Fabrication of Fe‒Al Coatings with Micro/Nanostructures for Antifouling Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fe–Al Electrode
2.2. Fe–Al Coating Fabrication Process
2.3. Antifouling Property Experiment
2.4. Characterization
3. Results
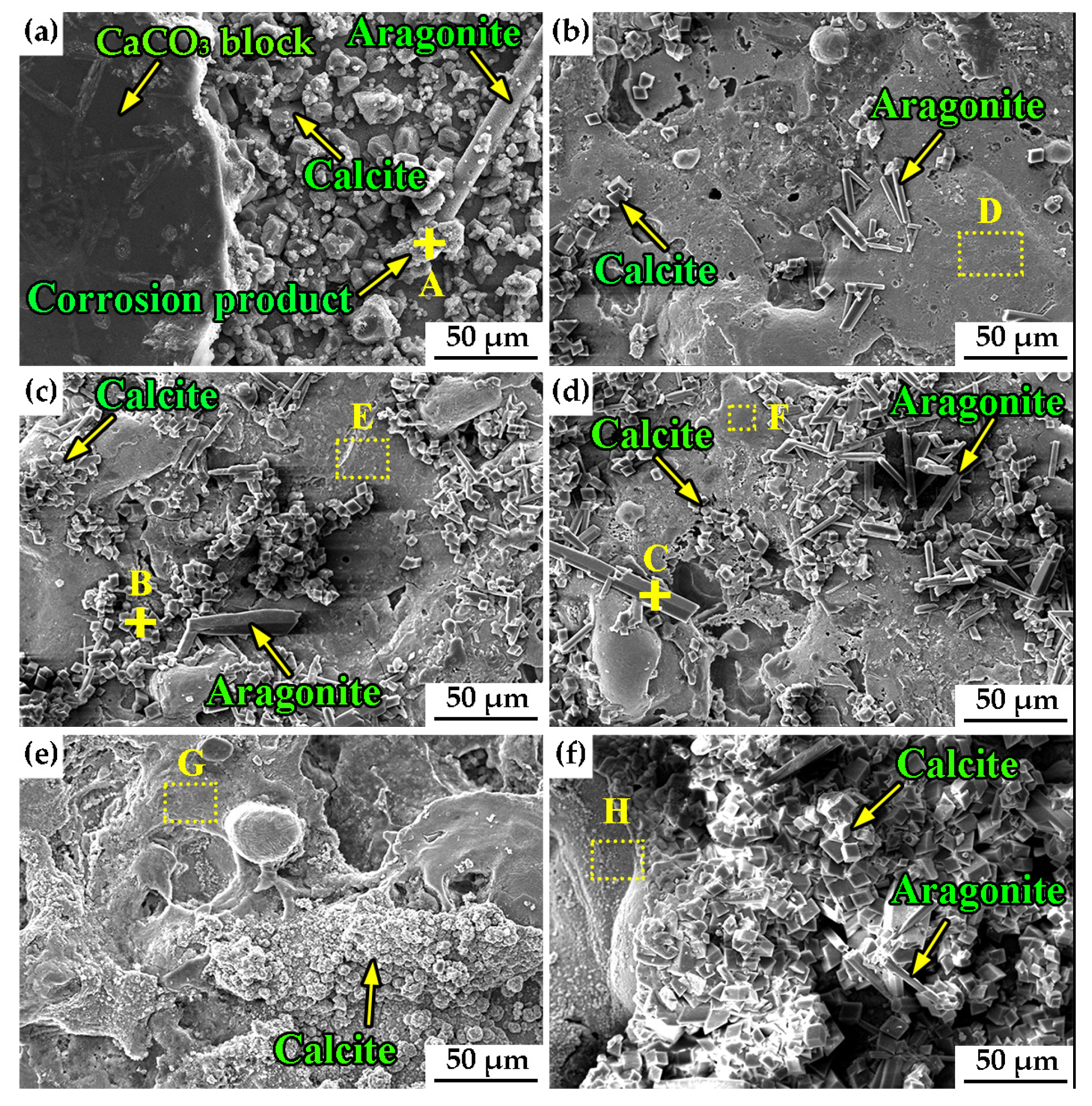
