Preparation and Synergistic Effect of Chitosan/Sodium Phytate/MgO Nanoparticle Fire-Retardant Coatings on Wood Substrate through Layer-By-Layer Self-Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
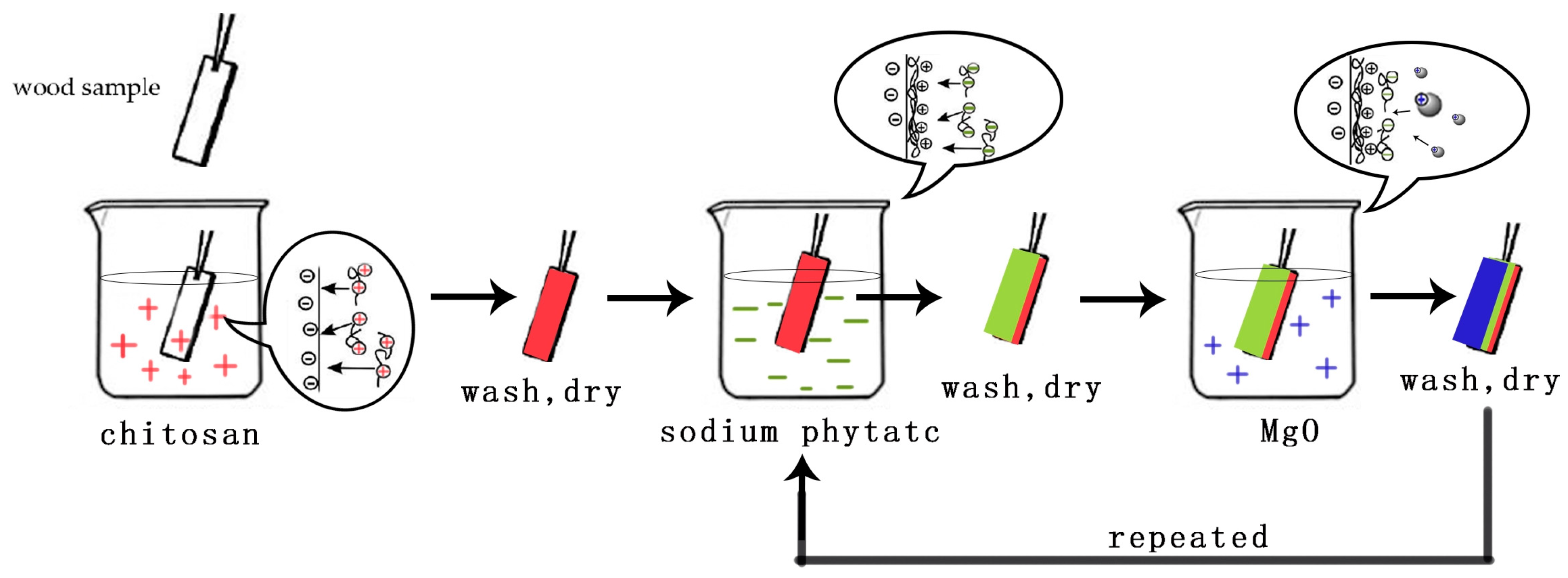
2.2. Fabrication of Fire Retardant Coatings on Wood Surface
2.3. Characterization
2.4. Thermogravimetric Analysis
2.5. Limiting Oxygen Index Test
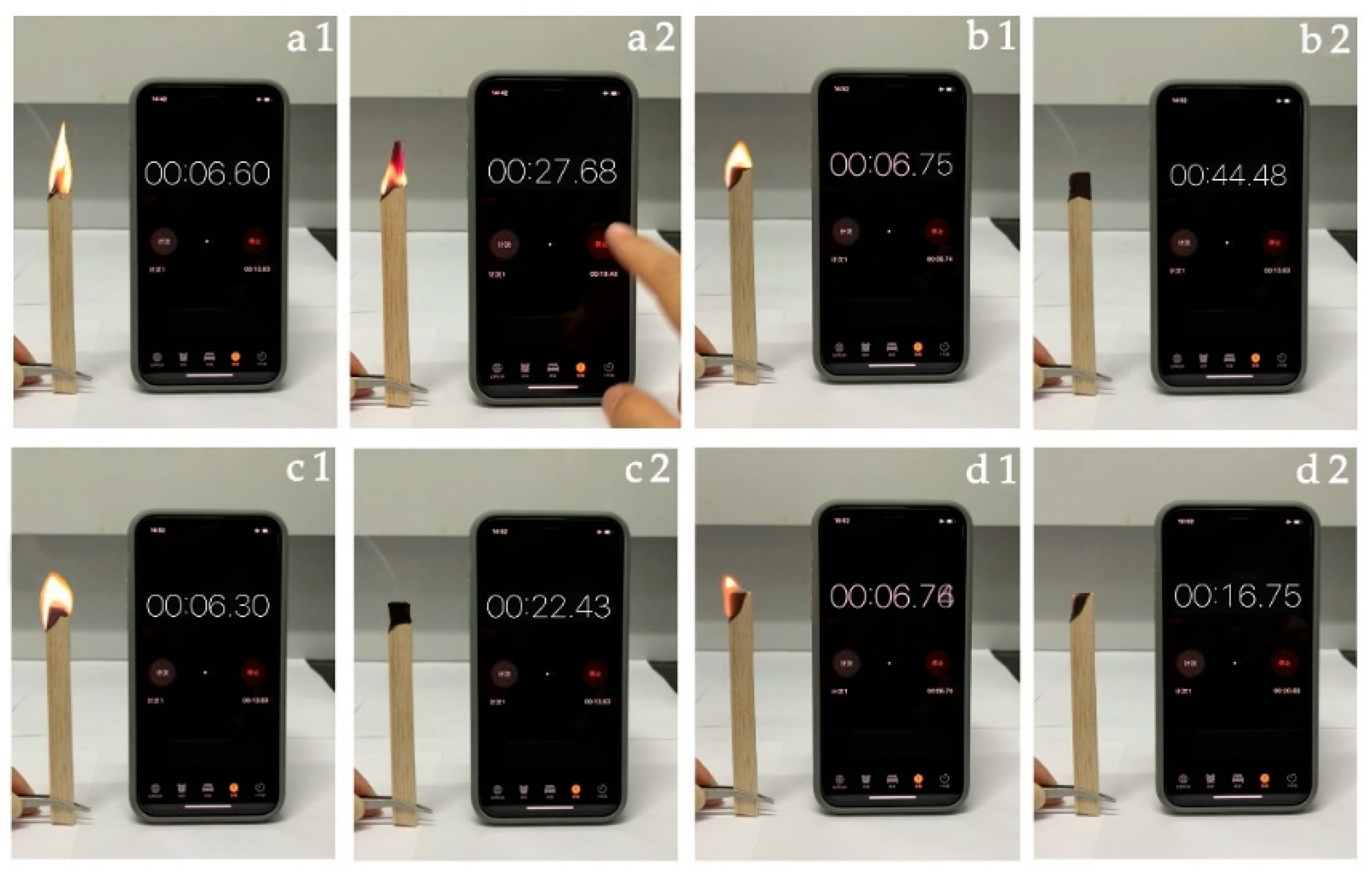
2.6. Exposure Combustion Test
3. Results
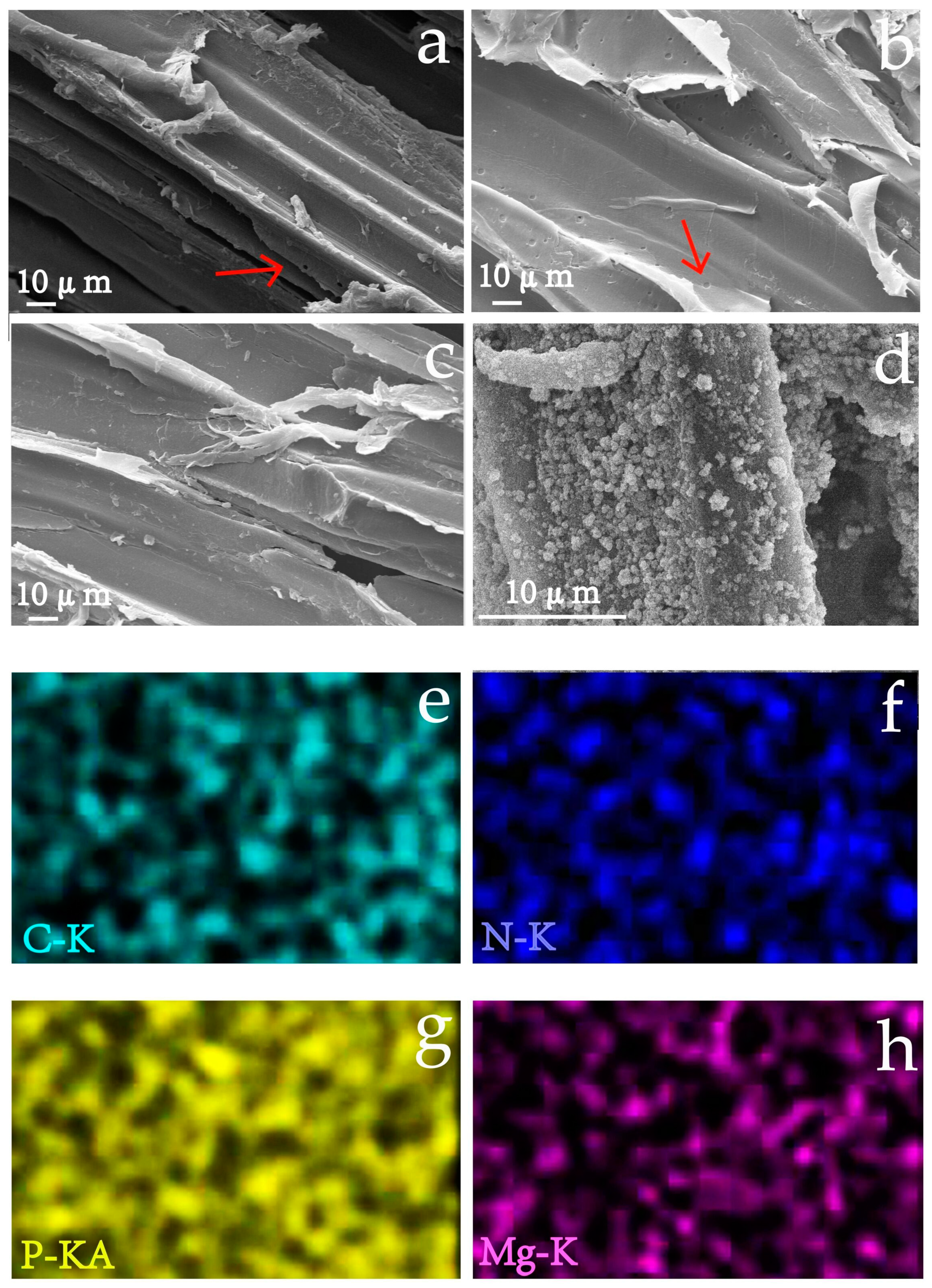
3.1. Surface Morphologies Analysis
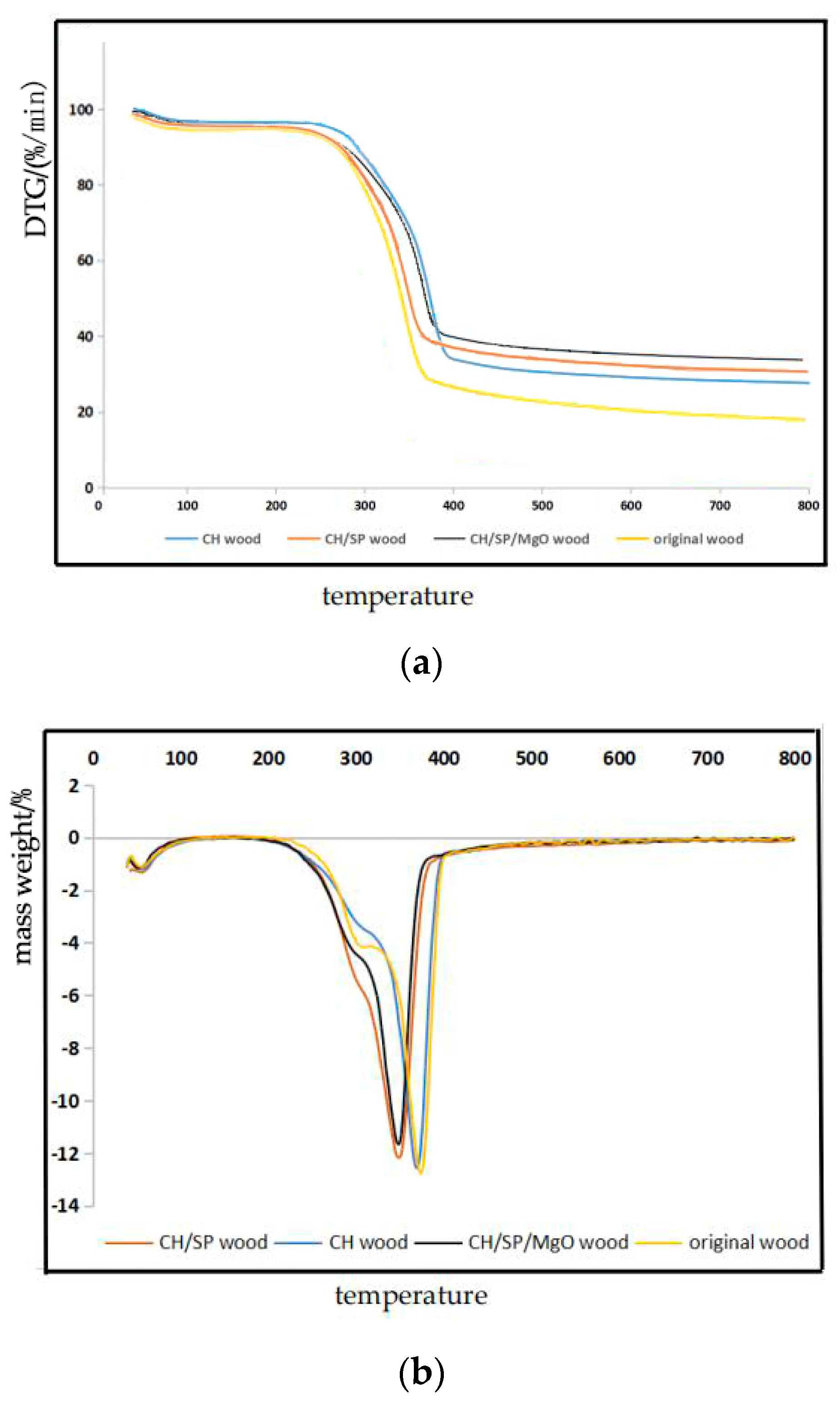
3.2. Thermal Stability Analysis
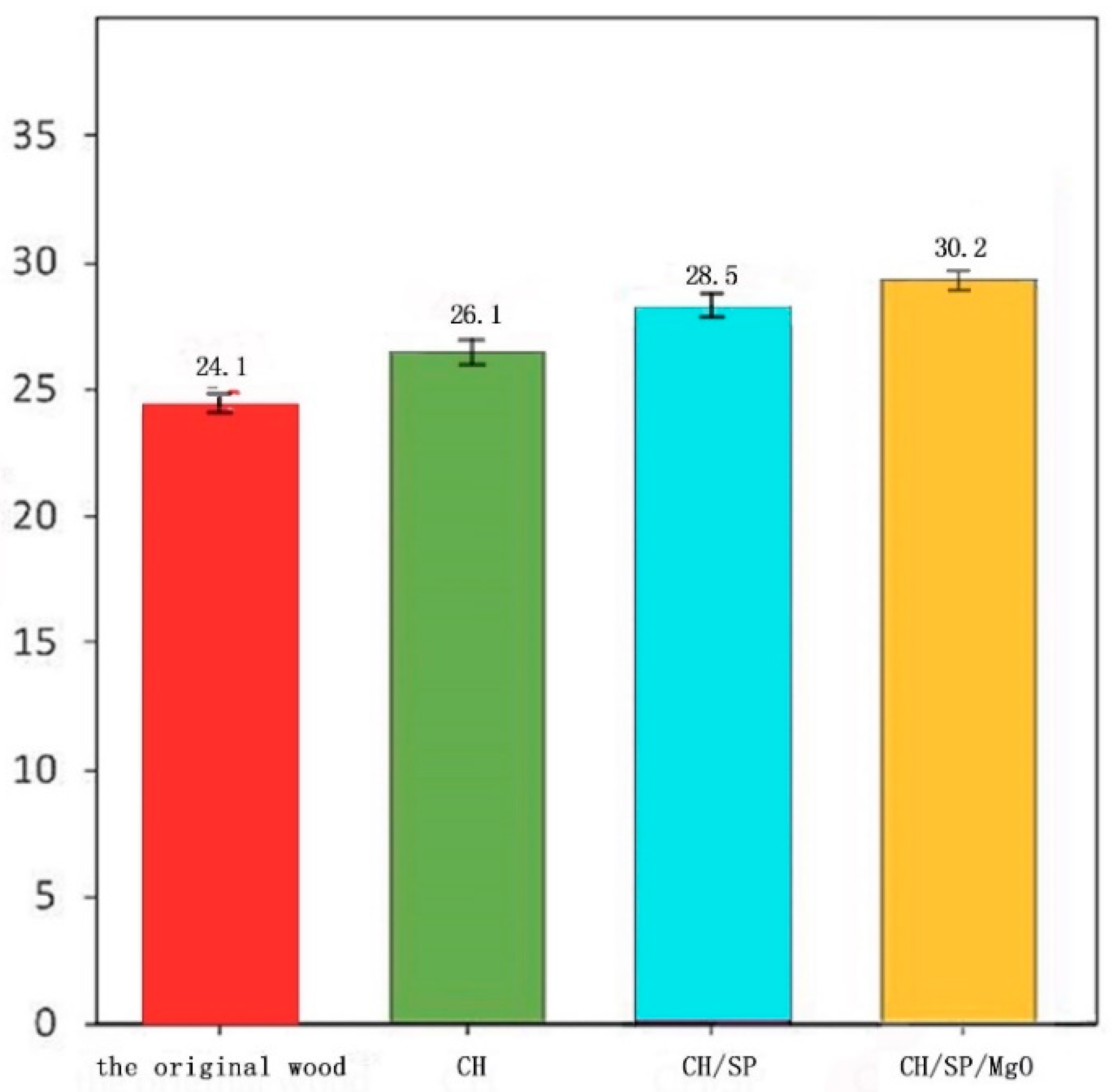
3.3. Fire Resistance Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, M.; Sun, C.; Wang, C. Thermal Degradation of Wood Treated with Flame Retardants. J. Therm. Anal. Calorim. 2006, 85, 765–769. [Google Scholar] [CrossRef]
- Son, Y.S. A Study on Flame Retardant Treatment of National Cultural Properties of Wood Structure. Fire Sci. Eng. 1988, 2, 31–41. [Google Scholar]
- Kin, I.B.; Hyun, S.H. A Study on the Flame Retardant Performance and Toxicity of the Painting Wood Painted with Flame Retardant Solution. Fire Sci. Eng. 2009, 23, 66–71. [Google Scholar]
