Investigating the Nano-Films Effect on Physical, Mechanical Properties, Chemical Changes, and Microbial Load Contamination of White Button Mushrooms during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Treatments
2.2. Analyses
2.2.1. Weight Loss, Acidity, and Total Soluble Solids Concentrations
2.2.2. Color Investigation Measurements
2.2.3. Cap Opening Percentage
2.2.4. Firmness and Electrolyte Leakage Rate
2.2.5. Headspace Gas Composition and PPO Activity Analysis
2.2.6. Microbial Load Contamination
2.2.7. Statistical Analysis
3. Results and Dissection
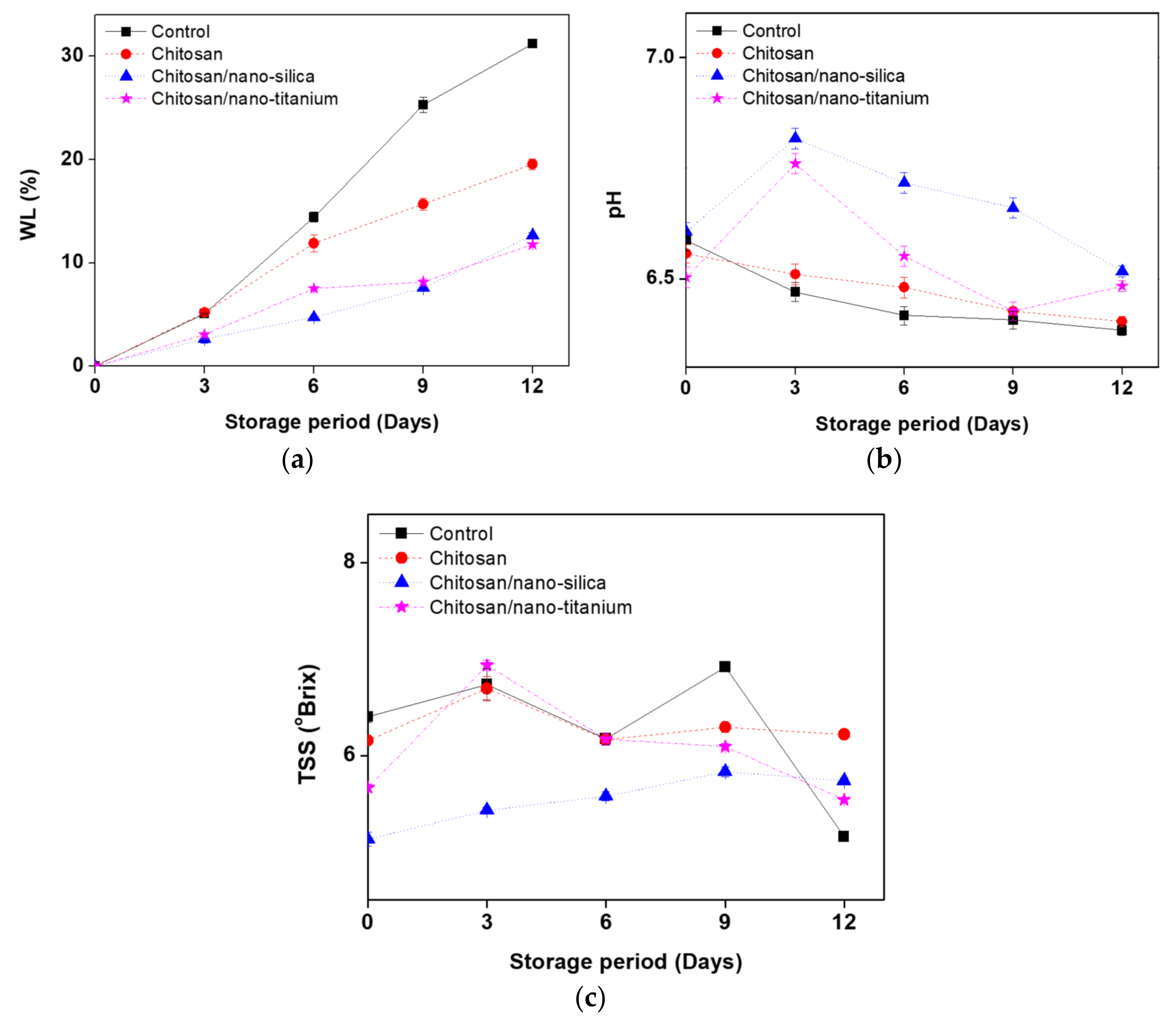
3.1. Weight Loss (WL), pH, TSS Changes
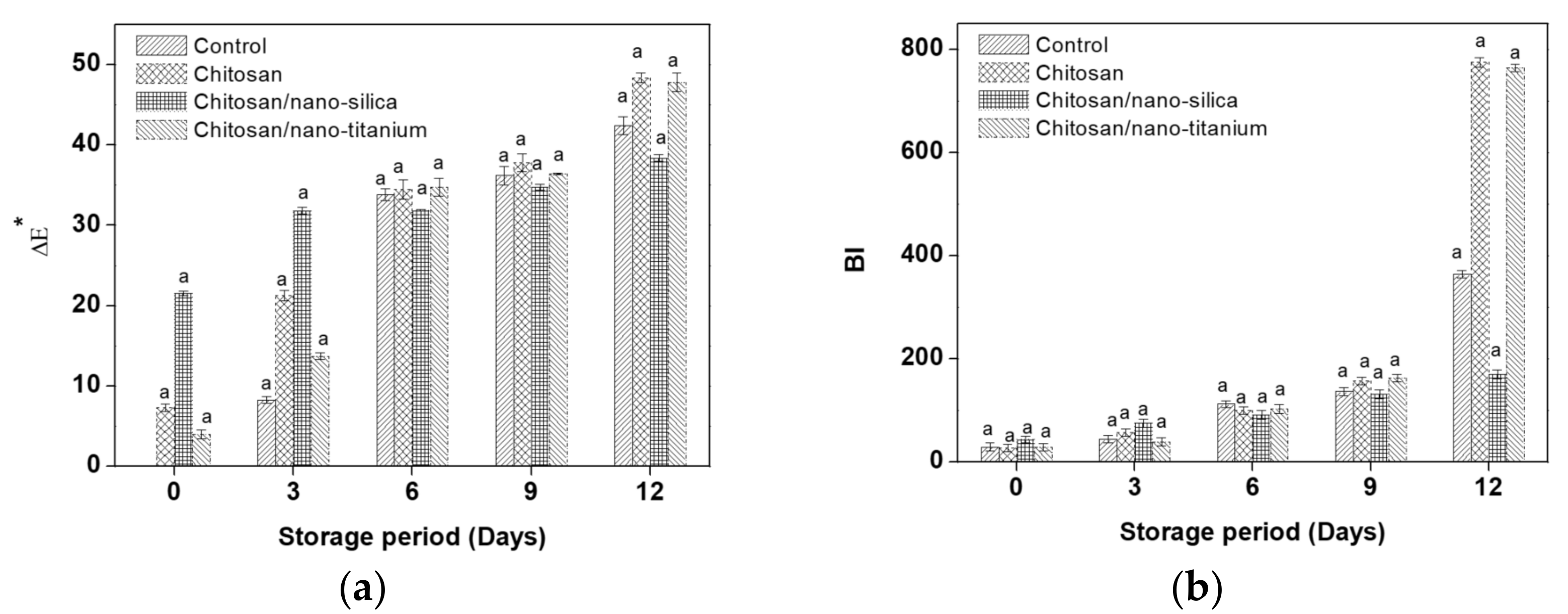
3.2. Colour Parameters Changes
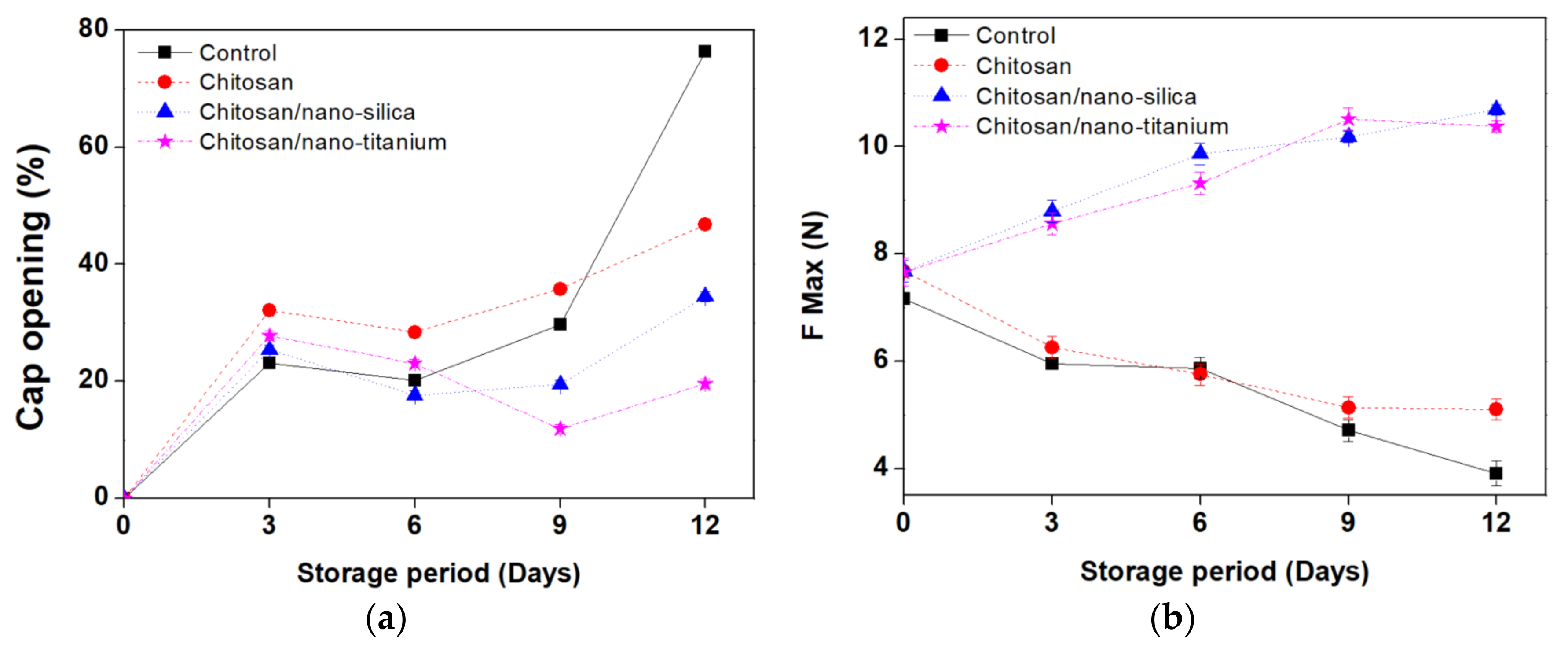
3.3. Cap Opening, Firmness, and Electrolyte Leakage Rate
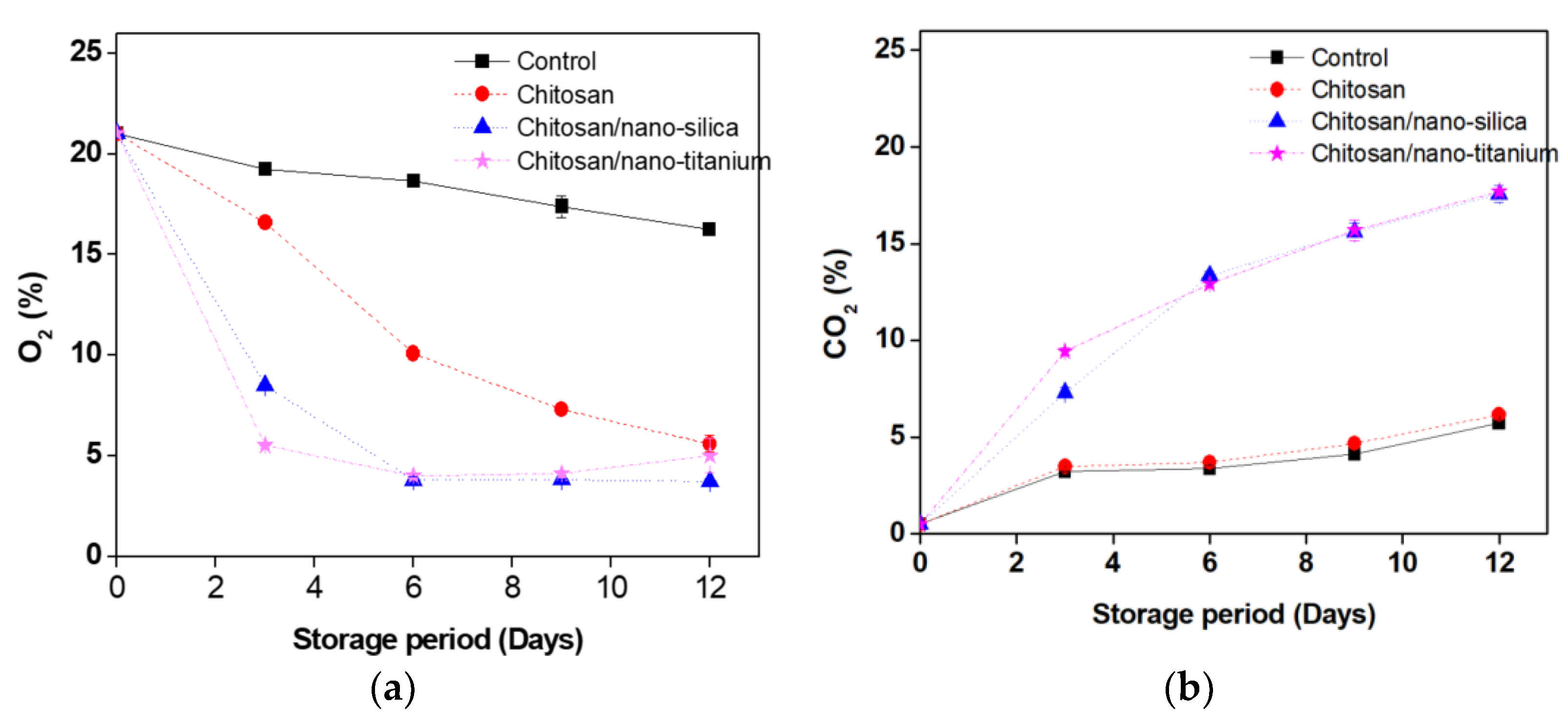
3.4. Changes in Headspace Gas Compositions and PPO Activity
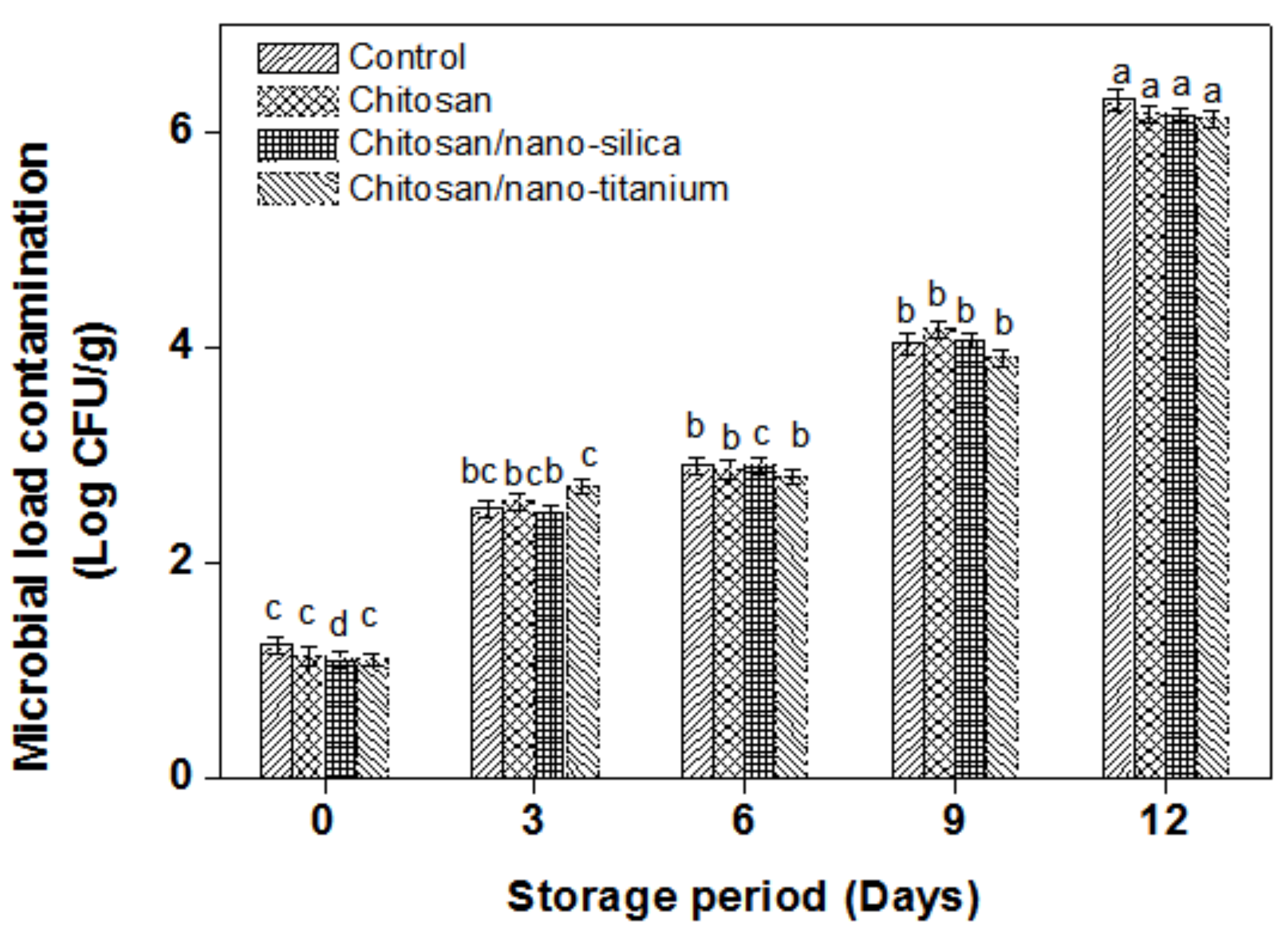
3.5. Microbial Load Contamination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, S.-T. Overview of Mushroom Cultivation and Utilization as Functional Foods. In Mushrooms as Functional Foods; Cheung, P.C., Ed.; Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 1–29. [Google Scholar]
- Lu, Y.; Zhang, J.; Wang, X.; Lin, Q.; Liu, W.; Xie, X.; Wang, Z.; Guan, W. Effects of UV-C irradiation on the physiological and antioxidant responses of button mushrooms (Agaricus bisporus) during storage. Int. J. Food Sci. Technol. 2016, 51, 1502–1508. [Google Scholar] [CrossRef]
