Application and Development Progress of Cr-Based Surface Coatings in Nuclear Fuel Element: I. Selection, Preparation, and Characteristics of Coating Materials
Abstract
1. Introduction
2. Current Status of Application of Zirconium Alloy Claddings
3. Proposal and Development of ATF Cladding
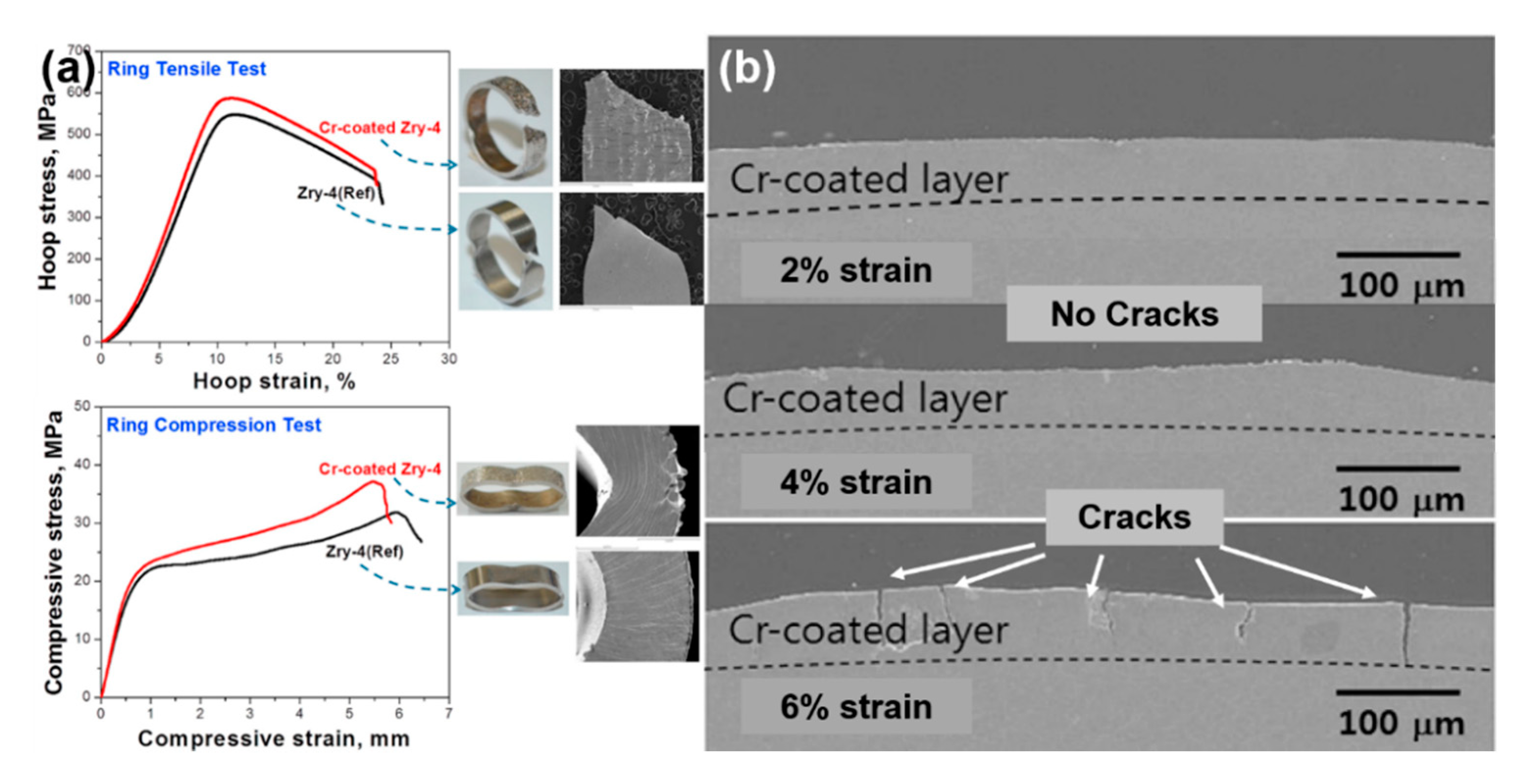
4. Development of ATF Coating Materials
5. Preparation of Cr-Based Coatings
5.1. Physical Vapor Deposition
5.2. Cold Spraying
5.3. 3D Laser-Melt Coating
6. Summary and Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, W.D. Materials for Nuclear Reactors; Atomic Energy Press: Beijing, China, 2000. [Google Scholar]
- Hirano, M.; Yonomoto, T.; Ishigaki, M.; Watanabe, N.; Maruyama, Y.; Sibamoto, Y.; Watanabe, T.; Moriyama, K. Insights from review and analysis of the Fukushima Dai-ichi accident. J. Nucl. Sci. Technol. 2012, 49, 1–17. [Google Scholar] [CrossRef]
- Carmack, J.; Goldner, F.; Bragg-Sitton, S.M.; Snead, L.L. Overview of the US DOE Accident Tolerant Fuel Development Program; Idaho National Laboratory (INL): Idaho Falls, IA, USA, 2013. [Google Scholar]
- Kurata, M. Research and development methodology for practical use of accident tolerant fuel in light water reactors. Nucl. Eng. Technol. 2016, 48, 26–32. [Google Scholar] [CrossRef]
- Bischoff, J.; Blanpain, P.; Brachet, J.-C.; Lorrette, C.; Ambard, A.; Strumpell, J.; Mckoy, K. Development of fuels with enhanced accident tolerance. In Proceedings of the Technical Meeting Held at Oak Ridge National Laboratories, Oak Ridge, TN, USA, 13–16 October 2014; No. IAEA-TECDOC-1797. pp. 22–30. [Google Scholar]
- Kim, H.G.; Yang, J.H.; Kim, W.J.; Koo, Y.H. Development status of accident-tolerant fuel for light water reactors in Korea. Nucl. Eng. Technol. 2016, 48, 1–15. [Google Scholar] [CrossRef]
- Koo, Y.-H.; Yang, J.-H.; Park, J.-Y.; Kim, K.S.; Kim, H.G.; Kim, D.J.; Jung, Y.I.; Song, K.W. KAERI’s development of LWR accident-tolerant fuel. Nucl. Technol. 2014, 186, 295–304. [Google Scholar] [CrossRef]
- Karoutas, Z.; Brown, J.; Atwood, A.; Hallstadius, L.; Lahoda, E.; Ray, S.; Bradfute, J. The maturing of nuclear fuel: Past to Accident Tolerant Fuel. Prog. Nucl. Energy 2018, 102, 68–78. [Google Scholar] [CrossRef]
- Zhang, P.J. Study progress on the accident tolerrent fuel. China Nucl. Power 2017, 10, 425–429. [Google Scholar]
- Liu, J.K.; Zhang, X.H.; Yun, D. A complete review and a prospect on the candidate materials for accident-tolerant fuel claddings. Mater. Rev. 2018, 32, 1757–1778. [Google Scholar]
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Bragg-Sitton, S.M.; Todosow, M.; Montgomery, R.; Stanek, C.R.; Montgomery, R.; Carmack, W.J. Metrics for the technical performance evaluation of light water reactor accident-tolerant fuel. Nucl. Technol. 2017, 195, 111–123. [Google Scholar] [CrossRef]
- Tang, C.; Stueber, M.; Seifert, H.J.; Steinbrueck, M. Protective coatings on zirconium-based alloys as accident-tolerant fuel (ATF) claddings. Corros. Rev. 2017, 35, 141–165. [Google Scholar] [CrossRef]
- Brachet, J.C.; Idarraga-Trujillo, I.; Flem, M.L.; Saux, M.L.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, V.; Tupin, M.; et al. Early studies on Cr-Coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Maier, B.; Yeom, H.; Johnson, G.; Dabney, T.; Walters, J.; Xu, P.; Romero, J.; Shah, H.; Sridharan, K. Development of cold spray chromium coatings for improved accident tolerant zirconium-alloy cladding. J. Nucl. Mater. 2019, 519, 247–254. [Google Scholar] [CrossRef]
- Ševecek, M.; Gurgen, A.; Phillips, B.; Che, Y.; Wagih, M.; Shirvan, K. Cold spray Cr-coated fuel cladding with enhanced accident tolerance. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza Jeju, Jeju Island, Korea, 10–14 September 2017; Available online: https://www.researchgate.net/publication/319728416 (accessed on 15 September 2017).
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. Adhesion property and High-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W.; et al. AREVA NP’s enhanced accident-tolerant fuel developments: Focus on Cr-coated M5 cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]
- Krejcí, J.; Ševecek, M.; Kabátová, J.; Manoch, F.; Kocí, J.; Cvrcek, L.; Málek, J.; Krum, S.; Šutta, P.; Bublíková, P.; et al. Experimental behavior of chromium-based coatings. In Proceedings of the TopFuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328066345 (accessed on 4 October 2018).