3.1. Surface Morphology and Chemical Composition
3.2. Phase Combination of Fe–Al Micro/Nanostructures (MNS)
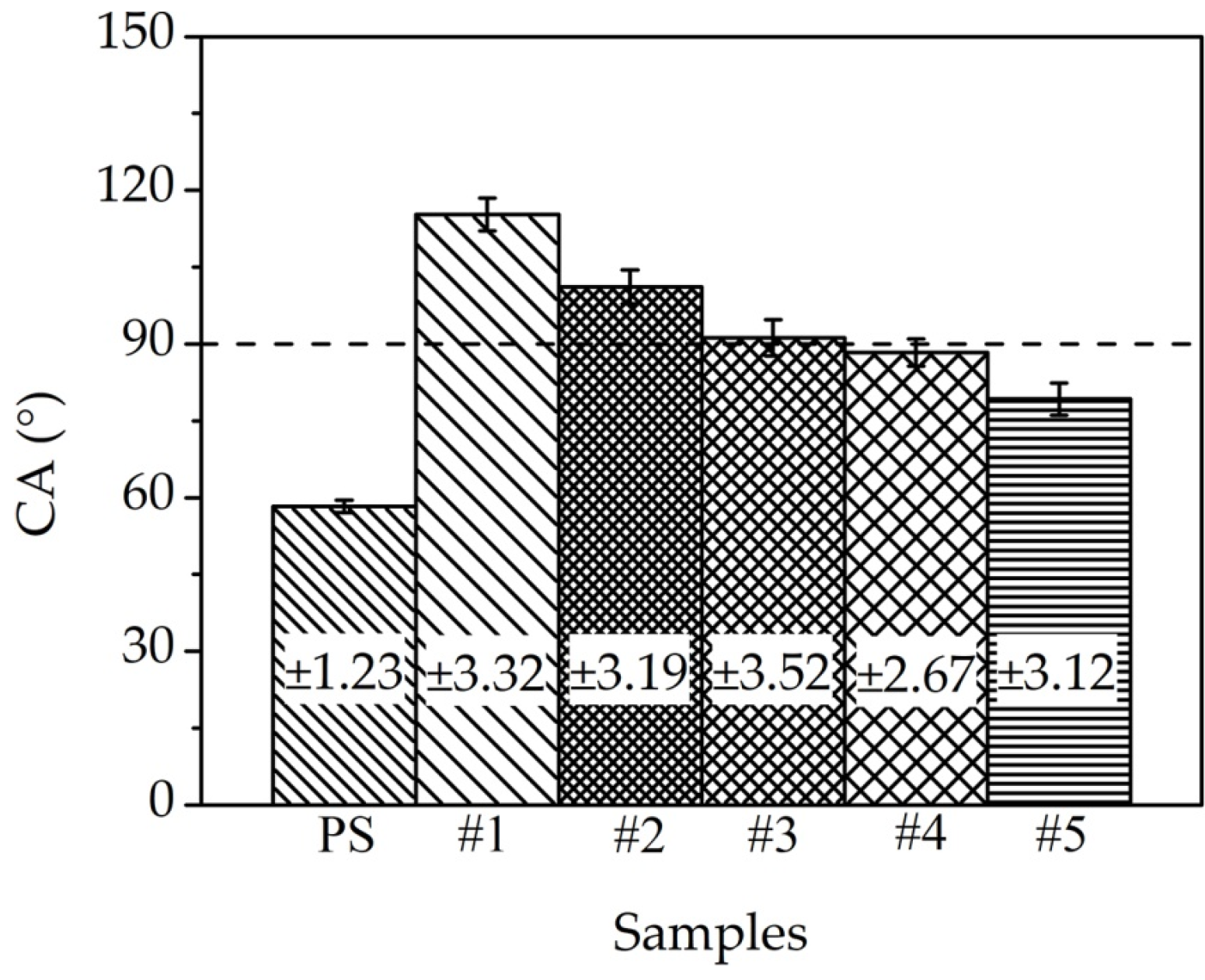
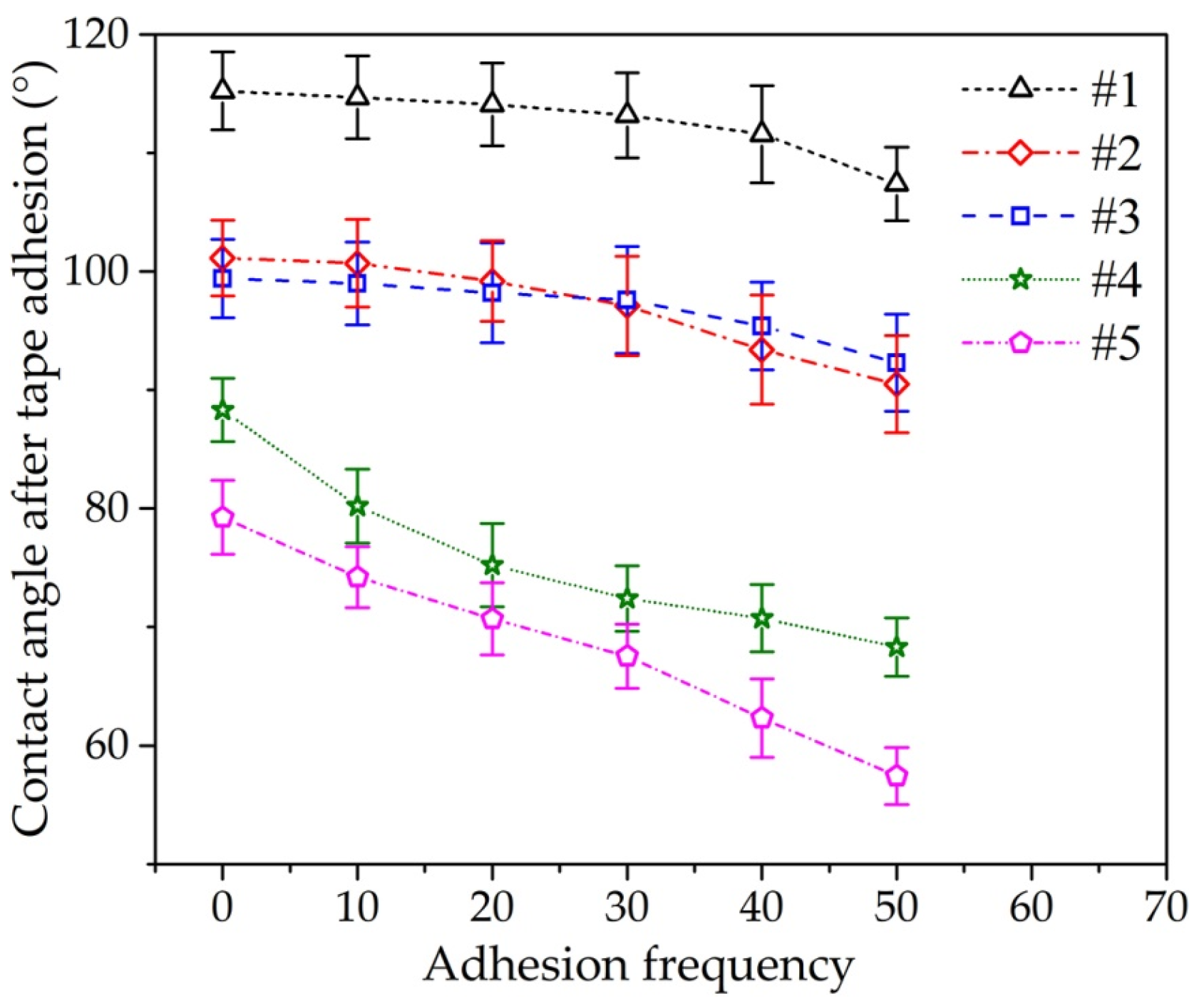
3.3. Surface Wettability
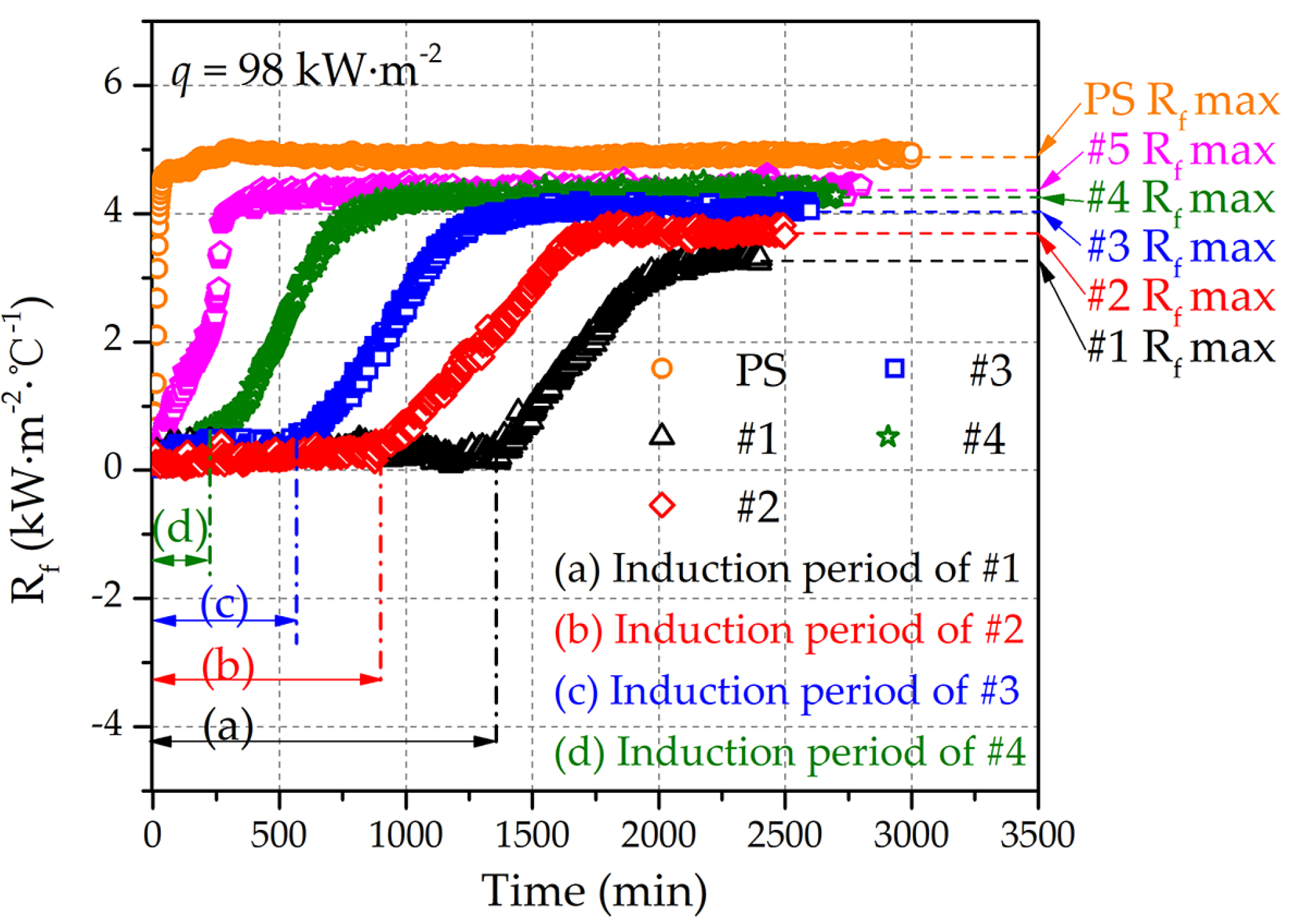
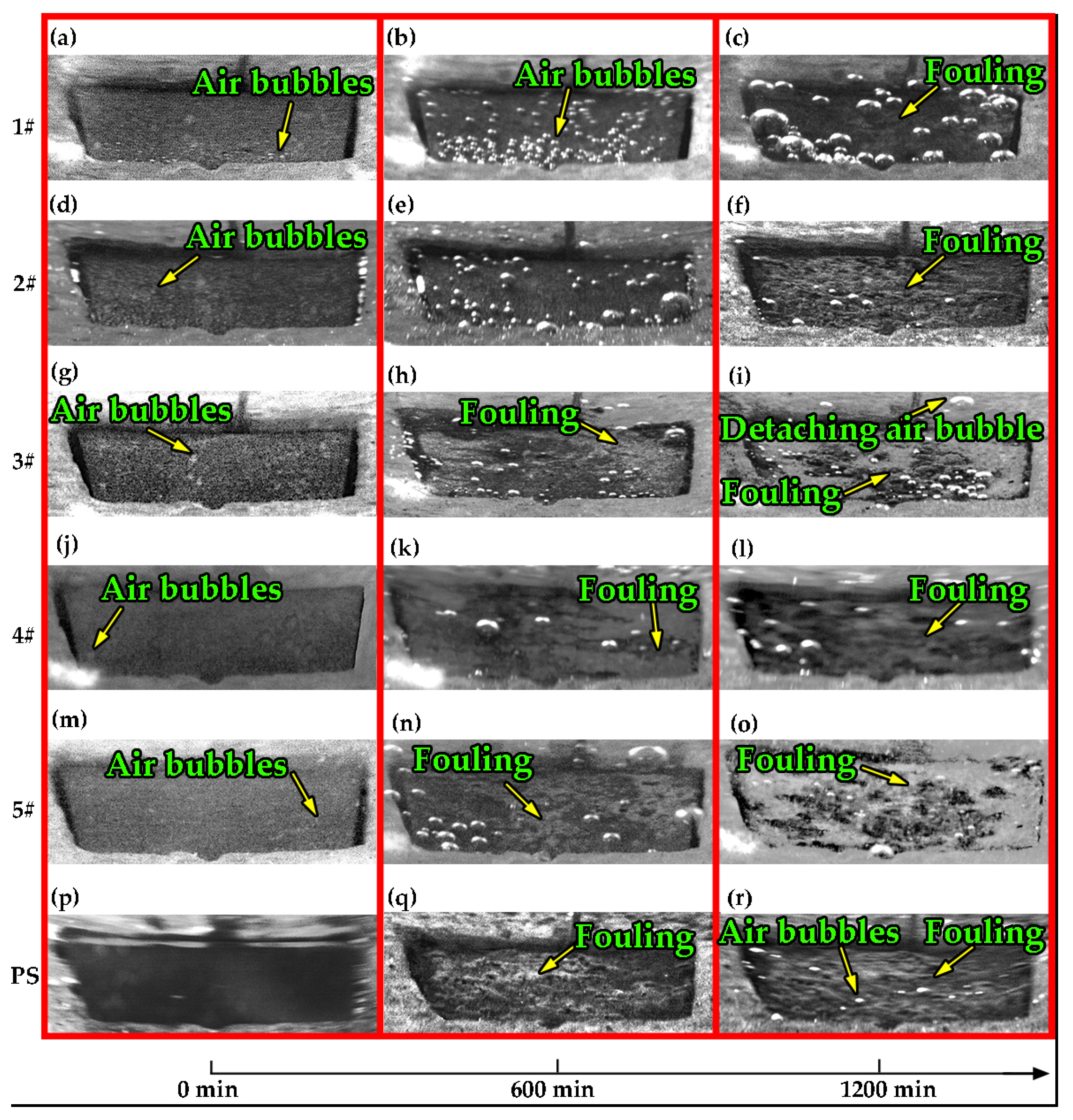
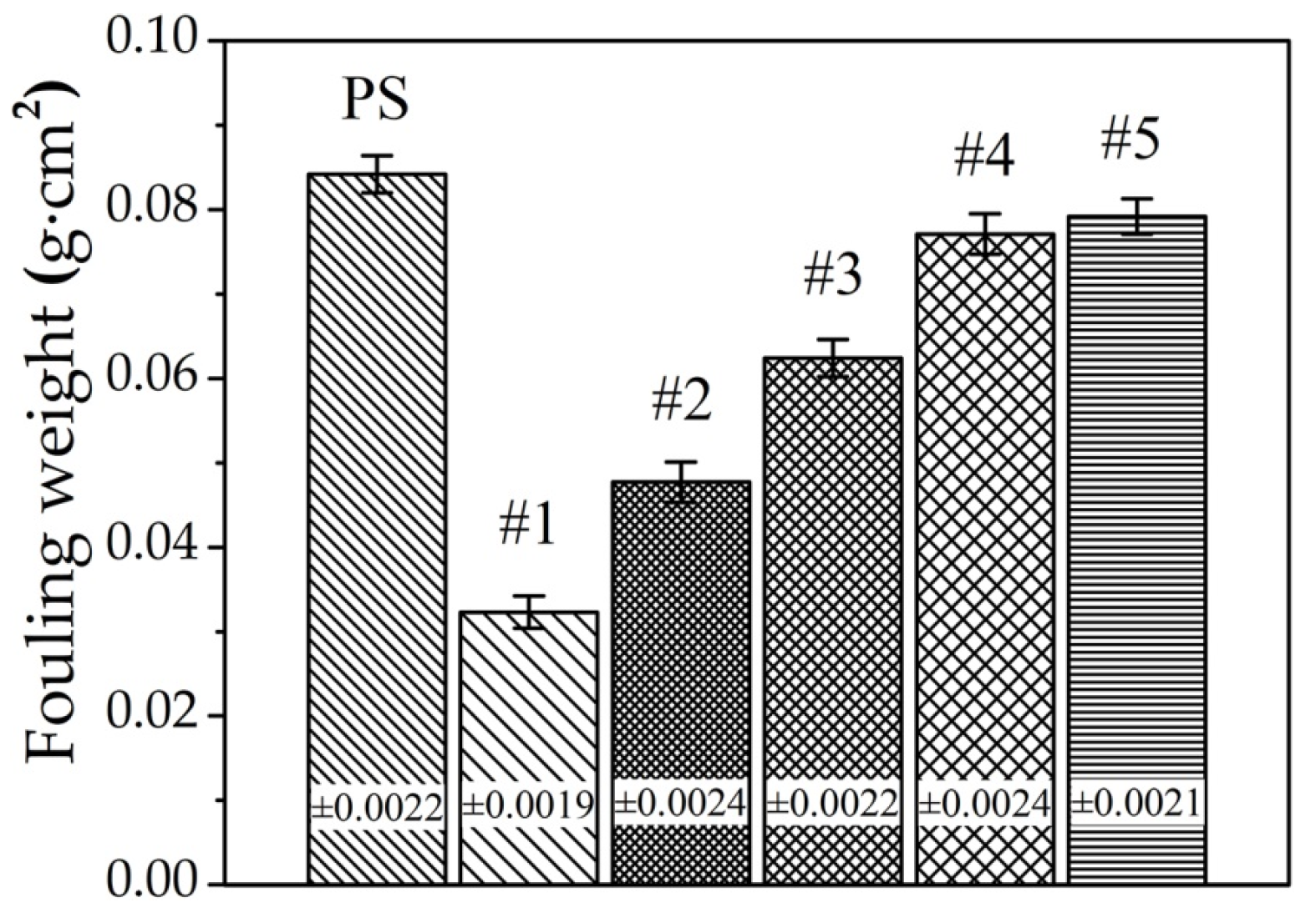