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.P.; Lyng, J.G.; Jacquier, J.-C.; Bhuyan, D.J. Recovery of ergosterol and vitamin D2 from mushroom waste—Potential valorization by food and pharmaceutical industries. Trends Food Sci. Technol. 2020, 99, 351–366. [Google Scholar] [CrossRef]
- Rai, R.D.; Arumuganathan, T. Post Harvest Technology of Mushrooms; National Research Centre for Mushroom, Indian Council of Agricultural Research: Chambaghat, India, 2008. [Google Scholar]
- Akbarriad, H.; Kazemeini, S.M.; Shariaty, M.A. Deterioration and some of applied preservation techniques for common mushrooms (Agaricus bisporus, followed by Lentinus edodes, Pleurotus spp.). J. Microbiol. Biotechnol. Food Sci. 2013, 2, 2398–2402. [Google Scholar]
- Ding, Y.; Zhu, Z.; Zhao, J.; Nie, Y.; Zhang, Y.; Sheng, J.; Meng, D.; Mao, H.; Tang, X. Effects of postharvest brassinolide treatment on the metabolism of white button mushroom (Agaricus bisporus) in relation to development of browning during storage. Food Bioprocess. Technol. 2016, 9, 1327–1334. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Chen, C.-M.; Xu, L.; Cui, Y.; Yu, X.-Y.; Gao, H.-J.; Wang, Q.; Liu, K.; Shi, Y.; Chen, Q.-X. Postharvest application of 4-methoxy cinnamic acid for extending the shelf life of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 104, 33–41. [Google Scholar] [CrossRef]
- Simón, A.; González-Fandos, E.; Vázquez, M. Effect of washing with citric acid and packaging in modified atmosphere on the sensory and microbiological quality of sliced mushrooms (Agaricus bisporus L.). Food Control. 2010, 21, 851–856. [Google Scholar] [CrossRef]