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 108077. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Wen, Q.; Ruan, X.; Luo, F.; Bai, G.; Qing, Y.; Zhu, D.; Huang, Z.; Zhang, Y.; et al. Behavior of plasma sprayed Cr coatings and FeCrAl coatings on Zr fuel cladding under loss-of-coolant accident conditions. Surf. Coat. Technol. 2018, 344, 141–148. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.G.; Park, J.Y.; Jung, Y.I.; Park, D.J.; Koo, Y.H. High temperature steam-oxidation behavior of arc ion plated Cr coatings for accident tolerant fuel claddings. Surf. Coat. Technol. 2015, 280, 256–259. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Chaari, N.; Vauglin, C.; Buchanan, K.; Barberis, P.; Monsifort, E.; Schuster, F.; Brachet, J.C.; Nimishakavi, K. Cr-coated cladding development at framatome. In Proceedings of the Top Fuel 2018, Prague, Czech Republic, 30 September–4 October 2018; p. A0152. [Google Scholar]
- Ševecek, M.; Gurgen, A.; Seshadri, A.; Che, Y.; Wagih, M.; Phillips, B.; Champagne, V.; Shirvan, K. Development of Cr cold spray–coated fuel cladding with enhanced accident tolerance. Nucl. Eng. Technol. 2018, 50, 229–236. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.H.; Park, J.Y.; Koo, Y.H. Oxidation behavior and mechanical property of Cr-coated zirconium cladding prepared by 3D laser coating. In Proceedings of the 2014 Water Reactor Fuel Performance Meeting (WRFPM 2014), Sendai, Japan, 14–17 September 2014. No. 100054. [Google Scholar]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Busby, J.T. Structural materials for fission & fusion energy. Materialstoday 2009, 12, 12–19. [Google Scholar]
- Azevedo, C.R.F. Selection of fuel cladding material for nuclear fission reactors. Eng. Fail. Anal. 2011, 18, 1943–1962. [Google Scholar] [CrossRef]
- Tunes, M.A.; de Oliveira, C.R.E.; Schön, C.G. Multi-objective optimization of a compact pressurized water nuclear reactor computational model for biological shielding design using innovative materials. Nucl. Eng. Des. 2017, 313, 20–28. [Google Scholar] [CrossRef]
- Motta, A.T.; Couet, A.; Comstock, R.J. Corrosion of Zirconium Alloys Used for Nuclear Fuel Cladding. Annu. Rev. Mater. Res. 2015, 45, 311–343. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Sun, C.; Yang, Z.; Wu, Z. Study on Corrosion Resistance of N36 Zirconium Alloy in LiOH Aqueous Solution. World J. Nucl. Sci. Technol. 2018, 8, 30–37. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.H.; Yang, J.H.; Koo, Y.H. Progress of surface modified Zr cladding development for ATF at KAERI. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza Jeju, Jeju Island, Korea, 10–14 September 2017; Available online: https://www.researchgate.net/publication/320074054 (accessed on 28 September 2017).
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.H.; Choi, B.K.; Lee, Y.H. Out-of-pile performance of surface-modified Zr cladding for accident tolerant fuel in LWRs. J. Nucl. Mater. 2018, 510, 93–99. [Google Scholar] [CrossRef]
- PWR Nuclear Power Plants Reactor System Design—Core—Part 3: Fuel Assembly; NB/T 20057.3-2012; National Energy Administration: Beijing, China, 2012.
- Standard Review Plan—4.2 Fuel System Design; NUREG-0800; Nuclear Regulatory Commission: Washington, DC, USA, 2007.
- Code on Design Limits for Fuel System of PWR Nuclear Power Plants; EJ/T 1029-1996; China National Nuclear Corporation: Beijing, China, 1996.
- Alam, T.; Khan, M.K.; Pathak, M.; Ravi, K.; Singh, R.; Gupta, S.K. A review on the clad failure studies. Nucl. Eng. Des. 2011, 241, 3658–3677. [Google Scholar] [CrossRef]
- Zielinsk, A.; Sobieszczyk, S. Hydrogen-enhanced degradation and oxide effects in zirconium alloys for nuclear applications. Int. J. Hydrog. Energy 2011, 36, 8619–8629. [Google Scholar] [CrossRef]
- Stuckert, J.; Grobe, M.; Rössger, C.; Steinbrück, M.; Walter, M. Influence of the temperature history on secondary hydriding and mechanical properties of zircaloy-4 claddings: An analysis of the QUENCH-LOCA bundle tests. In Proceedings of the 22nd International Conference on Nuclear Enginerring, Prague, Czech Republic, 7–11 July 2014; Available online: https://www.researchgate.net/publication/271762938 (accessed on 16 February 2018).
- Erbacher, F.; Leistikow, S. Zircaloy fuel cladding behavior in a loss-of-coolant accident: A review. Zircon. Nucl. Ind. Astm Int. 1987, 939, 451. [Google Scholar]
- Kim, H.G.; Jeong, Y.H.; Kim, K.T. The effects of creep and hydride on spent fuel integrity during interim dry storage. Nucl. Eng. Technol. 2010, 42, 249–258. [Google Scholar] [CrossRef]
- Billone, M.; Yan, Y.; Burtseva, T.; Daum, R. Cladding Embrittlement during Postulated Loss-of-Coolant Accidents; NUREG/CR-6967; ANL-07/04; Nuclear Regulatory Commission: Washington, DC, USA, 2008. [Google Scholar]
- Jang, K.-N.; Cha, H.-J.; Kim, K.-T. Allowable peak heat-up cladding temperature for spent fuel integrity during interim-dry storage. Nucl. Eng. Technol. 2017, 49, 1740–1747. [Google Scholar] [CrossRef]
- Cheng, B.; Kim, Y.J.; Chou, P. Improving Accident Tolerance of Nuclear Fuel with Coated Mo-alloy Cladding. Nucl. Eng. Technol. 2016, 48, 16–25. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 2nd ed.; Elsevier: San Francisco, CA, USA, 2016. [Google Scholar]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science Publishers: London, UK, 1988. [Google Scholar]
- Sarrazin, P.; Galerie, A.; Fouletier, J. Mechanisms of High Temperature Corrosion; Trans Tech Publications Ltd.: Uetikon-Zürich, Switzerland, 2008. [Google Scholar]
- Pint, B.A.; Terrani, K.A.; Yamamoto, Y.; Snead, L.L. Material Selection for Accident Tolerant Fuel Cladding. Metall. Mater. Trans. E 2015, 2, 190–196. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.G.; Park, J.Y.; Kim, W.J. Fabrication and measurement of hoop strength of SiC triplex tube for nuclear fuel cladding applications. J. Nucl. Mater. 2015, 458, 29–36. [Google Scholar] [CrossRef]
- Lu, H.R.; Zhang, M. Current status and recent research achievements in SiC composites for fuel cladding. China Nucl. Power 2016, 9, 306–312. [Google Scholar]
- Zhou, X.G.; Wang, H.L.; Zhao, S. Progress of SiCf/SiC Composites for Nuclear Application. Adv. Ceram. 2016, 37, 151–167. [Google Scholar]
- Brachet, J.C.; Lorrette, C.; Michaux, A.; Sauder, C.; Idarraga-Trujillo, I.; Le Saux, M.; Le Flem, M.; Schuster, F.; Billard, A.; Monsifrot, E.; et al. CEA studies on advanced nuclear fuel claddings for enhanced Accident Tolerant LWRs Fuel (LOCA and beyond LOCA conditions). In Proceedings of the Fontevraud 8: Conference on Contribution of Materials Investigations and Operating Experience to LWRs’ Safety, Performance and Reliability, Avignon, France, 15–18 September 2014; Available online: https://www.researchgate.net/publication/268444279 (accessed on 18 November 2014).
- Qiu, B.; Wang, J.; Deng, Y.; Wang, M.; Wu, Y.; Qiu, S.Z. A review on thermohydraulic and mechanical-physical properties of SiC, FeCrAl and Ti3SiC2 for ATF cladding. Nucl. Eng. Technol. 2020, 52, 1–13. [Google Scholar] [CrossRef]
- Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T.; Massey, C.P.; Pint, B.A. Development of low-Cr ODS FeCrAl alloys for accident-tolerant fuel cladding. J. Nucl. Mater. 2018, 501, 59–71. [Google Scholar] [CrossRef]
- Pint, B.A. Performance of FeCrAl for accident-tolerant fuel cladding in High-temperature steam. Corros. Rev. 2017, 35, 167–175. [Google Scholar] [CrossRef]
- Gamble, K.A.; Barani, T.; Pizzocri, D.; Hales, J.D.; Terrani, K.A.; Pastore, G. An investigation of FeCrAl cladding behavior under normal operating and loss of coolant conditions. J. Nucl. Mater. 2017, 491, 55–66. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Pint, B.A.; Terrani, K.A.; Field, K.G.; Snead, L.L. Development and property evaluation of nuclear grade wrought FeCrAl fuel cladding for light water reactors. J. Nucl. Mater. 2015, 467, 703–716. [Google Scholar] [CrossRef]
- Terrani, K.A.; Zinkle, S.J.; Snead, L.L. Advanced oxidation-resistant iron-based alloys for LWR fuel cladding. J. Nucl. Mater. 2014, 448, 420–435. [Google Scholar] [CrossRef]
- Cheng, B. Fuel behavior in severe accidents and Mo-alloy based cladding designs to improve accident tolerance. Atw. Int. Z. Fuer Kernenerg. 2013, 59, 158–160. [Google Scholar]
- Nelson, A.T.; Sooby, E.S.; Kim, Y.J.; Cheng, B.; Maloy, S.A. High temperature oxidation of molybdenum in water vapor environments. J. Nucl. Mater. 2014, 448, 441–447. [Google Scholar] [CrossRef]
- Cheng, B.; Chou, P.; Kim, Y.J. Evaluations of Mo-alloy for light water reactor fuel cladding to enhance accident tolerance. Epj Nucl. Sci. Technol. 2016, 2, 5. [Google Scholar] [CrossRef]
- Cheng, B.; Chou, P.; Kim, Y.J. Development of Mo-Based Accident Tolerant LWR Fuel Cladding; No. IAEA-TECDOC-1797: Vienna, Austria, 2016. [Google Scholar]
- Pint, B.A.; Tettani, K.A.; Brady, M.P.; Cheng, T.; Keiser, J.R. High temperature oxidation of fuel cladding candidate materials in steam–hydrogen environments. J. Nucl. Mater. 2013, 440, 420–427. [Google Scholar] [CrossRef]
- Galvin, T.; Hyatt, N.C.; Rainforth, W.M.; Reaney, L.M.; Shepherd, D. Slipcasting of MAX phase tubes for nuclear fuel cladding applications. Nucl. Mater. Energy 2020, 22, 100725. [Google Scholar] [CrossRef]
- Ward, J.; Bowden, D.; Prestat, E.; Holdsworth, S.; Stewart, D.; Barsoum, M.W.; Preuss, M.; Frankel, P. Corrosion performance of Ti3SiC2, Ti3AlC2, Ti2AlC and Cr2AlC MAX phases in simulated primary water conditions. Corros. Sci. 2018, 139, 444–453. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Yang, J.H.; Koo, Y.H. Development of surface modified Zr cladding by coating technology for ATF. In Proceedings of the Top Fuel 2016, Boise, ID, USA, 11–15 September 2016; pp. 1157–1163. [Google Scholar]
- Bai, G.H.; Chen, Z.L.; Zhang, Y.W.; Liu, E.W.; Xue, J.X.; Yu, W.W.; Wang, R.S.; Li, R.; Liu, T. Research progress of coating on zirconium alloy for nuclear fuel cladding. Rare Met. Mater. Eng. 2017, 46, 2035–2040. [Google Scholar]
- Yang, H.Y.; Zhang, R.Q.; Peng, X.M.; Wang, M.L. Research progress regarding surface coating of zirconium alloy cladding. Surf. Technol. 2017, 46, 69–77. [Google Scholar]
- Shah, H.; Romero, J.; Xu, P.; Maier, B.; Johnson, G.; Walters, J.; Dabney, T.; Yeom, H.; Sridharan, K. Development of surface coatings for enhanced accident tolerant fuel. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza Jeju, Jeju Island, Korea, 10–14 September 2017; Available online: https://www.researchgate.net/publication/320190475 (accessed on 3 October 2017).
