Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches
Abstract
1. Introduction
2. Native Bioactive Polymers as Packaging Materials
3. Nanostructured Polymers as Packaging Materials
4. Drug Delivery Systems as Packaging Materials
5. Probiotic Loaded Food Packaging
6. Bioactive Packaging Materials with Multiple Mechanisms of Activity
7. Active Food Packaging Materials and Their Sensorial Activity
- Critical indicators for temperature which indicate only if a product has been exposed to a higher temperature (or sometimes a lower one) than the reference value;
- Critical TTIs which indicate the cumulative effect of the temperature change and time passed with such higher temperature;
- Indicators with complete history which can accomplish a continuous monitoring of the temperature change in time [137].
8. Conclusions
Funding
Conflicts of Interest
References
- Marsh, K.; Bugusu, B. Food packaging-Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Oprea, O.; Andronescu, E.; Ficai, D.; Ficai, A.; Oktar, F.N.; Yetmez, M. ZnO Applications and Challenges. Curr. Org. Chem. 2014, 18, 192–203. [Google Scholar] [CrossRef]
- Ficai, A.; Sonmez, M.; Ficai, D.; Andronescu, E. Graphene based materials for environmental applications. Adv. Mater. Technol. Environ. Appl. 2017, 1, 79–85. [Google Scholar]
- Kamran, F.; Reddy, N. Bioactive Peptides from Legumes: Functional and Nutraceutical Potential. Recent Adv. Food Sci. 2018, 1, 134–149. [Google Scholar]
- Nedelcu, I.A.; Ficai, A.; Sonmez, M.; Ficai, D.; Oprea, O.; Andronescu, E. Silver Based Materials for Biomedical Applications. Curr. Org. Chem. 2014, 18, 173–184. [Google Scholar] [CrossRef]
- Spoiala, A.; Ficai, D.; Gunduz, O.; Ficai, A.; Andronescu, E. Silver nanoparticles used for water purification. Adv. Mater. Technol. Environ. Appl. 2018, 2, 220–234. [Google Scholar]
- Fu, P.P. Introduction to the Special Issue: Nanomaterials-Toxicology and medical applications. J. Food Drug. Anal. 2014, 22, 1–2. [Google Scholar] [CrossRef]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K.; Maurya, P.K.; Chandra, P. Prospects of Nanostructure Materials and Their Composites as Antimicrobial Agents. Front. Microbiol. 2018, 9, 422. [Google Scholar] [CrossRef]
- Brandelli, A. The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Sci. Hum. Wellness 2020, 9, 8–20. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef]
- Krasniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications-A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Jamroz, E.; Kopel, P. Polysaccharide and Protein Films with Antimicrobial/Antioxidant Activity in the Food Industry: A Review. Polymers 2020, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Tetik, I.; Dudys, E. Nanotechnology and its applications in the food industry. Recent Adv. Food Sci. 2020, 3, 247–258. [Google Scholar]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug. Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council. Directive 94/62/EC of 20 December 1994 on Packaging and Packaging Waste; European Parliament and Council: Brussels, Belgium, 2018. [Google Scholar]