- Hajjar, S.E.; Massantini, R.; Botondi, R.; Kefalas, P.; Mencarelli, F. Influence of high carbon dioxide and low oxygen on the postharvest physiology of fresh truffles. Postharvest Biol. Technol. 2010, 58, 36–41. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Fang, T.; Zhao, Y.; Duan, Y.; Lin, Q. Effects of nitric oxide treatment on flavour compounds and antioxidant enzyme activities of button mushroom (Agaricus bisporus) during storage. Food Qual. Saf. 2020, 4, 135–142. [Google Scholar] [CrossRef]
- Villaescusa, R.; Gil, M.I. Quality improvements of Pleurotus mushrooms by modified atmosphere packaging and moisture absorbers. Postharvest Biol. Technol. 2003, 28, 169–179. [Google Scholar] [CrossRef]
- Jiang, T.; Zheng, X.; Li, J.; Jing, G.; Cai, L.; Ying, T. Integrated application of nitric oxide and modified atmosphere packaging o improve quality of retention of button mushroom (Agaricus bisporus). Food Chem. 2011, 126, 1693–1699. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Aguiló-Aguayo, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments on quality and antioxidant properties of fresh-cut mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2010, 56, 216–222. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, M.; Hangqing, Y.; Jincai, S. Effects of different storage conditions on the chemical and physical properties of white mushrooms after vacuum cooling. J. Food Eng. 2006, 77, 545–549. [Google Scholar] [CrossRef]