- Maier, B.; Yeom, H.; Johnson, G.; Dabney, T.; Walters, J.; Romero, J.; Shah, H.; Xu, P.; Sridharan, K. Development of cold spray coatings for accident-tolerant fuel cladding in light water reactors. Jom 2018, 70, 198–202. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Park, J.Y.; Koo, Y.H. Application of coating technology on zirconium-based alloy to decrease high-temperature oxidation. In Proceedings of the ASTM 17th International Symposium on Zirconium in the Nuclear Industry, Hyderabad, India, 3–7 February 2013; Available online: https://www.researchgate.net/publication/280534853 (accessed on 29 July 2015).
- Bragg-Sitton, S.M. Update on development of enhanced accident tolerant fuel for light water reactors in the United States. In Start-Up Meeting of the Expert Group on Accident Tolerant Fuels for Light Water Reactors; OECD/NEA: Paris, France, 2014. [Google Scholar]
- Forgeron, T.; Guédeney, P.; Brachet, J.-C.; Michaux, A. ATF R&D status and perspectives. In Start-Up Meeting of the Expert Group on Accident Tolerant Fuels for Light Water Reactors; OECD/NEA: Paris, France, 2014. [Google Scholar]
- Koo, Y.-H.; Yang, J.-H.; Kim, H.-G. Accident tolerant fuel (ATF) development: KAERI’s R&D status. In Start-Up Meeting of the Expert Group on Accident Tolerant Fuels for Light Water Reactors; OECD/NEA: Paris, France, 2014. [Google Scholar]
- Kurata, M. Overview on ATF R&D in Japan. In Start-up Meeting of the Expert Group on Accident Tolerant Fuels for Light Water Reactors; OECD/NEA: Paris, France, 2014. [Google Scholar]
- Field, K.G.; Hu, X.; Littrell, K.C.; Yamamoto, Y.; Snead, L.L. Radiation tolerance of neutron-irradiated model Fe-Cr-Al alloys. J. Nucl. Mater. 2015, 465, 746–755. [Google Scholar] [CrossRef]
- Parish, C.M.; Unocic, K.A.; Tan, L.; Zinkle, S.J.; Kondo, S.; Snead, L.L.; Hoelzer, D.T.; Katoh, Y. Helium sequestration at nanoparticle-matrix interfaces in helium + heavy ion irradiated nanostructured ferritic alloys. J. Nucl. Mater. 2017, 483, 21–34. [Google Scholar] [CrossRef]
- Byun, T.S.; Li, M.; Cockeram, B.V.; Snead, L.L. Deformation and fracture properties in neutron irradiated pure Mo and Mo alloys. J. Nucl. Mater. 2008, 376, 240–246. [Google Scholar] [CrossRef]
- Daghbouj, N.; Li, B.S.; Callisti, M.; Sen, H.S.; Karlik, M.; Polcar, T. Microstructural evolution of helium-irradiated 6H–SiC subjected to different irradiation conditions and annealing temperatures: A multiple characterization study. Acta Mater. 2019, 181, 160–172. [Google Scholar] [CrossRef]
- Daghbouj, N.; Li, B.S.; Callisti, M.; Sen, H.S.; Lin, J.; Ou, X.; Karlik, M.; Polcar, T. The structural evolution of light-ion implanted 6H-SiC single crystal: Comparison of the effect of helium and hydrogen. Acta Mater. 2020, 188, 609–622. [Google Scholar] [CrossRef]
- Koyanagi, T.; Nozawa, T.; Katoh, Y.; Snead, L.L. Mechanical property degradation of high crystalline SiC fiber–reinforced SiC matrix composite neutron irradiated to ∼100 displacements per atom. J. Eur. Ceram. Soc. 2018, 38, 1087–1094. [Google Scholar] [CrossRef]
- Ang, C.; Silva, C.; Shih, C.; Koyanagi, T.; Katoh, Y.; Zinkle, S.J. Anisotropic swelling and microcracking of neutron irradiated Ti3AlC2–Ti5Al2C3 materials. Scr. Mater. 2016, 114, 74–78. [Google Scholar] [CrossRef]
- Ang, C.; Zinkle, S.; Shih, C.; Shih, C.; Silva, C.; Cetiner, N.; Katoh, Y. Phase stability, swelling, microstructure and strength of Ti3SiC2-TiC ceramics after low dose neutron irradiation. J. Nucl. Mater. 2017, 483, 44–53. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.J.; Yang, Y.Y.; Liu, N. Preparation, structure, and properties of high-entropy alloy multilayer coatings for nuclear fuel cladding: A case study of AlCrMoNbZr/(AlCrMoNbZr)N. J. Nucl. Mater. 2018, 512, 15–24. [Google Scholar] [CrossRef]
- Brady, M.; Wright, I.; Gleeson, B. Alloy design strategies for promoting protective oxide-scale formation. Jom 2000, 52, 16–21. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Ceramic coating for corrosion (c3) resistance of nuclear fuel cladding. Surf. Coat. Technol. 2015, 281, 133–143. [Google Scholar] [CrossRef]
- Van Nieuwenhove, R.; Andersson, V.; Balak, J.; Oberländer, B. In-Pile testing of CrN, TiAlN, and AlCrN coatings on zircaloy cladding in the Halden reactor. In Proceedings of the Zirconium in the Nuclear Industry: 18th International Symposium, Hilton Head, SC, USA, 5–19 May 2016; Available online: https://www.researchgate.net/publication/322519179 (accessed on 16 January 2018).