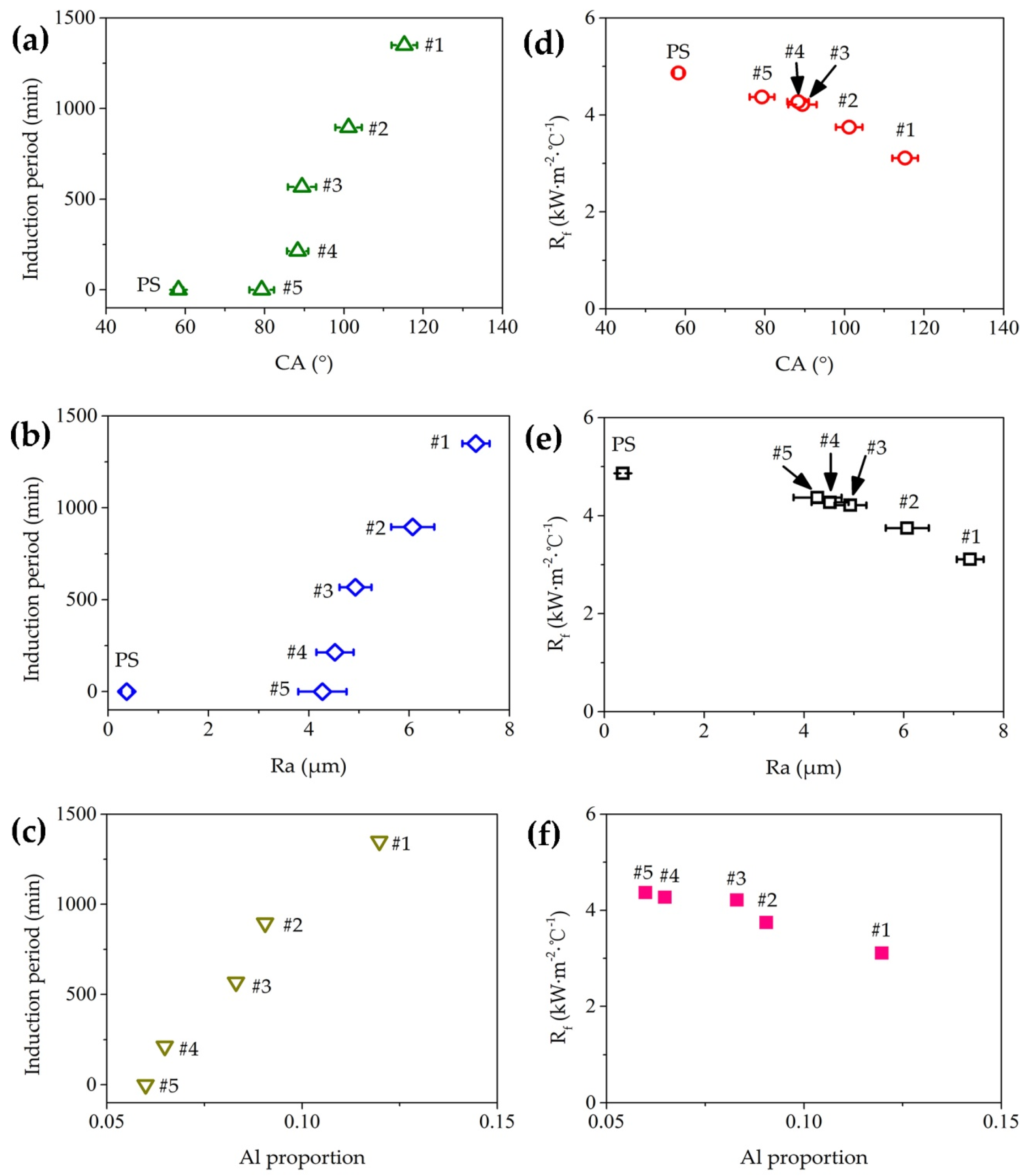
3.4. Antifouling Property of Fe–Al MNS Sample
3.5. Durability Test of Fe–Al MNS
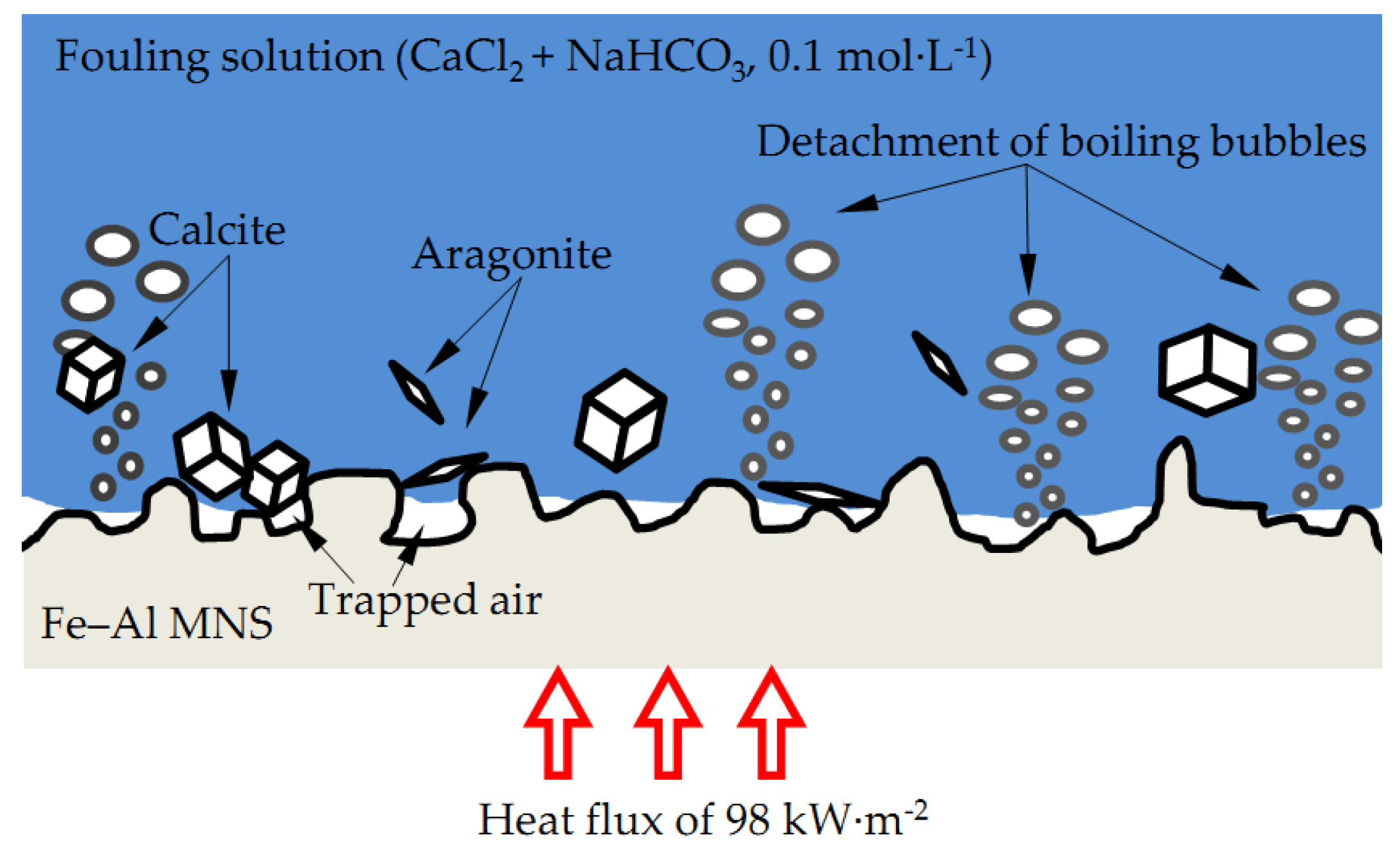
4. Discussion
5. Conclusions
- According to the EDC technology and Fe–Al electrode, the Fe–Al coating with micro/nanostructures is fabricated on a low-carbon steel substrate. The roughness and coating thickness of Fe–Al MNS decreased with the increasing gap voltage of EDC. The porosity of Fe–Al MNS decreased sharply from a low gap voltage to a high gap voltage. The main phases of Fe–Al MNS were combined by Fe3Al, Fe7C3, and AlFe3C0.5.