- Wei, W.; Lv, P.; Xia, Q.; Tan, F.; Sun, F.; Yu, W.; Jia, L.; Cheng, J. Fresh-keeping effects of three types of modified atmosphere packaging of pine-mushrooms. Postharvest Biol. Technol. 2017, 132, 62–70. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Golding, J.B. Aloe vera gel treatment delays postharvest browning of white button mushroom (Agaricus bisporus). J. Food Meas. Charact. 2019, 13, 1250–1256. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Li, J. Changes in microbial and postharvest quality of shiitake mushroom (Lentinus edodes) treated with chitosan–glucose complex coating under cold storage. Food Chem. 2012, 131, 780–786. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef]
- Eldib, R. Application of nano-coating and chitosan combination films on cantaloupe preservation. Pak. J. Biol. Sci. 2020, 23, 1037–1043. [Google Scholar] [CrossRef]
- Ctibor, P.; Nevrlá, B.; Neufuss, K.; Petrášek, J.; Sedláček, J. Plasma spray coatings of natural ores from structural, mechanical, thermal, and dielectric viewpoints. Coatings 2019, 10, 3. [Google Scholar] [CrossRef]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Application of chitosan nanoparticles containing Cuminum cyminum oil as a delivery system for shelf life extension of Agaricus bisporus. LWT 2019, 106, 218–228. [Google Scholar] [CrossRef]
- Iqbal, A.; Sun, D.-W.; Allen, P. Prediction of moisture, color and pH in cooked, pre-sliced turkey hams by NIR hyperspectral imaging system. J. Food Eng. 2013, 117, 42–51. [Google Scholar] [CrossRef]
- Sultana, S.; Iqbal, A. Hossain comparative study of chitosan extracted from shrimp and crab shell and evaluation of its effect as a preservative on pineapple juice quality. J. Bangladesh Agric. Univ. 2020. [Google Scholar] [CrossRef]