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Tunes, M.A.; da Silva, F.C.; Camara, O.; Schön, C.G.; Sagás, J.C.; Fontana, L.C.; Donnelly, S.E.; Greaves, G.; Edmondson, P.D. Energetic particle irradiation study of TiN coatings: Are these films appropriate for accident tolerant fuels? J. Nucl. Mater. 2018, 512, 239–245. [Google Scholar] [CrossRef]
- Terrani, K.A.; Parish, C.M.; Shin, D.; Pint, B.A. Protection of zirconium by alumina- and chromia-forming iron alloys under High-temperature steam exposure. J. Nucl. Mater. 2013, 438, 64–71. [Google Scholar] [CrossRef]
- Zhong, W.; Mouche, P.A.; Han, X.; Heuser, B.J.; Mandapaka, K.K.; Was, G.S. Performance of iron-chromium-aluminum alloy surface coatings on Zircaloy 2 under high-temperature steam and normal BWR operating conditions. J. Nucl. Mater. 2016, 470, 327–338. [Google Scholar] [CrossRef]
- Park, D.J.; Kim, H.G.; Jung, Y.I.; Park, J.H.; Yang, J.H.; Koo, Y.H. Behavior of an improved Zr fuel cladding with oxidation resistant coating under loss-of-coolant accident conditions. J. Nucl. Mater. 2016, 482, 75–82. [Google Scholar] [CrossRef]
- Daub, K.; Van Nieuwenhove, R.; Nordin, H. Investigation of the impact of coatings on corrosion and hydrogen uptake of Zircaloy-4. J. Nucl. Mater. 2015, 467, 260–270. [Google Scholar] [CrossRef]
- Roberts, D.A. Magnetron Sputtering and Corrosion of Ti-Al-C and Cr-Al-C Coatings for Zr-Alloy Nuclear Fuel Cladding. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2016. [Google Scholar]
- Tunes, M.A.; Harrison, R.W.; Donnelly, S.E.; Edmondson, P.D. A transmission electron microscopy study of the neutron-irradiation response of Ti-based MAX phases at high temperatures. Acta Mater. 2019, 169, 237–247. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Su, R.; Gao, J.; Shi, L. Effects of irradiation damage on the structure in Cr2AlC thin film. Nucl. Inst. Methods Phys. Res. B 2019, 450, 286–290. [Google Scholar] [CrossRef]
- Imtyazuddin, M.; Mir, A.H.; Tunes, M.A.; Vishnyakov, V.M. Radiation resistance and mechanical properties of magnetron-sputtered Cr2AlC thin films. J. Nucl. Mater. 2019, 526, 151742. [Google Scholar] [CrossRef]
- Pantano, M.; Avincola, V.; de Seze, P.A.; McKrell, T.; Kazimi, M.H. High temperature steam oxidation performance of MAX phase (Ti2AlC) coated ZIRLO. In Proceedings of the International Congress on Advances in Nuclear Power Plants, ICAPP 2014, Charlotte, NC, USA, 6–9 April 2014; pp. 2126–2135. [Google Scholar]
- Yeom, H.; Hauch, B.; Cao, G.; Garcia-Diaz, B.; Martinez-Rodriguez, M.; Colon-Mercado, H.; Olson, L.; Sridharan, K. Laser surface annealing and characterization of Ti2AlC plasma vapor deposition coating on zirconium-alloy substrate. Thin Solid Film. 2016, 615, 202–209. [Google Scholar] [CrossRef]
- Tang, C.; Steinbrueck, M.; Stueber, M.; Grosse, M.; Yu, X.; Ulrich, S.; Seifert, H.J. Deposition, characterization and high-temperature steam oxidation behavior of single-phase Ti2AlC-coated Zircaloy-4. Corrossion Sci. 2018, 135, 87–98. [Google Scholar] [CrossRef]
- Brachet, J.C.; Le Saux, M.; Le Flem, M.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Cobac, C.; Lahogue, F.; Rousselot, J.; Tupin, M. On-going studies at CEA on chromium coated zirconium based nuclear fuel claddings for enhanced accident tolerant LWRs fuel. In Proceedings of the TopFuel 2015, Zurich, Switzerland, 13–17 September 2015; Available online: https://www.researchgate.net/publication/283446965 (accessed on 3 November 2015).
- Wu, A.; Ribis, J.; Brachet, J.C.; Clouet, E.; Lepretre, F.; Bordas, E.; Arnal, B. HRTEM and chemical study of an ion-irradiated chromium/zircaloy-4 interface. J. Nucl. Mater. 2018, 504, 289–299. [Google Scholar] [CrossRef]
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Vasilenko, R.L.; Ovcharenko, V.D.; Tolstolutskaya, G.D.; Kopanets, I.E.; Kolodiy, I. Irradiation resistance of vacuum arc chromium coatings for zirconium alloy fuel claddings. J. Nucl. Mater. 2018, 510, 163–167. [Google Scholar] [CrossRef]
- Maier, B.R.; Yeom, H.; Johnson, G.; Dabney, T.; Hu, J.; Baldo, P.; Li, M.M.; Sridharan, K. In situ TEM investigation of irradiation-induced defect formation in cold spray Cr coatings for accident tolerant fuel applications. J. Nucl. Mater. 2018, 512, 320–323. [Google Scholar] [CrossRef]
- Idarraga-Trujillo, I.; Le Flem, M.; Brachet, J.C.; Le Saux, M. Assessment at CEA of coated nuclear fuel cladding for LWRs with increasing margins in LOCA and beyond LOCA conditions. In Proceedings of the TopFuel 2013, Charlotte, NC, USA, 15–19 September 2013; pp. 860–867. [Google Scholar]
- Brachet, J.C.; Guilbert, T.; Le Saux, M.; Rousselot, J.; Nony, G.; Toffolon-Masclet, C.; Michau, A.; Schuster, F.; Palancher, H.; Bischoff, J. Behavior of Cr-coated M5 claddings during and after high temperature steam oxidation from 800 °C up to 1500 °C (Loss-of-Coolant Accident & Design Extension Conditions). In Proceedings of the Topfuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328095354 (accessed on 5 October 2018).
- Brachet, J.C.; Le Saux, M.; Lezaud-Chaillioux, V.; Dumerval, M.; Houmaire, Q.; Lomello, F.; Schuster, F.; Monsifrot, E.; Bischoff, J.; Pouillier, E. Behavior under LOCA conditions of enhanced accident tolerant chromium coated zircaloy-4 claddings. In Proceedings of the TopFuel 2016 Conference, Boise, ID, USA, 11–15 September 2016; pp. 1173–1178. [Google Scholar]
- Brachet, J.C.; Dumerval, M.; Lezaud-Chaillioux, V.; Le Saux, M.; Rouesne, E.; Hamon, D.; Urvoy, S.; Guilbert, T.; Houmaire, Q.; Cobac, C. Behavior of chromium coated M5TM claddings under LOCA conditions. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza Jeju, Jeju Island, Korea, 10–14 September 2017; Available online: https://www.researchgate.net/publication/319988324 (accessed on 22 September 2017).
- Dumerval, M.; Houmaire, Q.; Brachet, J.C.; Palancher, H.; Bischoff, J.; Pouillier, E. Behavior of Chromium Coated M5 Claddings upon Thermal Ramp Tests under Internal Pressure (Loss-of-Coolant Accident Conditions). In Proceedings of the TopFuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328095355 (accessed on 5 October 2018).
- Brova, M.J.; Alat, E.; Pauley, M.A.; Sherbondy, R.; Motta, A.T.; Wolfe, D.E. Undoped and ytterbium-doped titanium aluminum nitride coatings for improved oxidation behavior of nuclear fuel cladding. Surf. Coat. Technol. 2017, 331, 163–171. [Google Scholar] [CrossRef]
- Ougier, M.; Michau, A.; Lomello, F.; Schuster, F.; Maskrot, H.; Schlegel, M.L. High-temperature oxidation behavior of HiPIMS as-deposited Cr–Al–C and annealed Cr2AlC coatings on Zr-based alloy. J. Nucl. Mater. 2020, 528, 151855. [Google Scholar] [CrossRef]
- Bourdon, G.; Ševecek, M.; Krejcí, J.; Cvrcek, L. High-temperature steam and air oxidation of chromium-coated optimized ZIRLO™. Acta Polytech. Ctu Proc. 2019, 24, 1–8. [Google Scholar] [CrossRef]
- Chalupová, A.; Krejcí, J.; Cvrcek, L.; Ševecek, M.; Rozkošný, V.; Pribyl, A.; Halodová, P.; Gávelová, P. Coated cladding behavior during high-temperature transients. Acta Polytech. Ctu Proc. 2019, 24, 9–14. [Google Scholar] [CrossRef]
- Ševecek, M.; Krejcí, J.; Shahin, M.; Shahin, M.H.; Petrik, J.; Ballinger, R.G.; Shirvan, K. Fatigue behavior of cold spray-coated accident tolerant cladding. In Proceedings of the TopFuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328066444 (accessed on 4 October 2018).
- Ševecek, M.; Shirvan, K.; Ballinger, R.G. Study of thermal creep of coated cladding materials. Acta Polytech. Ctu Proc. 2018, 19, 22–29. [Google Scholar] [CrossRef]
- Yeom, H.; Dabney, T.; Johnson, G.; Maier, B.; Lenling, M.; Sridharan, K. Improving deposition efficiency in cold spraying chromium coatings by powder annealing. Int. J. Adv. Manuf. Technol. 2018, 100, 1373–1382. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. High-temperature oxidation behavior of Cr-coated zirconium alloy, Proceeding of LWR Fuel Performance Meeting. In Proceedings of the TopFuel 2013, Charlotte, NC, USA, 15–19 September 2013; pp. 842–846. [Google Scholar]
- Greene, J.E. Review Article: Tracing the recorded history of thin-film sputter deposition: From the 1800s to 2017. J. Vac. Sci. Technol. A: Vac. Surf. Film. 2017, 35, 05C204. [Google Scholar] [CrossRef]
- Anders, A. A review comparing cathodic arcs and high power impulse magnetron sputtering (HiPIMS). Surf. Coat. Technol. 2014, 257, 308–325. [Google Scholar] [CrossRef]
- Anders, A. Tutorial: Reactive high power impulse magnetron sputtering (R-HiPIMS). J. Appl. Phys. 2017, 121, 171101. [Google Scholar] [CrossRef]
- Ribis, J.; Wu, A.; Brachet, J.C.; Barcelo, F.; Arnal, B. Atomic-scale interface structure of a Cr-coated Zircaloy-4 material. J. Mater. Sci. 2018, 53, 9879–9895. [Google Scholar] [CrossRef]
- Ribis, J.; Wu, A.; Brachet, J.C.; Clouet, E.; Arnal, B.; Rouesne, E.; Urvoy, S.; Barcelo, F.; Gentiles, A.; Baumier, C. Chromium hardening and Zr-Cr interface stability of irradiated chromium-coated Zircaloy-4 alloy. In Proceedings of the Numat 2018, Seattle, WA, USA, 14–18 October 2018; p. cea-02400192. [Google Scholar]
- Gärtner, F.; Stoltenhoff, T.; Schmidt, T.; Kreye, H. The cold spray process and its potential for industrial applications. J. Therm. Spray Technol. 2006, 15, 223–232. [Google Scholar] [CrossRef]
- Michau, A.; Maury, F.; Schuster, F.; Boichot, R.; Pons, M. Evidence for a Cr metastable phase as a tracer in DLI-MOCVD chromium hard coatings usable in high temperature environment. Appl. Surf. Sci. 2017, 422, 198–206. [Google Scholar] [CrossRef]
- Michau, A.; Maskrot, H.; Gazal, Y.; Maury, F.; Duguet, T.; Boichot, R.; Pons, M.; Brachet, J.-C.; Monsifrot, E.; Schuster, F. Inner surface protection of nuclear fuel cladding, towards a full-length treatment by dli-mocvd, an optimized coating process. In Proceedings of the TopFuel 2018, Prague, Czech Republic, 30 September–4 October 2018; Available online: https://www.researchgate.net/publication/328073036 (accessed on 4 October 2018).