- The Fe–Al MNS showed a hydrophobic property. The contact angle of Fe–Al MNS decreased with the increasing EDC gap voltage. Due to the effect of micro/nanostructures of Fe–Al MNS, water droplets could be isolated by tapped air or infiltration in the coating, resulting in the regulation of the hydrophobic property.
- Compared with the polished surface of low-carbon steel, Fe–Al MNS shows better antifouling performance, with an extended induction period and lower Rf max value. The antifouling property of Fe–Al MNS samples decreased with the increasing EDC gap voltage. One potential reason is that the hydrophobic property of the Fe–Al MNS inhibited the adhesion of fouling crystals. Another reason could be that the micro/nanostructures of Fe–Al MNS also increased the nuclear area of boiling bubbles, which can dislodge the loose fouling crystals from the Fe–Al MNS surface by a wiping action when the boiling bubbles detach. Finally, due to the activity of the Al element in the Fe–Al MNS, a passive film was formed on the Fe–Al MNS surface to mitigate the corrosion products, which could easily absorb the fouling crystals. As a result, the adhesion behavior of the fouling crystals was inhibited. Thus, the co-effect of the above reasons supports the fact that Fe–Al MNS showed a superior antifouling property. Meanwhile, after the durability test, the hydrophobic property of Fe–Al MNS decreased slightly, indicating that there is an industrial application prospect for Fe–Al MNS.
- According to the results of this study, micro/nanostructures enhance the antifouling property of Fe–Al MNS. This indicates that there is a relationship between the scale effect of micro/nanostructures and the antifouling property, which is worth investigating further.
Author Contributions
Funding
Conflicts of Interest
References
- Wei, L.; Kan, Z.; Manglik, R.M.; Li, G.Q.; Bergles, A.E. Investigation of CaCO3 fouling in plate heat exchangers. Heat Mass Transfer. 2016, 52, 2401–2414. [Google Scholar]
- Kazi, S.N. Fouling and fouling mitigation of calcium compounds on heat exchangers by novel colloids and surface modifications. Rev. Chem. Eng. 2019, 36, 653–685. [Google Scholar] [CrossRef]
- Hu, M.; Zheng, S.; Mi, B. Organic fouling of graphene oxide membranes and its implications for membrane fouling control in engineered osmosis. Environ. Sci. Technol. 2016, 50, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.O.; Jaramillo, H.; Xie, M.; Ude, M.; Nghiem, L.D.; Elimelech, M. Biofouling mitigation in forward osmosis using graphene oxide functionalized thin-film composite membranes. Environ. Sci. Technol. 2016, 50, 5840–5848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fei, G.; Xia, H.; Zuilhof, H. Dual water-healable zwitterionic polymer coatings for anti-biofouling surfaces. J. Mater. Chem. B. 2018, 6, 6930–6935. [Google Scholar] [CrossRef] [PubMed]
- He, Z.R.; Liu, C.S.; Jie, X.H.; Lian, W.Q.; Luo, S.T. Preparation of anti-fouling heat transfer surface by magnetron sputtering a-C film on electrical discharge machining Cu surface. Surf. Coat. Tech. 2019, 369, 44–51. [Google Scholar] [CrossRef]
- Weeranoppanant, N.; Amar, L.; Tong, E.; Faria, M.; Hill, M.I.; Leonard, E.F. Modeling of fouling in cross-flow microfiltration of suspensions. AIChE J. 2019, 65, 207–213. [Google Scholar] [CrossRef]