- Dhalsamant, K.; Dash, S.K.; Bal, L.M.; Panda, M.K. Effect of perforation mediated MAP on shelf life of mushroom (Volvariella volvacea). Sci. Hortic. 2015, 189, 41–50. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, X.; Dong, S.; Sun, Y.; Zhao, Z. The properties of chitosan/zein blend film and effect of film on quality of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 155, 47–56. [Google Scholar] [CrossRef]
- Oz, A.T.; Ulukanli, Z.; Bozok, F.; Baktemur, G. The postharvest quality, sensory and shelf life of Agaricus bisporus in active map. J. Food Process. Preserv. 2015, 39, 100–106. [Google Scholar] [CrossRef]
- Peng, Y.; Li, T.; Jiang, H.; Gu, Y.; Chen, Q.; Yang, C.; Qi, W.L.; Liu, S.-Q.; Zhang, X. Postharvest biochemical characteristics and ultrastructure of Coprinus comatus. PeerJ 2020, 8, e8508. [Google Scholar] [CrossRef]
- Thakur, R.R.; Shahi, N.C.; Mangaraj, S.; Lohani, U.C.; Chand, K. Effect of apple peel based edible coating material on physicochemical properties of button mushrooms (Agaricus bisporus) under ambient condition. Int. J. Chem. Stud. 2020, 8, 2362–2370. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.; Niakousari, M. Application of tragacanth gum impregnated with satureja khuzistanica essential oil as a natural coating for enhancement of postharvest quality and shelf life of button mushroom (Agaricus bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef]
- Hosseini, A.; Moradinezhad, F. Effect of short-term high CO2 treatment on quality and shelf life of button mushroom (Agaricus bisporus) at refrigerated storage. Postharvest Biol. Technol. 2018, 1, 37–48. [Google Scholar]