- Michau, A.; Maury, F.; Schuster, F.; Boichot, R.; Pons, M.; Monsifrot, E. Chromium carbide growth at low temperature by a highly efficient DLI-MOCVD process in effluent recycling mode. Surf. Coat. Technol. 2017, 332, 96–104. [Google Scholar] [CrossRef]
- Michau, A.; Maury, F.; Schuster, F.; Nuta, I.; Gazal, Y.; Boichot, R.; Pons, M. Chromium carbide growth by direct liquid injection chemical vapor deposition in long and narrow tubes, experiments, modeling and simulation. Coatings 2018, 8, 220. [Google Scholar] [CrossRef]
- Michau, A.; Maury, F.; Schuster, F.; Lomello, F.; Brachet, J.-C.; Rouesne, E.; Le Saux, M.; Boichot, R.; Pons, M. High-temperature oxidation resistance of chromium-based coatings deposited by DLI-MOCVD for enhanced protection of the inner surface of long tubes. Surf. Coat. Technol. 2018, 349, 1048–1057. [Google Scholar] [CrossRef]
- Kim, J.M.; Ha, T.H.; Kim, I.H.; Kim, H.G. Microstructure and oxidation behavior of CrAl laser-coated Zircaloy-4 alloy. Metals 2017, 7, 59. [Google Scholar] [CrossRef]
- Dong, Y.; Ge, F.; Meng, F.; Zhao, G.; Zhou, J.; Deng, Z.; Huang, Q.; Huang, F. Improved oxidation resistance of zirconium at high-temperature steam by magnetron sputtered Cr-Al-Si ternary coatings. Surf. Coat. Technol. 2018, 350, 841–847. [Google Scholar] [CrossRef]
- Zhong, W.; Mouche, P.A.; Heuser, B.J. Response of Cr and Cr-Al coatings on Zircaloy-2 to high temperature steam. J. Nucl. Mater. 2018, 498, 137–148. [Google Scholar] [CrossRef]