- Merighi, S.; Mazzocchetti, L.; Benelli, T.; Maccaferri, E.; Giorgini, L. A new wood surface flame-retardant based on poly-m-aramid electrospun nanofibers. Polym. Eng. Sci. 2019, 59, 2541–2549. [Google Scholar] [CrossRef]
- Phromsaen, A.; Prinya, C.; Hiziroglu, S.; Kasemsiri, P. Thermal degradation and fire retrandancy of wood impregnated with nitrogen phosphorus flame retardant. Adv. Mater. Res. 2014, 931–932, 152–156. [Google Scholar] [CrossRef]
- Salman, S.; Pétrissans, A.; Thévenon, M.F.; Dumarçay, S.; Perrin, D.; Pollier, B.; Pollier, D.; Gérardin, P. Development of new wood treatments combining boron impregnation and thermo modification: Effect of additives on boron leachability. Eur. J. Wood Wood Prod. 2015, 72, 335–365. [Google Scholar] [CrossRef]
- Guo, C.; Wang, S.; Wang, Q. Synergistic effect of treatment with disodium octaborate tetrahydrateand guanyl urea phosphate on flammability of pine wood. Eur. J. Wood Wood Prod. 2018, 76, 213–220. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.S.; Liu, D.L. Synthesis and application of novel magnesium phosphate ester flame retardants for transparent intumescent fire retardant coatings applied on wood substrates. Prog. Org. Coatins 2019, 129, 327–337. [Google Scholar] [CrossRef]
- Lewin, M.; Endo, M. Catalysis of intumescent flame retardancy of polypropylene by metallic compounds. Polym. Adv. Technol. 2010, 14, 3–11. [Google Scholar] [CrossRef]
- Wu, N.; Yang, R. Effects of metal oxides on intumescent flame-retardant polypropylene. Polym. Adv. Technol. 2011, 22, 495–501. [Google Scholar] [CrossRef]
- Gao, W.; Yu, Y.; Chen, T.T.; Zhang, Q.W.; Chen, Z.W.; Chen, Z.Q.; Jiang, J.C. Enhanced flame retardancy of unsaturated polyester resin composites containing ammonium polyphosphate and metal oxides. J. Appl. Polym. Ence 2020, 137, 49148. [Google Scholar] [CrossRef]
- Sain, M.; Park, S.H.; Suhara, F.; Law, S. Flame retardant and mechanical properties of natural fibre-PP composites containing magnesium hydroxide. Polym. Degrad. Stab. 2004, 83, 363–367. [Google Scholar] [CrossRef]