- Rok, E.; Ebtihal, K.; Abeer, E.; Nada, B.; Mahmoud, H. Chitosan, nisin, silicon dioxide nanoparticles coating films effects on blueberry (Vaccinium myrtillus) quality. Coatings 2020, 10, 1–12. [Google Scholar]
- Gholami, R.; Ahmadi, E.; Ahmadi, S. Investigating the effect of chitosan, nanopackaging, and modified atmosphere packaging on physical, chemical, and mechanical properties of button mushroom during storage. Food Sci. Nutr. 2020, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Eissa, H.A. Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. J. Food Qual. 2007, 30, 623–645. [Google Scholar] [CrossRef]
- Khan, Z.U.; Aisikaer, G.; Khan, R.U.; Bu, J.; Jiang, Z.; Ni, Z.; Ying, T. Effects of composite chemical pretreatment on maintaining quality in button mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2014, 95, 36–41. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, M.; Lee, Y.S. Thermally buffered corrugated packaging for preserving the postharvest freshness of mushrooms (Agaricus bisporus). J. Food Eng. 2018. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Zhou, G.; Yuan, M.; Qin, Y. Biodegradable poly(lactic-acid)/poly(trimethylene-carbonate)/laponite composite film: Development and application to the packaging of mushrooms (Agaricus bisporus). Polym. Adv. Technol. 2015. [Google Scholar] [CrossRef]
- Rachappa, P.; Sudharma, D.C.; Chauhan, O.P.; Patki, P.E.; Nagaraj, R.; Naik, S.; Naik, R. Development and evaluation of white button mushroom based snacks. J. Food Process. Technol. 2020, 1–5. [Google Scholar] [CrossRef]