- Wei, D.H.; Wu, Y.W.; He, X.J.; Ma, X.F.; Wang, H.; Shi, H.J. High-temperature oxidation and tensile behaviors of CrAl coating on zirconium alloy. China Surf. Eng. 2019, 32, 44–53. [Google Scholar]
- Park, D.J.; Jung, Y.I.; Park, J.H.; Lee, Y.H.; Choi, B.K.; Kim, H.G. Microstructural characterization of accident tolerant fuel cladding with Cr-Al alloy coating layer after oxidation at 1200 °C in a steam environment. Nucl. Eng. Technol. 2020. [Google Scholar] [CrossRef]
- Zhang, J. Initial study on composition demixing effect of alloy coating/cathode material depostied by multi-arc ion plating. Vacuum 1994, 4, 44–47. [Google Scholar]
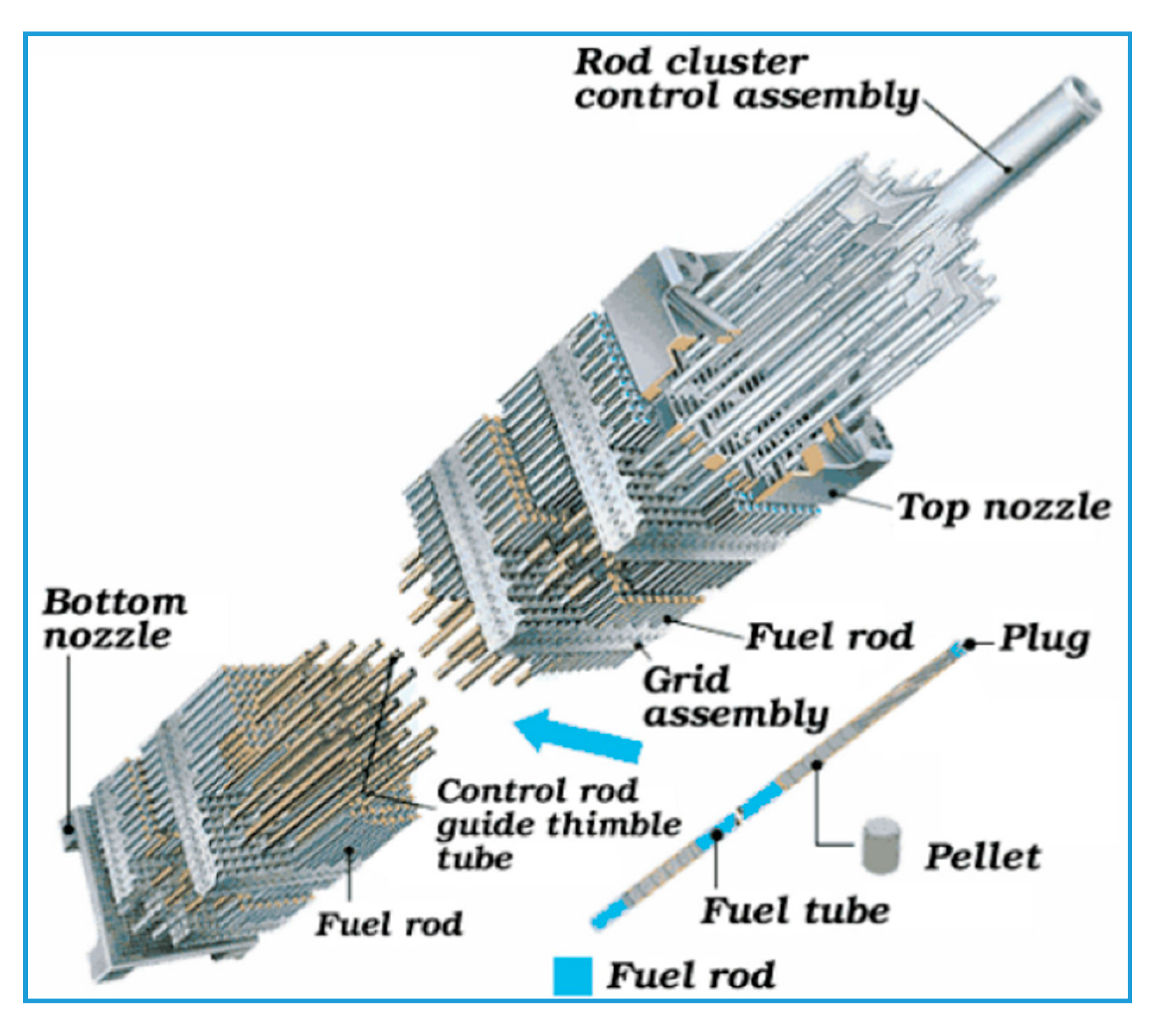
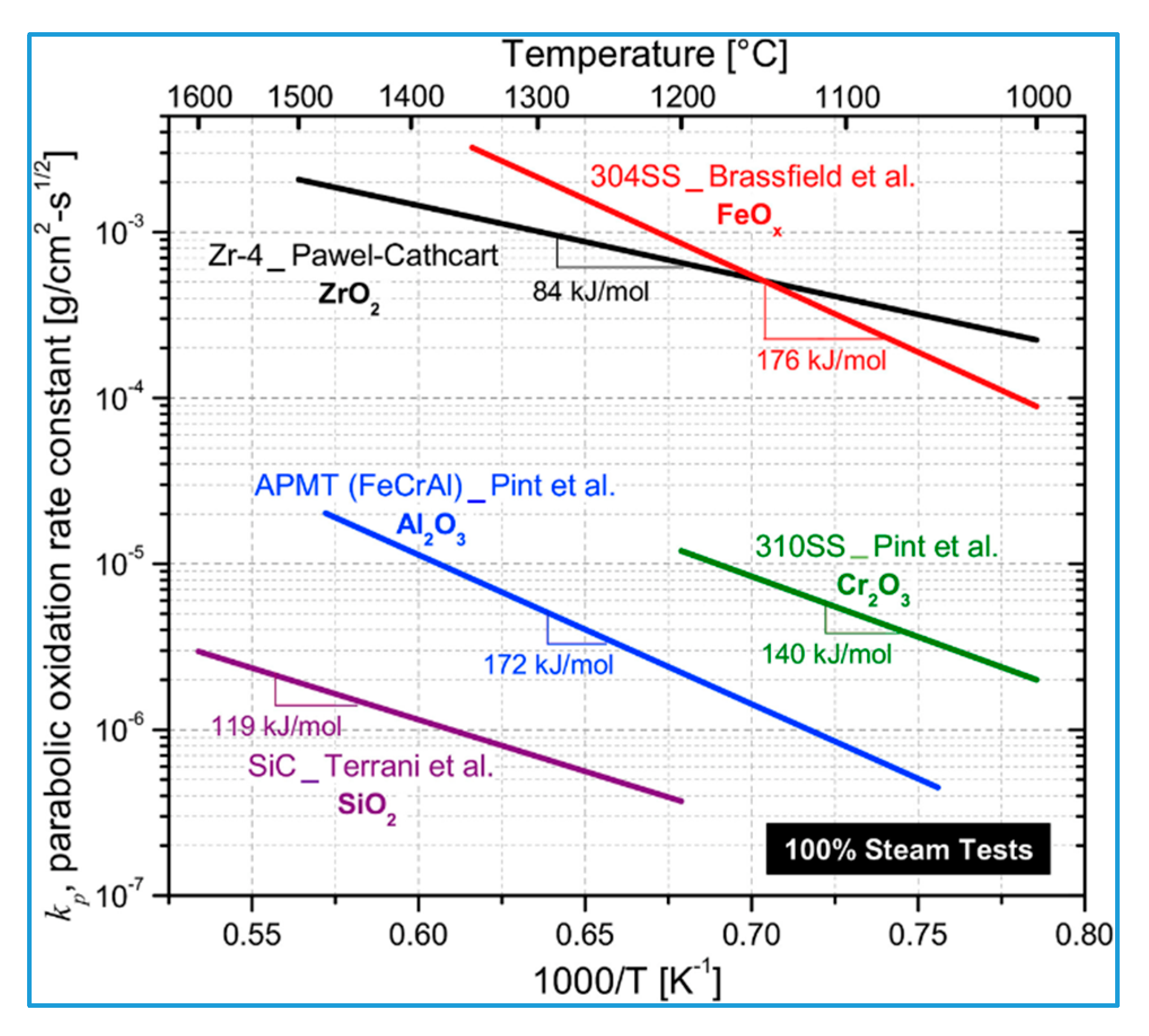
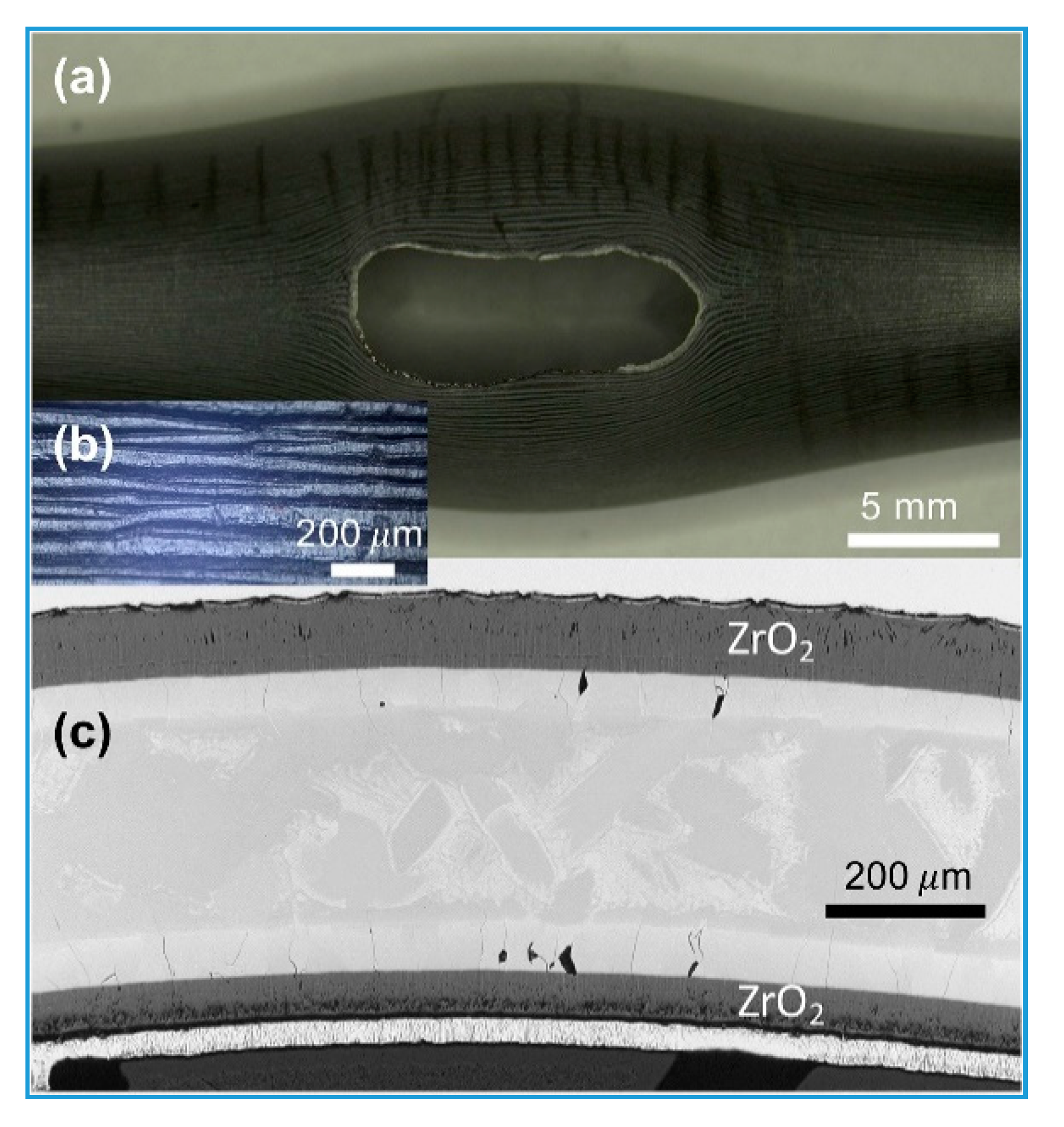
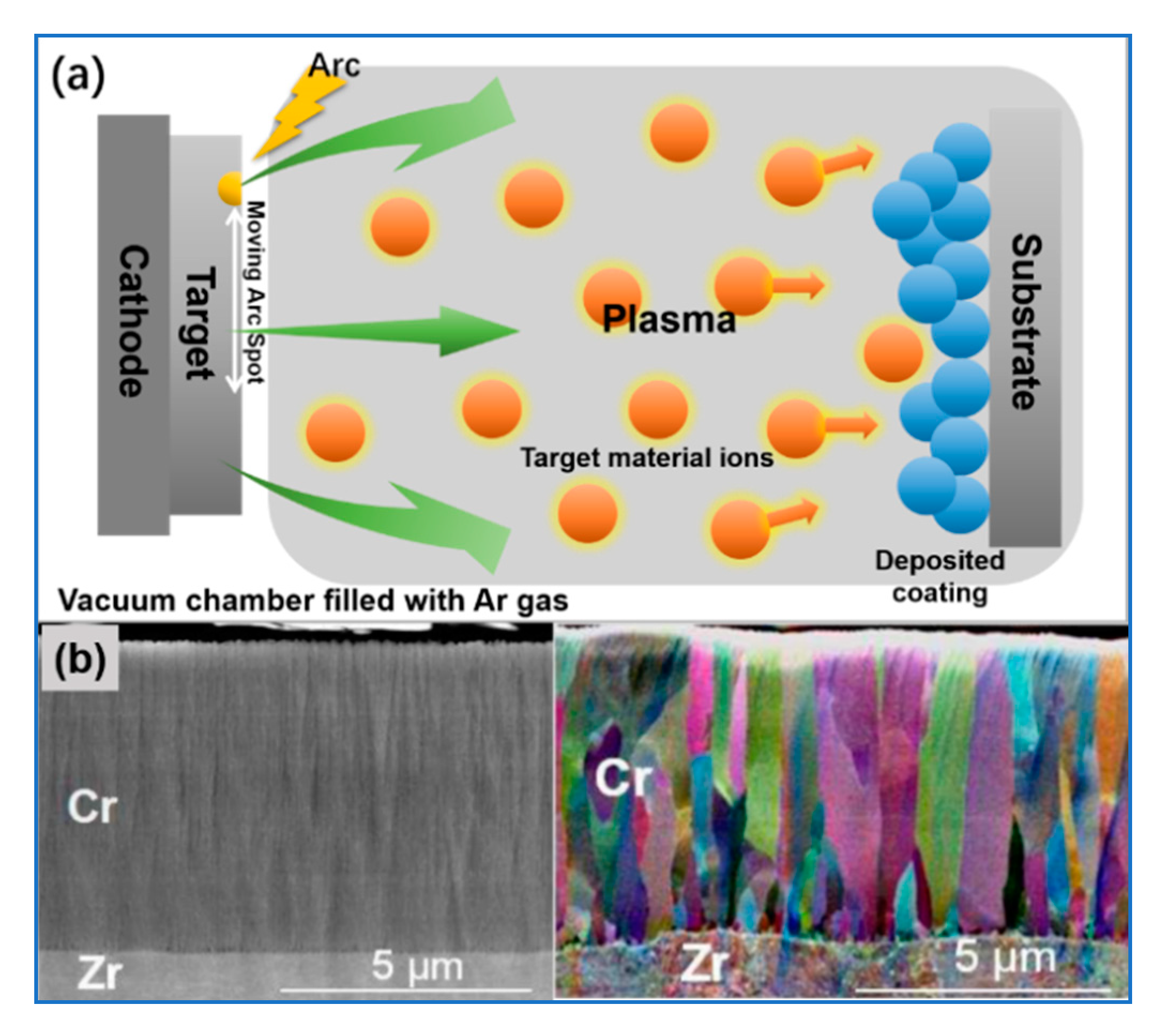

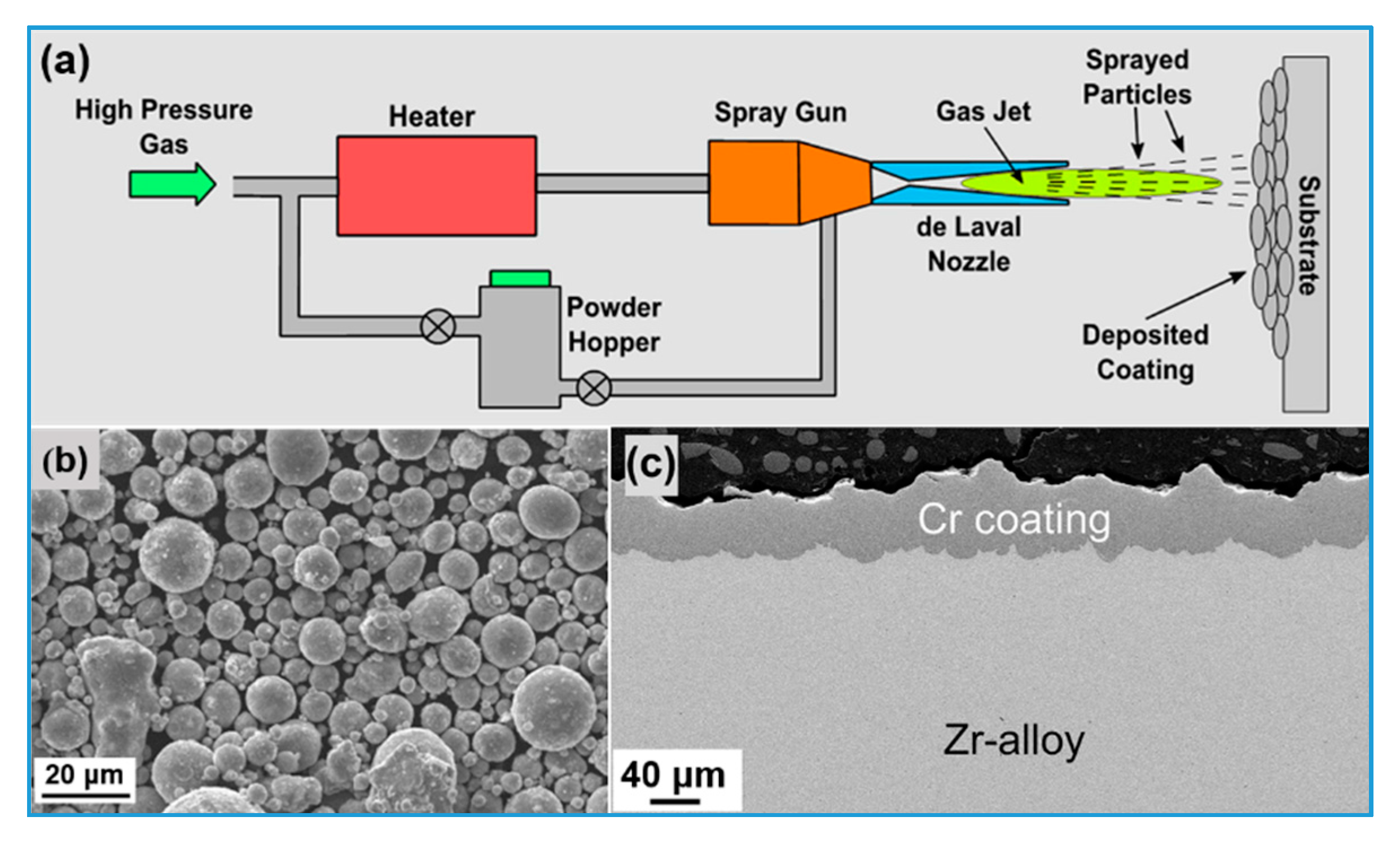
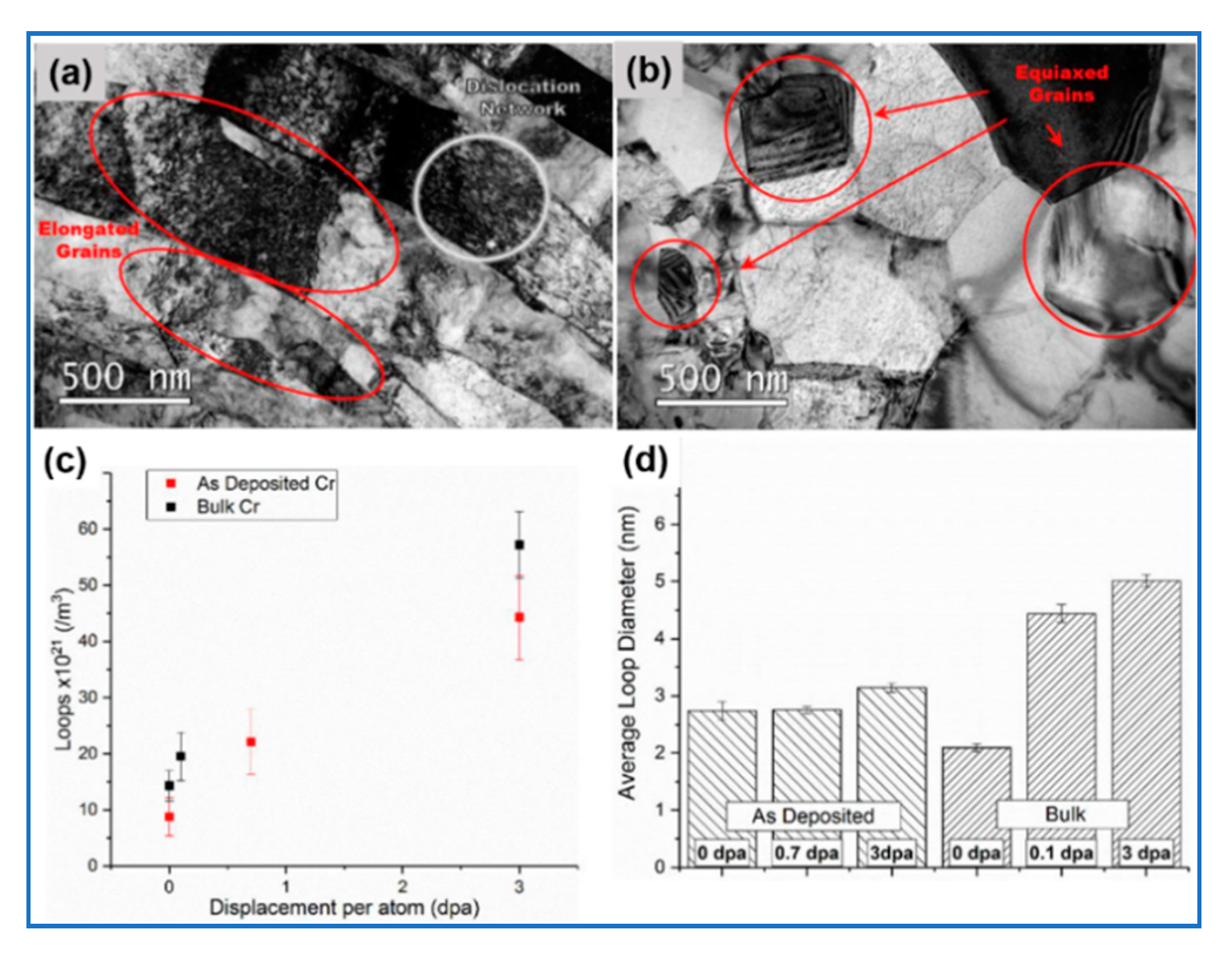
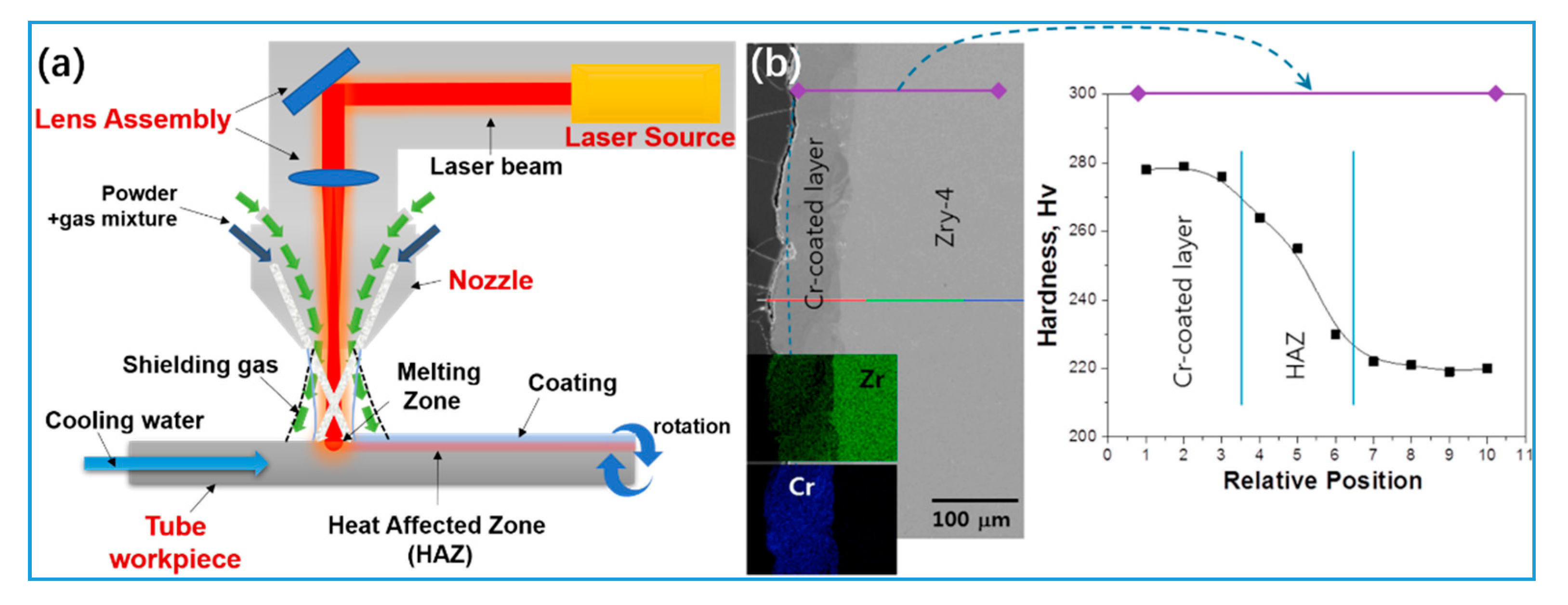
| Performance Parameters | Commercial Zirconium Alloy Cladding | Limit Values | |
|---|---|---|---|
| Manufacturability | Optimized | - | |
| Normal operating conditions | Corrosion | No problem | Corrosion depth < 10% of cladding thickness [35,37] |
| Creep | No problem | Hoop strain < 1% [35,36,37] | |
| Irradiation growth | No problem | Shoulder gap closure | |
| Wear resistance | No problem | Wear damage < 10% of cladding thickness [37] | |
| PCMI/PCI [36,38] | Failure related to hydrides (corrosion and hydrogen absorption) | - | |
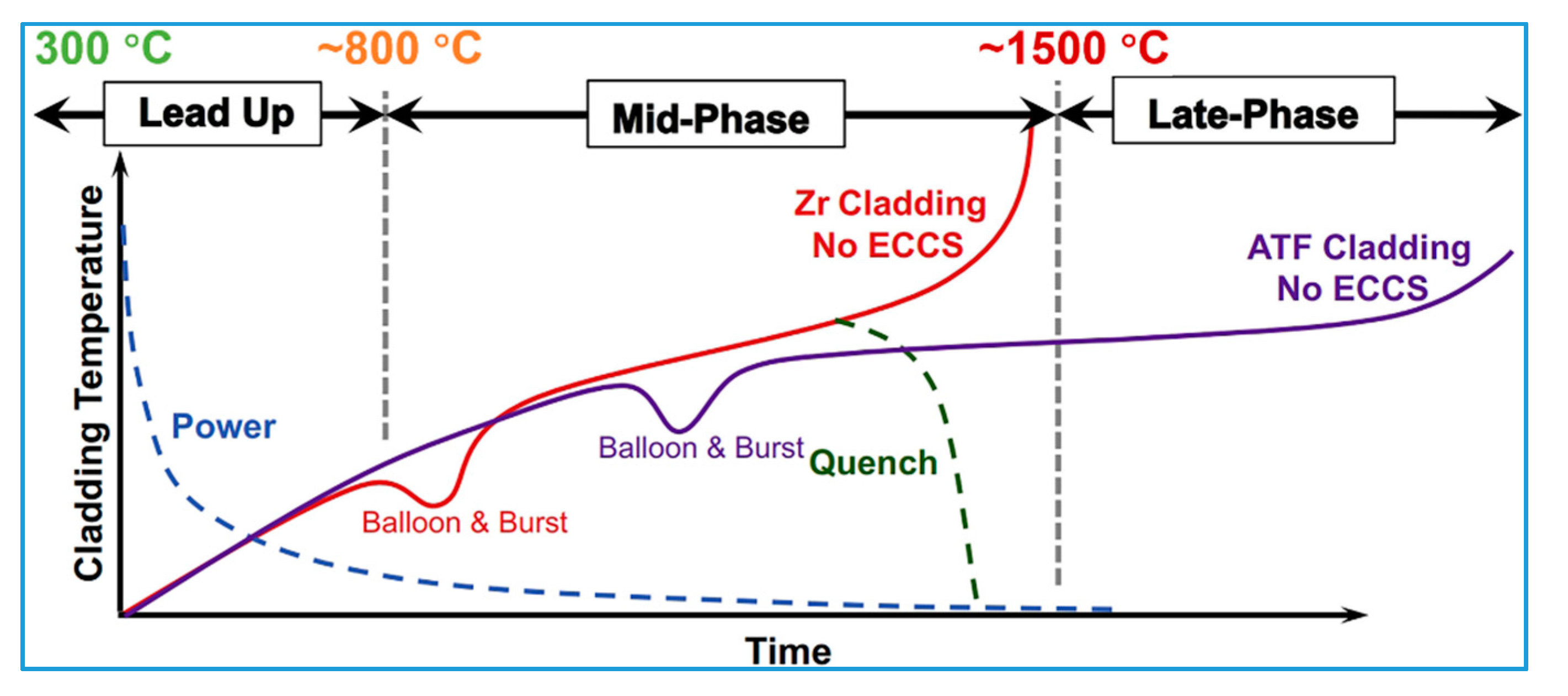
| DBA | LOCA [36,38] | Severe oxidation and hydrogen generation; Balloon and burst | PCT < 1204 °C; ECR < 17% [37,43] |