- Guo, B.T.; Liu, Y.Z.; Zhang, Q.; Wang, F.Q.; Wang, Q.W.; Liu, Y.X.; Li, J.; Yu, H.P. Efficient Flame-Retardant and Smoke-Suppression Properties of Mg-Al-Layered Double-Hydroxide Nanostructures on Wood Substrate. ACS Appl. Mater. Interfaces 2017, 9, 23039–23047. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, S.F.; Wang, K.H.; Li, Q. Study on the Effects of Microwave Pretreatment on the Fire Retardant Permeability of Fast-Growing Wood. Appl. Mech. Mater. 2013, 2746, 1334–1338. [Google Scholar] [CrossRef]
- Zhang, K.K.; Zong, L.; Tan, Y.Q.; Ji, Q.; Yun, W.C.; Shi, R.; Xian, Y.Z. Improve the flame retardancy of cellulose fibers by grafting zinc ion. Carbohydr. Polym. 2016, 136, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Zhang, K.K.; Shi, R.; Ma, X.M.; Tan, L.W.; Ji, Q.; Xian, Y.Z. Enhanced flame-retardant properties of cellulose fibers by incorporation of acid-resistant magnesium-oxide microcapsules. Carbohydr. Polym. 2017, 176, 246–256. [Google Scholar] [CrossRef]
- Rao, X.; Liu, Y.; Fu, Y.; Liu, Y.; Yu, H. Formation and properties of polyelectrolytes/TiO2 composite coating on wood surfaces through layer-by-layer assembly method. Holzforschung 2016, 70, 361–367. [Google Scholar] [CrossRef]