- Malayeri, M.R.; Evangelidou, M. Enhanced heat transfer and fouling propensity of DLC coated smooth and finned tubes during external nucleate boiling. J. Heat Transfer. 2016, 138, 081502. [Google Scholar] [CrossRef]
- He, Z.R.; Liu, C.S.; Gao, H.Y.; Jie, X.H.; Lian, W.Q. Experimental study on the anti-fouling effects of EDM machined hierarchical micro/nano structure for heat transfer surface. Appl. Therm. Eng. 2019, 162, 114248. [Google Scholar] [CrossRef]
- He, Z.R.; Luo, S.T.; Liu, C.S.; Jie, X.H.; Lian, W.Q. Hierarchical micro/nano structure surface fabricated by electrical discharge machining for anti-fouling application. J. Mater. Res. Technol. 2019, 8, 3878–3890. [Google Scholar] [CrossRef]
- Bogacz, W.; Lemanowicz, M.; Rashed, M.H.A.; Nakonieczny, D.; Piotrowski, T.; Wójcik, J. Impact of roughness, wettability and hydrodynamic conditions on the incrustation on stainless steel surfaces. Appl. Therm. Eng. 2017, 112, 352–361. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, M.; Xu, Y. Corrosion and Fouling Behaviors on modified stainless steel surfaces in simulated oilfield geothermal water. Prot. Met. Phys. Chem. Surf. 2018, 54, 526–535. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, M. Corrosion and fouling behaviours of copper-based superhydrophobic coating. Surf. Eng. 2018, 35, 542–549. [Google Scholar] [CrossRef]
- Steinhagen, H.M.; Malayeri, M.R.; Watkinson, A.P. Fouling of heat exchangers-new approaches to solve an old problem. Heat Transfer Eng. 2005, 26, 1–4. [Google Scholar] [CrossRef]
- Steinhagen, H.M.; Malayeri, M.R.; Watkinson, A.P. Heat exchanger fouling: Environmental impacts. Heat Transfer Eng. 2009, 30, 773–776. [Google Scholar] [CrossRef]
- Li, N.N.; Wang, M.Z.; Li, Y.S.; Chen, G.; Li, P. Corrosion behavior of Fe–Al coatings fabricated by pack aluminizing method. Acta Metall. Sin. 2016, 29, 813–819. [Google Scholar] [CrossRef]
- Reichardt, A.; Shapiro, A.A.; Otis, R.; Dillon, R.P.; Borgonia, J.P.; McEnerney, B.W.; Hosemann, P.; Beese, A.M. Advances in additive manufacturing of metal-based functionally graded materials. Int. Mater. Rev. 2020, 1–29. [Google Scholar] [CrossRef]
- Senderowski, C.; Chodala, M.; Bojar, Z. Corrosion behavior of detonation gun sprayed Fe–Al type intermetallic coating. Materials 2015, 8, 1108–1123. [Google Scholar] [CrossRef]
- Vasylyev, M.A.; Mordyuk, B.N.; Sidorenko, S.I.; Voloshko, S.M.; Burmak, A.P. Corrosion of 2024 alloy after ultrasonic impact cladding with iron. Surf. Eng. 2017, 34, 324–329. [Google Scholar] [CrossRef]
- Gao, H.; Xie, W.; Zhang, H.; Shen, W.; He, Y. Modification of the reactive synthesis of porous FeAl with addition of Si. Mater. High Temp. 2019, 36, 1–8. [Google Scholar] [CrossRef]
- Tyagi, R.; Mahto, N.K.; Das, A.K.; Mandal, A. Preparation of MoS2 + Cu coating through the EDC process and its analysis. Surf. Eng. 2020, 36, 86–93. [Google Scholar] [CrossRef]
- Xie, Z.J.; Mai, Y.J.; Lian, W.Q.; He, S.L.; Jie, X.H. Titanium carbide coating with enhanced tribological properties obtained by EDC using partially sintered titanium electrodes and graphite powder mixed dielectric. Surf. Coat Tech. 2016, 300, 50–57. [Google Scholar] [CrossRef]