| RIA [36,38] | PCMI failure related to hydrides | - | |
| BDBA [11,36,38] | Severe oxidation and hydrogen generation; Melting point problem | - | |
| Spent fuel storage [42,44] | Hydrogen-induced embrittlement behavior Hydride reorientation problem | Fuel-cladding interface temperature: drying < 570 °C, storage < 400 °C; Clad hoop stress < 90 MPa [42] | |
| Leading Institute | Abbreviation | Country | Preferred Research Directions |
|---|---|---|---|
| Oak Ridge National Laboratory | ORNL | US | FeCrAl, SiCf/SiC, MAX phases, coated Zr alloy |
| Los Alamos National Laboratory | LANL | US | FeCrAl, Mo alloy |
| Westinghouse Electric Corporation | WHE | US | Coated Zr alloy, SiCf/SiC |
| General Electric Company | GE | US | FeCrAl |
| Electric Power Research Institute | EPRI | US | Mo-alloy |
| Areva S.A. | AREVA | France | Coated Zr alloy |
| French Alternative Energies and Atomic Energy Commission | CEA | France | Coated Zr alloy, SiCf/SiC |
| Korea Atomic Energy Research Institute | KAERI | South Korea | Coated Zr alloy, SiCf/SiC |
| Japan Atomic Energy Agency | JAEA | Japan | FeCrAl, SiCf/SiC |
| Nuclear Power Institute of China | NPIC | China | Coated Zr alloy, FeCrAl, SiCf/SiC |
| Type | Potential High-Temperature-Resistant Coating Materials |
|---|---|
| Metals | Cr, CrAl, AlTiCr, FeCrAl, 310SS, and high-entropy alloys |
| Carbides | MAX phase carbides (Cr2AlC, Zr2AlC, Ti2AlC, and Ti3SiC2), CrxCy, and SiC |
| Nitrides | CrN, AlCrN, TiAlN, TiAlCrN, and TiAlSiN |
| Silicides | ZrSi2 |
| Multilayer | Cr-Zr/Cr/CrN, CrN/Cr, Cr/CrAl, Cr/FeCrAl, Mo/FeCrAl, and TiN/TiAlN |