- Tang, T.L.; Fu, Y.C. Formation of Chitosan/Sodium Phytate/Nano-Fe3O4 Magnetic Coatings on Wood Surfaces via Layer-by-Layer Self-Assembly. Coatings 2020, 10, 51. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, Y.C. Flame-Retardant Wood Composites Based on Immobilizing with Chitosan/Sodium Phytate/Nano-TiO2-ZnO Coatings via Layer-by-Layer Self-Assembly. Coatings 2020, 10, 296. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Tian, M.; Hu, X.; Qu, L.; Du, M.; Zhu, S.; Sun, Y.; Han, G. Ultraviolet protection cotton fabric achieved via layer-by-layer self-assembly of graphene oxide and chitosan. Appl. Surf. Sci. 2016, 377, 141–148. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Wang, W.; Song, L.; Fan, W.; Gao, X.; Xiong, C. Synthesis and characterization of pH-responsive mesoporous chitosan microspheres loaded with sodium phytate for smart water-based coatings. Mater. Corros. 2018, 69, 736–748. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.Q.; Jiang, Z.M.; Zhang, C.J.; Li, Z.F.; Chen, H.Q.; Zhu, P. Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. J. Anal. Appl. Pyrolysis 2018, 135, 289–298. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Manivasakan, P.; Karthik, A.; Rajendran, V. Hydrophobicity, flame retardancy and antibacterial properties of cotton fabrics functionalised with MgO/methyl silicate nanocomposites. RSC Adv. 2014, 4, 32161–32173. [Google Scholar] [CrossRef]
- Gariépy, E.R.; Leroux, J.C. Chitosan: A Natural Polycation with Multiple Applications. ACS Symp. Ser. 2006, 934, 243–259. [Google Scholar]
- Yang, J.; Lu, H.; Li, M.; Liu, J.; Zhang, S.L.; Xiong, L.; Sun, Q.J. Development of chitosan-sodium phytate nanoparticles as a potent antibacterial agent. Carbohydr. Polym. 2017, 178, 311–321. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Z.M.; Deng, C.; Zhu, M.F.; Wang, D.Y.; Li, K.; Deng, Y.; Jiang, M.M. Microporous Nano-MgO/Diatomite Ceramic Membrane with High Positive Surface Charge for Tetracycline Removal. J. Hazard. Mater. 2016, 320, 495–503. [Google Scholar] [CrossRef]
| Sample Name | Assembled Structure |
|---|---|
| CH | Wood/CH/CH/… |
| CH/SP | Wood/CH/SP/CH/SP/… |
| CH/SP/nano-MgO | Wood/CH/SP/MgO/SP/MgO/… |
| Invariants | |
