g and a holding time of 10 s. Each sample was tested at seven different locations. The JB-4C surface roughness meter (Shanghai Optical Instrument, Shanghai, China) was used to measure the surface roughness of the coatings. The sampling length was 0.8 cm. Data were collected for each sample thrice, and the average value was finally obtained. The dry sliding friction and wear performance of the composite coatings were tested using a material surface performance comprehensive tester (CFT-1, Zhongke Kaihua, Lanzhou, China). The grinding parts were GCr15 alloy steel balls with diameters of 4 mm. The load was 330 g, the reciprocating sliding stroke was 5 mm, the reciprocating speed was 500 r/min, the wear time was 20 min and the sampling frequency was 1 Hz. A laser scanning confocal microscope (LSCM) (OLS4100, Olympus Corporation, Tokyo, Japan) was used to characterize the 3D morphology of the coatings after wear and to measure the volume and depth of the wear scar.
4. Conclusions
In this study, Ni–Mn alloy coatings and Ni–Mn–SiC composite coatings were electrodeposited on the surface of type 45 steel substrate under varying sodium citrate and SiC particle concentrations. The changes in the concentration of sodium citrate and SiC particles significantly influenced the surface morphology and mechanical properties of the deposited coatings, including:
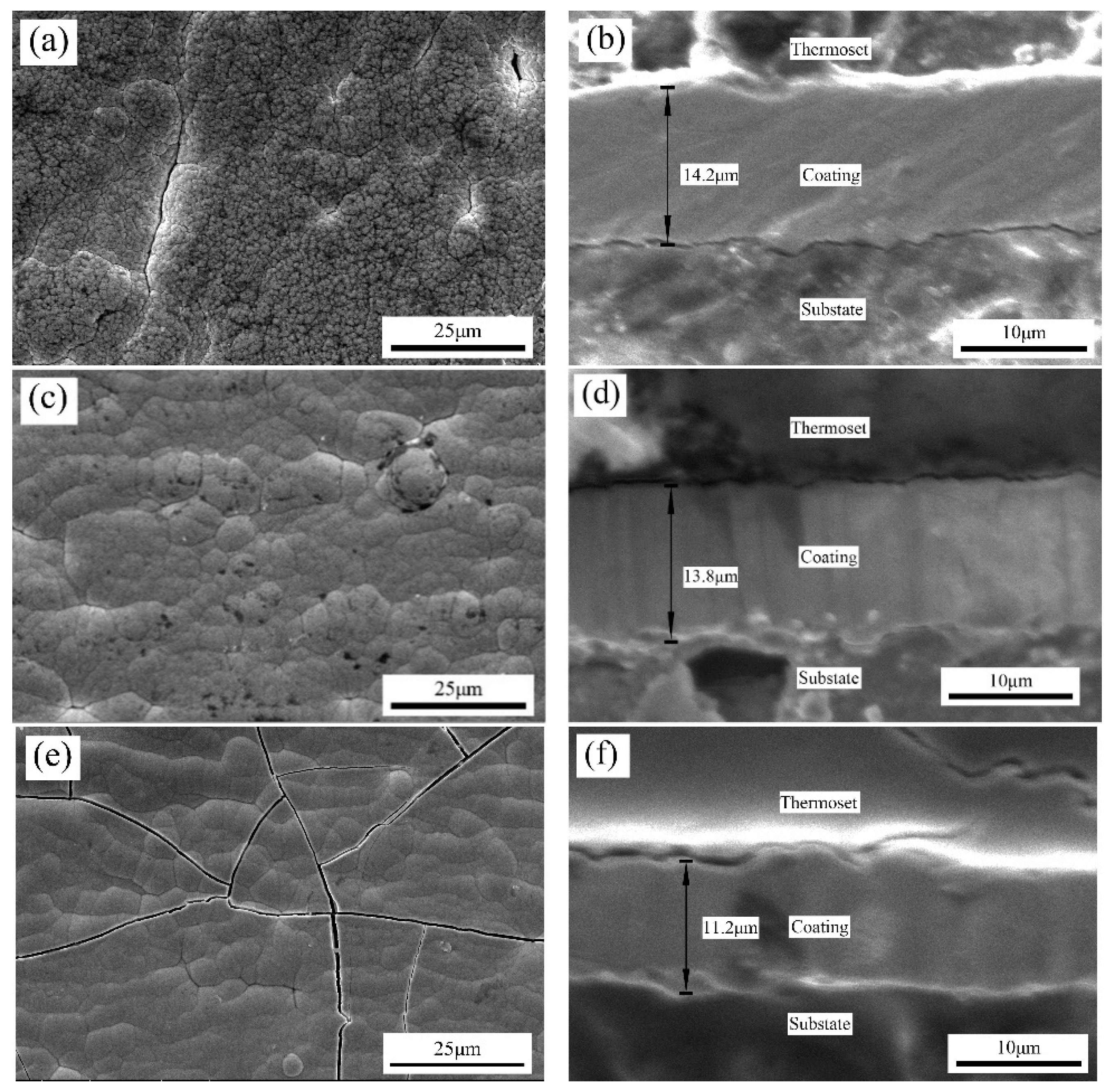
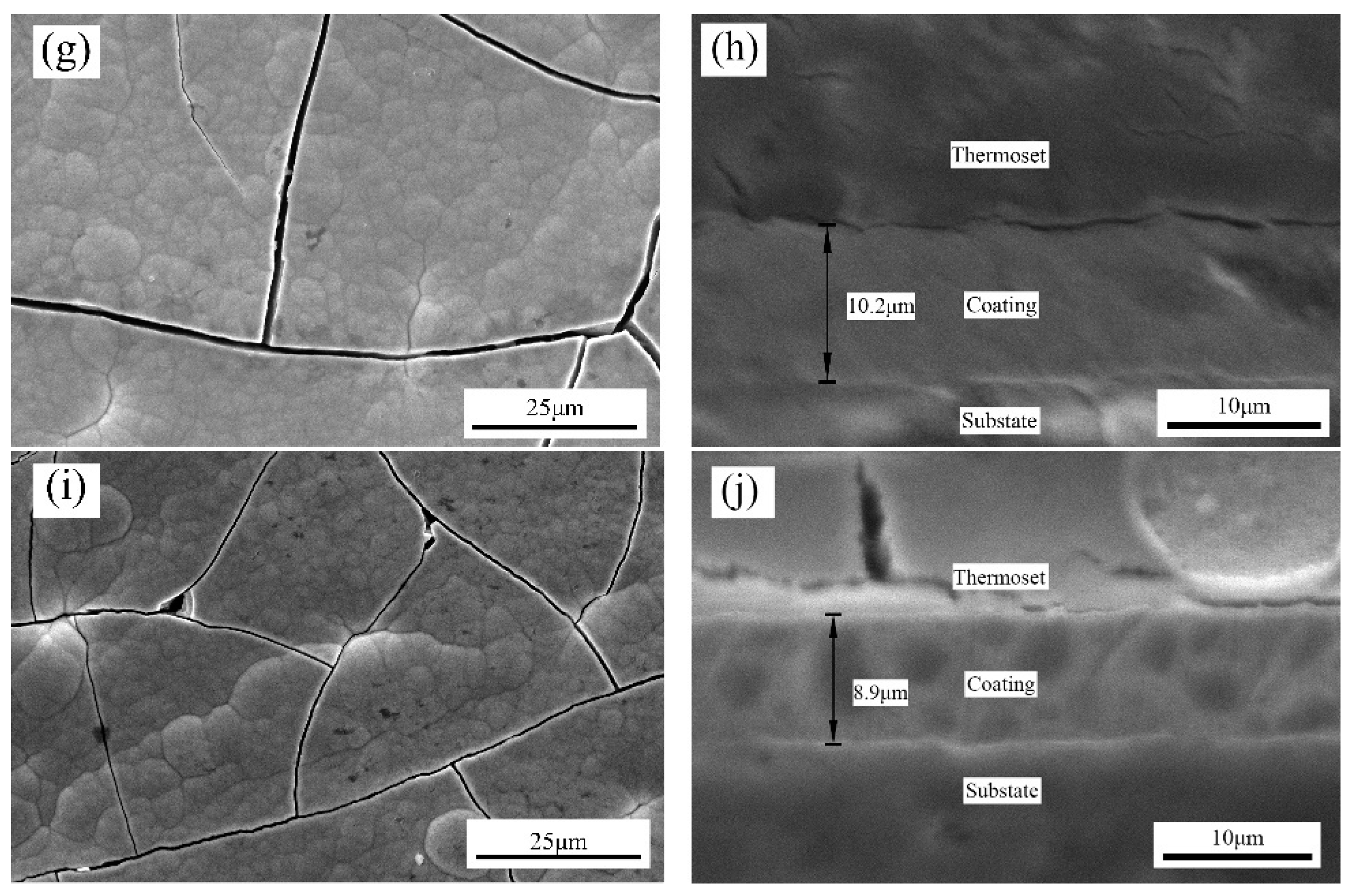
Sodium citrate promoted the grain refinement of the Ni–Mn alloy coatings and improved the coating density; however, it induced cracks due to the high residual stress. When the sodium citrate concentration was 40 g/L, the mechanical properties of the coatings were the best. When the sodium citrate concentration reached 60 g/L and above, the Ni–Mn coatings exhibited amorphous structures;
Based on the Ni–Mn alloy coatings, the addition of SiC particles could effectively reduce the residual stress, improve the surface integrity and further improve the microhardness and wear resistance of the composite coatings. The effect was best when the SiC particle concentration was 4 g/L.