| Key Performance | Cr | TiAlN | TiN/TiAlN | FeCrAl | CrN | Ti2AlC | Ti3SiC2 | Cr2AlC |
|---|---|---|---|---|---|---|---|---|
| Corrosion behavior | √ | × | √ | √ | √ | × | - | × |
| [14] | [91] | [91] | [34] | [96] | [97] | [97] | ||
| Irradiation behavior | √ | - | × | - | √ | × | × | × |
| [104] | [92] | [90] | [98] | [85] | [100] | |||
| Oxidation behavior | √ | √ | - | × | × | √ | × | √ |
| [14] | [113] | [93] | [46] | [73] | [51] | [114] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, X.; Zhang, R. Application and Development Progress of Cr-Based Surface Coatings in Nuclear Fuel Element: I. Selection, Preparation, and Characteristics of Coating Materials. Coatings 2020, 10, 808. https://doi.org/10.3390/coatings10090808
Chen H, Wang X, Zhang R. Application and Development Progress of Cr-Based Surface Coatings in Nuclear Fuel Element: I. Selection, Preparation, and Characteristics of Coating Materials. Coatings. 2020; 10(9):808. https://doi.org/10.3390/coatings10090808
Chicago/Turabian StyleChen, Huan, Xiaoming Wang, and Ruiqian Zhang. 2020. "Application and Development Progress of Cr-Based Surface Coatings in Nuclear Fuel Element: I. Selection, Preparation, and Characteristics of Coating Materials" Coatings 10, no. 9: 808. https://doi.org/10.3390/coatings10090808
APA StyleChen, H., Wang, X., & Zhang, R. (2020). Application and Development Progress of Cr-Based Surface Coatings in Nuclear Fuel Element: I. Selection, Preparation, and Characteristics of Coating Materials. Coatings, 10(9), 808. https://doi.org/10.3390/coatings10090808