- Ban, Z.; Li, L.; Guan, J.; Feng, J.; Wu, M.; Xu, X.; Li, J. Modified atmosphere packaging (MAP) and coating for improving the preservation of whole and sliced Agaricus bisporus. J. Food Sci. Technol. 2014. [Google Scholar] [CrossRef][Green Version]
- Cliffe-Byrnes, V.; O’Beirne, D. Effects of gas atmosphere and temperature on the respiration rates of whole and sliced mushrooms (Agaricus bisporus)? Implications for film permeability in modified atmosphere packages. J. Food Sci. 2007, 72, E197–E204. [Google Scholar] [CrossRef]


| Storage (Days) | Control | Chitosan | Chitosan/Nano-Silica | Chitosan/Nano-Titanium | |
|---|---|---|---|---|---|
| L * | 0 | 60.35 ± 0.22 a | 53.66 ± 0.87 c | 38.89 ± 0.42 d | 56.79 ± 0.32 b |
| 3 | 52.92 ± 1.48 a | 39.26 ± 0.44 c | 28.53 ± 0.75 d | 46.64 ± 0.95 b | |
| 6 | 26.59 ± 0.90 b | 25.70 ± 1.12 b | 28.46 ± 0.70 a | 25.45 ± 0.63 b | |
| 9 | 24.60 ± 0.28 b | 23.07 ± 0.35 c | 26.18 ± 0.60 a | 24.80 ± 0.26 b | |
| 12 | 19.82 ± 0.73 b | 12.81 ± 0.57 c | 22.84 ± 0.17 a | 13.45 ± 0.15 c | |
| a * | 0 | 5.78 ± 0.25 a | 5.95 ± 0.23 a | 6.29 ± 0.59 a | 6.24 ± 0.85 a |
| 3 | 6.91 ± 0.20 a,b | 7.64 ± 0.65 a | 6.47 ± 0.57 b | 6.53 ± 0.14 b | |
| 6 | 8.58 ± 0.90 a | 7.77 ± 0.38 a,b | 8.66 ± 0.17 a | 7.07 ± 0.18 b | |
| 9 | 8.37 ± 0.84 a | 8.56 ± 0.93 a | 8.56 ± 0.38 a | 8.25 ± 0.28 a | |
| 12 | 9.12 ± 0.34 b,c | 9.71 ± 0.65 b | 11.44 ± 0.08 b | 8.40 ± 0.30 c | |
| b * | 0 | 12.44 ± 0.42 a | 9.55 ± 0.35 c | 10.97 ± 0.21 b | 11.08 ± 0.16 b |
| 3 | 15.75 ± 0.93 a | 14.06 ± 0.24 b | 13.02 ± 0.12 c | 12.08 ± 0.37 c | |
| 6 | 16.31 ± 0.16 a | 14.34 ± 0.33 b | 14.68 ± 0.59 b | 14.80 ± 0.31 b | |