| Immersion time: 90 min; Assembly cycle: 5; Mass volume concentration (chitosan, sodium phytate, nano-MgO): 1%. | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Tang, T.; Hou, S.; Fu, Y. Preparation and Synergistic Effect of Chitosan/Sodium Phytate/MgO Nanoparticle Fire-Retardant Coatings on Wood Substrate through Layer-By-Layer Self-Assembly. Coatings 2020, 10, 848. https://doi.org/10.3390/coatings10090848
Zhao F, Tang T, Hou S, Fu Y. Preparation and Synergistic Effect of Chitosan/Sodium Phytate/MgO Nanoparticle Fire-Retardant Coatings on Wood Substrate through Layer-By-Layer Self-Assembly. Coatings. 2020; 10(9):848. https://doi.org/10.3390/coatings10090848
Chicago/Turabian StyleZhao, Feiyue, Tingli Tang, Sijie Hou, and Yanchun Fu. 2020. "Preparation and Synergistic Effect of Chitosan/Sodium Phytate/MgO Nanoparticle Fire-Retardant Coatings on Wood Substrate through Layer-By-Layer Self-Assembly" Coatings 10, no. 9: 848. https://doi.org/10.3390/coatings10090848
APA StyleZhao, F., Tang, T., Hou, S., & Fu, Y. (2020). Preparation and Synergistic Effect of Chitosan/Sodium Phytate/MgO Nanoparticle Fire-Retardant Coatings on Wood Substrate through Layer-By-Layer Self-Assembly. Coatings, 10(9), 848. https://doi.org/10.3390/coatings10090848