- So, J.Y.; Bae, W.G. Fabrication of superhydrophobic metallic surface by wire electrical discharge machining for seamless roll-to-roll printing. Metals 2018, 8, 228. [Google Scholar]
- Tyagi, R.; Das, A.K.; Mandal, A. Electrical discharge coating using WS2 and Cu powder mixture for solid lubrication and enhanced tribological performance. Tribol. Int. 2018, 120, 80–92. [Google Scholar] [CrossRef]
- Mandal, P.; Mondal, S.C. Surface characteristics of mild steel using EDM with Cu-MWCNT composite electrode. Mater. Manuf. Process. 2019, 34, 1326–1332. [Google Scholar] [CrossRef]
- Wismogroho, A.S.; Widayatno, W.B.; Suryadi; Zaini Thosin, K.A.; Rochman, N.T.; Sueyoshi, H. Iron aluminide coating on Al by mechanical alloying. Surf. Eng. 2011, 27, 126–133. [Google Scholar] [CrossRef]
- Muralidharan, B.; Chelladurai, H.; Singh, P.; Roy, M.K. Single-spark analysis of electro-discharge deposition process. Mater. Manuf. Process. 2015, 31, 1853–1864. [Google Scholar] [CrossRef]
- Peng, Z.; Feng, T.; Wei, Z.; Zhang, Y.; Li, Y. Directly writing patterning of conductive material by high voltage induced weak electric arc machining (HV-μEAM). Coatings 2019, 9, 538. [Google Scholar] [CrossRef]
- Yue, X.; Yang, X. Molecular dynamics simulation of single pulse discharge process: Clarifying the function of pressure generated inside the melting area in EDM. Mol. Simulat. 2017, 43, 935–944. [Google Scholar] [CrossRef]
- Robert, N.W. Resistance of solid surfaces to weting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, M.Y. Pool boiling fouling and corrosion properties on liquid-phase-deposition TiO2 coatings with copper substrate. AIChE J. 2011, 57, 1710–1718. [Google Scholar] [CrossRef]
- Oldani, V.; Sergi, G.; Pirola, C.; Bianchi, C.L. Use of a sol-gel hybrid coating composed by a fluoropolymer and silica for the mitigation of mineral fouling in heat exchangers. Appl. Therm. Eng. 2016, 106, 427–431. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Han, Z.; Wang, J. Numerical simulation of calcium sulfate (CaSO4) fouling in the plate heat exchanger. Heat Mass Transfer. 2018, 54, 1867–1877. [Google Scholar] [CrossRef]
- Zou, A.; Maroo, S.C. Critical height of micro/nano structures for pool boiling heat transfer enhancement. Appl. Phys. Lett. 2013, 103, 221602. [Google Scholar] [CrossRef]
- Yao, Z.; Lu, Y.-W.; Kandlikar, S.G. Pool boiling heat transfer enhancement through nanostructures on silicon microchannels. J. Nanotechnol. Eng. Med. 2013, 3, 031002. [Google Scholar] [CrossRef]
- Kumar, S.; Chang, Y.W.; Chen, P.-H. Pool-boiling heat-transfer enhancement on cylindrical surfaces with hybrid wettable patterns. JOVE-J. Vis. Exp. 2017, 122, e55387. [Google Scholar] [CrossRef]
- Arya, M.; Khandekar, S.; Pratap, D.; Ramakrishna, S.A. Pool boiling of water on nano-structured micro wires at sub-atmospheric conditions. Heat Mass Transfer. 2016, 52, 1725–1737. [Google Scholar] [CrossRef]
- Roy, P.; Kisslinger, R.; Farsinezhad, S.; Mahdi, N.; Bhatnagar, A.; Hosseini, A.; Bu, L.; Hua, W.; Wiltshire, B.D.; Eisenhawer, A.; et al. All-solution processed, scalable superhydrophobic coatings on stainless steel surfaces based on functionalized discrete titania nanotubes. Chem. Eng. J. 2018, 351, 482–489. [Google Scholar] [CrossRef]