| 9 | 17.53 ± 0.54 b | 18.09 ± 0.47 b | 18.27 ± 0.34 b | 20.14 ± 0.54 a | |
| 12 | 24.56 ± 0.25 a | 20.57 ± 0.31 c | 18.29 ± 0.10 d | 21.46 ± 0.17 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokayya, S.; Khojah, E.; Elhakem, A.; Benajiba, N.; Chavali, M.; Vivek, K.; Iqbal, A.; Helal, M. Investigating the Nano-Films Effect on Physical, Mechanical Properties, Chemical Changes, and Microbial Load Contamination of White Button Mushrooms during Storage. Coatings 2021, 11, 44. https://doi.org/10.3390/coatings11010044
Rokayya S, Khojah E, Elhakem A, Benajiba N, Chavali M, Vivek K, Iqbal A, Helal M. Investigating the Nano-Films Effect on Physical, Mechanical Properties, Chemical Changes, and Microbial Load Contamination of White Button Mushrooms during Storage. Coatings. 2021; 11(1):44. https://doi.org/10.3390/coatings11010044
Chicago/Turabian StyleRokayya, Sami, Ebtihal Khojah, Abeer Elhakem, Nada Benajiba, Murthy Chavali, Kambhampati Vivek, Abdullah Iqbal, and Mahmoud Helal. 2021. "Investigating the Nano-Films Effect on Physical, Mechanical Properties, Chemical Changes, and Microbial Load Contamination of White Button Mushrooms during Storage" Coatings 11, no. 1: 44. https://doi.org/10.3390/coatings11010044
APA StyleRokayya, S., Khojah, E., Elhakem, A., Benajiba, N., Chavali, M., Vivek, K., Iqbal, A., & Helal, M. (2021). Investigating the Nano-Films Effect on Physical, Mechanical Properties, Chemical Changes, and Microbial Load Contamination of White Button Mushrooms during Storage. Coatings, 11(1), 44. https://doi.org/10.3390/coatings11010044