- Ozden, H.O.; Puri, V.M. Computational analysis of fouling by low energy surfaces. J. Food. Eng. 2010, 99, 250–256. [Google Scholar] [CrossRef]
- Xu, P.; Li, Q.; Xuan, Y. Enhanced boiling heat transfer on composite porous surface. Int. J. Heat Mass Tran. 2015, 80, 107–114. [Google Scholar] [CrossRef]
- Rishi, A.M.; Gupta, A.; Kandlikar, S.G. Improving aging performance of electrodeposited copper coatings during pool boiling. Appl. Therm. Eng. 2018, 140, 406–414. [Google Scholar] [CrossRef]
- Esawy, M.; Malayeri, M.R. Modeling of CaSO4 crystallization fouling of finned tubes during nucleate pool boiling. Chem. Eng. Res. Des. 2017, 118, 51–60. [Google Scholar] [CrossRef]
- Wang, G.G.; Zhu, L.Q.; Liu, H.C.; Li, W.P. Galvanic corrosion of Ni–Cu–Al composite coating and its anti-fouling property for metal pipeline in simulated geothermal water. Surf. Coat. Tech. 2012, 206, 3728–3732. [Google Scholar] [CrossRef]
- Kazi, S.N.; Duffy, G.G.; Chen, X.D. Fouling and fouling mitigation on heated metal surfaces. Desalination 2012, 288, 126–134. [Google Scholar] [CrossRef]


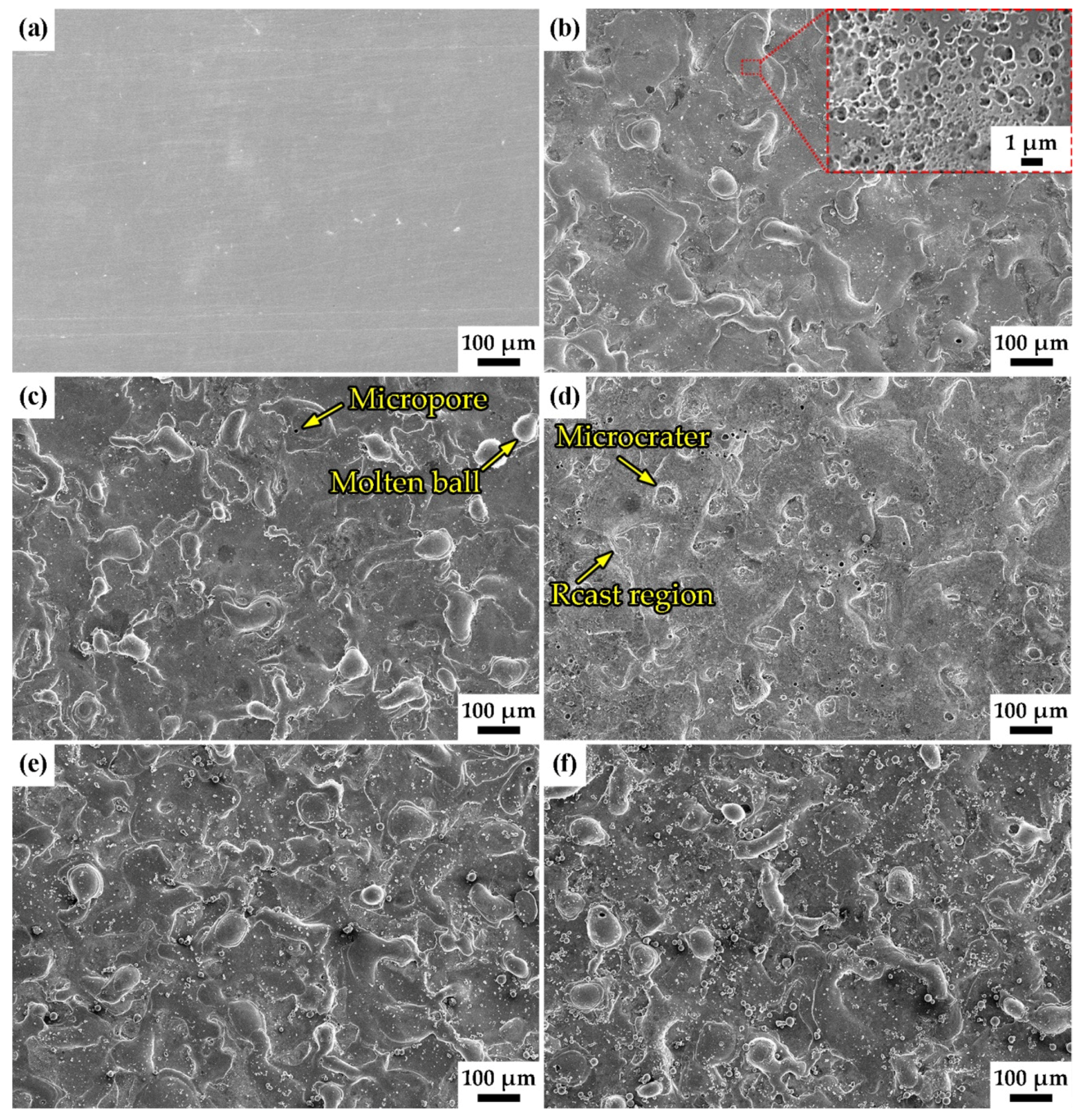
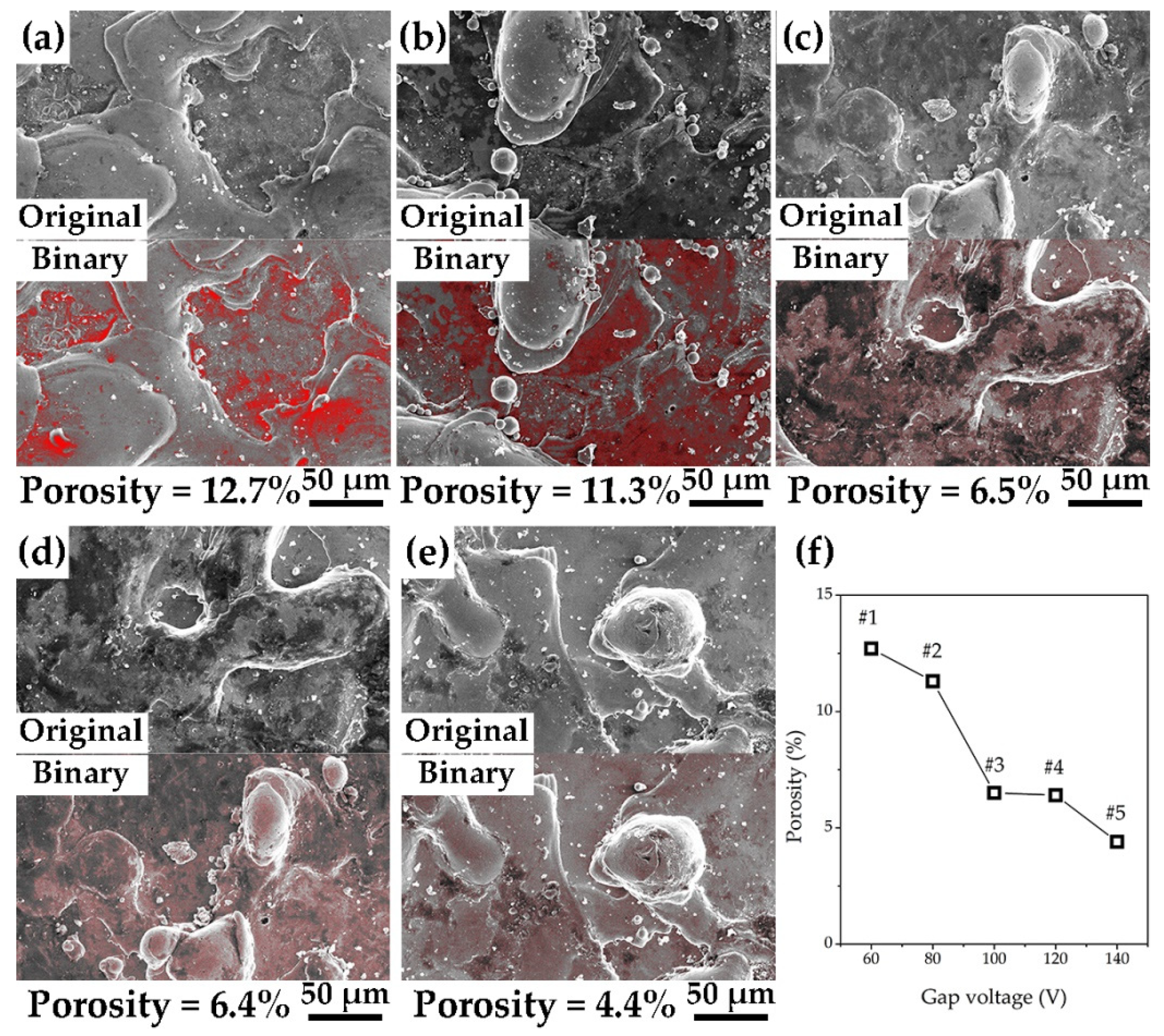
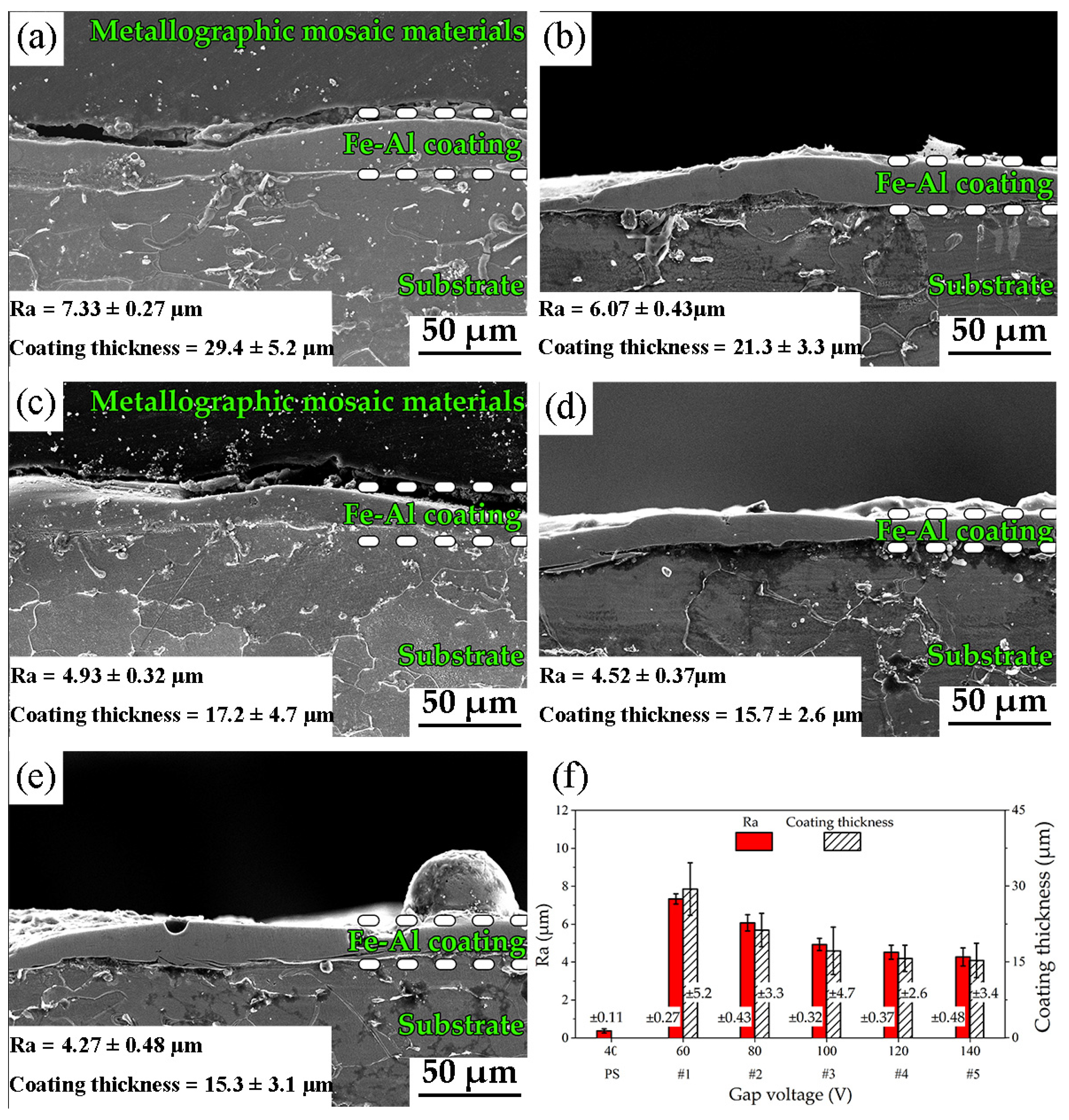
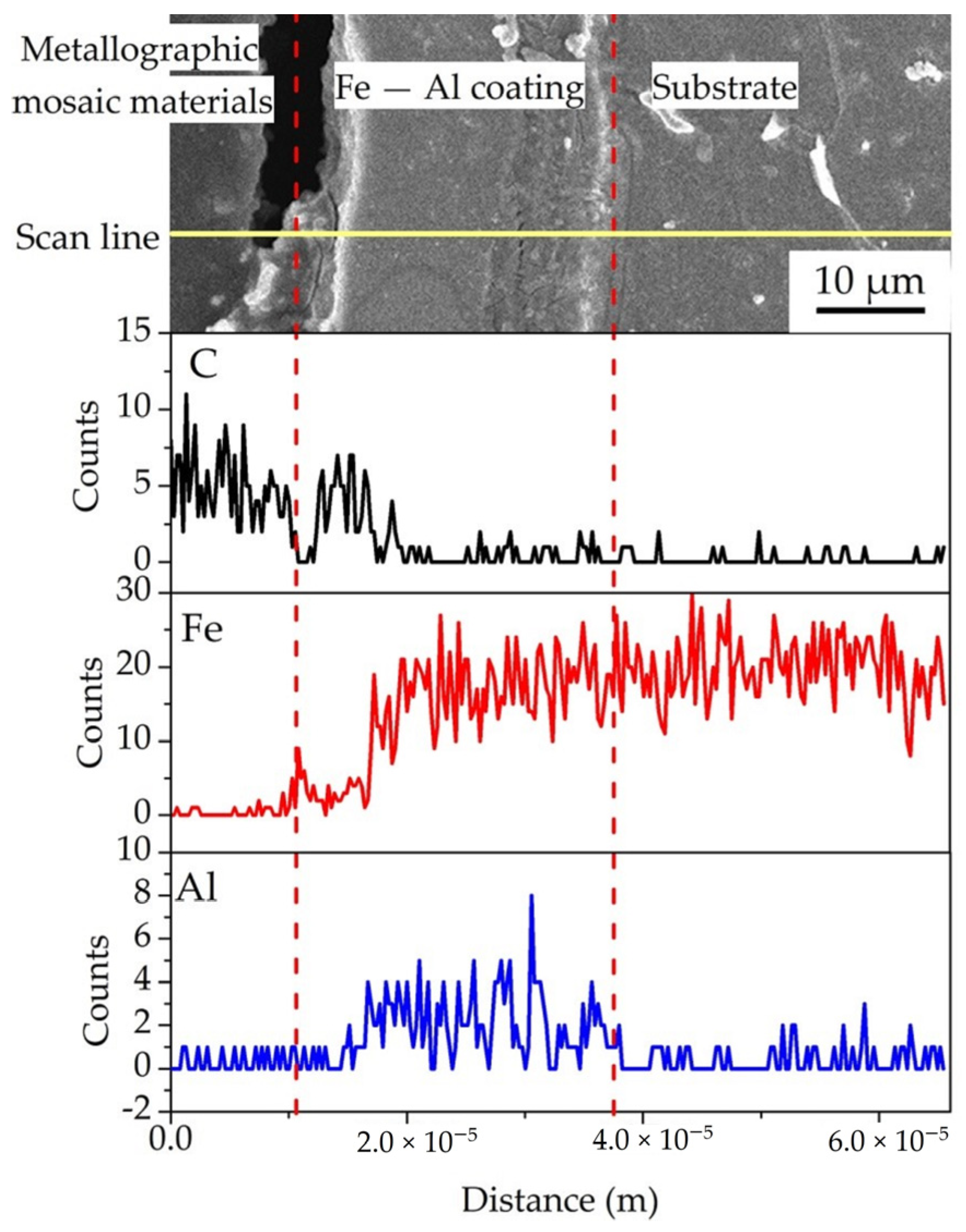

| Sample | Current (A) | Pulse Width (μs) | Duty Ratio (%) | Gap Voltage (V) |
|---|---|---|---|---|
| #1 | 12 | 50 | 20 | 60 |
| #2 | 12 | 50 | 20 | 80 |
| #3 | 12 | 50 | 20 | 100 |
| #4 | 12 | 50 | 20 | 120 |
| #5 | 12 | 50 | 20 | 140 |
| Sample | C | Fe | Al | O | Fe:Al |
|---|---|---|---|---|---|
| #1 | – | 89.30 | 10.70 | – | 8.35:1 |
| #2 | 8.16 | 84.22 | 7.62 | – | 11.05:1 |
| #3 | 3.65 | 88.96 | 7.39 | – | 12.04:1 |
| #4 | 10.64 | 82.13 | 5.44 | – | 15.43:1 |
| #5 | 11.21 | 83.77 | 5.02 | – | 16.69:1 |
| Average value of Fe/Al ratio | 12.71:1 | ||||
| Marks | C (wt.%) | Fe (wt.%) | Al (wt.%) | O (wt.%) | Ca (wt.%) |
|---|---|---|---|---|---|
| A spot | 0.50 | 72.59 | 0.47 | 26.44 | – |
| B spot | 4.00 | 4.58 | 9.87 | 38.32 | 43.23 |
| C spot | 5.79 | 4.38 | 3.66 | 43.64 | 42.53 |
| D region | 2.28 | 71.82 | 6.45 | 19.45 | – |
| E region | 2.31 | 67.35 | 6.56 | 23.78 | – |
| F region | 2.23 | 80.30 | 8.48 | 8.99 | – |
| G region | 2.92 | 70.43 | 6.89 | 19.76 | – |
| H region | 1.50 | 72.59 | 5.87 | 20.04 | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Wang, D.; Fan, Z.; Chen, Y.; Li, S.; Mo, C. Fabrication of Fe‒Al Coatings with Micro/Nanostructures for Antifouling Applications. Coatings 2020, 10, 902. https://doi.org/10.3390/coatings10090902
He Z, Wang D, Fan Z, Chen Y, Li S, Mo C. Fabrication of Fe‒Al Coatings with Micro/Nanostructures for Antifouling Applications. Coatings. 2020; 10(9):902. https://doi.org/10.3390/coatings10090902
Chicago/Turabian StyleHe, Zhaorong, Dacheng Wang, Zhiqing Fan, Yingjun Chen, Shidong Li, and Caisong Mo. 2020. "Fabrication of Fe‒Al Coatings with Micro/Nanostructures for Antifouling Applications" Coatings 10, no. 9: 902. https://doi.org/10.3390/coatings10090902
APA StyleHe, Z., Wang, D., Fan, Z., Chen, Y., Li, S., & Mo, C. (2020). Fabrication of Fe‒Al Coatings with Micro/Nanostructures for Antifouling Applications. Coatings, 10(9), 902. https://doi.org/10.3390/coatings10090902