- IETC. Plastic Shopping Bags Ban. In Primary Industries Water and Environment; International Environmental Technology Centre: Osaka, Japan, 2013. [Google Scholar]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based antimicrobial films for food packaging applications. E Polym. 2008, 8. [Google Scholar] [CrossRef]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systematic study needs for food applications. Food Sci. Technol. Int. 2012, 18, 3–34. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Mojaddar Langroodi, A. Chitosan coatings incorporated with propolis extract and Zataria multiflora Boiss oil for active packaging of chicken breast meat. Int. J. Biol. Macromol. 2019, 141, 401–409. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Partovi, R.; Talebi, F.; Babaei, A. Chitosan/TiO2 nanoparticle/Cymbopogon citratus essential oil film as food packaging material: Physico-mechanical properties and its effects on microbial, chemical, and organoleptic quality of minced meat during refrigeration. J. Food Process. Pres. 2020, 44. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-acorn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Y.M.; Yetmez, M.; Oktar, F.N.; Gunduz, O.; Agathopoulos, S.; Andronescu, E.; Ficai, D.; Sonmez, M.; Ficai, A. Nanostructured Biomaterials with Antimicrobial Properties. Curr. Med. Chem. 2014, 21, 3391–3404. [Google Scholar] [CrossRef] [PubMed]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Nicolescu, A.; Ascenzi, P.; Moise, L.G.; Tudor, L.; Trusca, V.G.; Gafencu, A.V.; Ficai, D.; et al. Simple and dual cross-linked chitosan millicapsules as a particulate support for cell culture. Int. J. Biol. Macromol. 2020, 143, 200–212. [Google Scholar] [CrossRef]
- Mutlu, E.C.; Ficai, A.; Ficai, D.; Yildirim, A.B.; Yildirim, M.; Oktar, F.N.; Demir, A. Chitosan/poly(ethylene glycol)/hyaluronic acid biocompatible patches obtained by electrospraying. Biomed. Mater. 2018, 13, 055011. [Google Scholar] [CrossRef]
- Injorhor, P.; Ruksakulpiwat, Y.; Ruksakulpiwat, C. Effect of shrimp shell chitosan loading on antimicrobial, absorption and morphological properties of natural rubber composites reinforced with silica-chitosan hybrid filler. Biointerface Res. Appl. Chem. 2020, 10, 5656–5659. [Google Scholar]
- Ullah, F.; Javed, F.; Zakaria, M.R.; Jamila, N.; Khattak, R.; Khan, A.N.; Akil, H.M. Determining the molecular-weight and interfacial properties of chitosan built nanohydrogel for controlled drug delivery applications. Biointerface Res. Appl. Chem. 2019, 9, 4452–4457. [Google Scholar]
- Ullah, F.; Javed, F.; Khan, A.N.; Kudus, M.H.A.; Jamila, N.; Minhaz, A.; Akil, H.M. Synthesis and surface modification of chitosan built nanohydrogel with antiviral and antimicrobial agent for controlled drug delivery. Biointerface Res. Appl. Chem. 2019, 9, 4439–4445. [Google Scholar]
- Arkoun, M.; Ardila, N.; Heuzey, M.C.; Ajji, A. Chitosan-Based Structures/Coatings with Antibacterial Properties. Handb. Antimicrob. Coat. 2018, 357–389. [Google Scholar] [CrossRef]
- Belalia, R.; Grelier, S.; Benaissa, M.; Coma, V. New bioactive biomaterials based on quaternized chitosan. J. Agric. Food Chem. 2008, 56, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Cha, R.T.; Mou, K.W.; Zhao, X.H.; Long, K.Y.; Luo, H.Z.; Zhou, F.S.; Jiang, X.Y. Nanocellulose-Based Antibacterial Materials. Adv. Healthc. Mater. 2018, 7, 1800334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Qiao, M.Y.; Ren, X.H.; Huang, T.S.; Buschle-Diller, G. Antibacterial membranes based on chitosan and quaternary ammonium salts modified nanocrystalline cellulose. Polym. Adv. Technol. 2017, 28, 1629–1635. [Google Scholar] [CrossRef]
- Nechita, P.; Bobu, E.; Parfene, G.; Dinica, R.M.; Balan, T. Antimicrobial Coatings Based on Chitosan Derivatives and Quaternary Ammonium Salts for Packaging Paper Applications. Cell. Chem. Technol. 2015, 49, 625–632. [Google Scholar]
- Hu, D.Y.; Wang, H.X.; Wang, L.J. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Hu, D.Y.; Wang, L.J. Fabrication of antibacterial blend film from poly (vinyl alcohol) and quaternized chitosan for packaging. Mater. Res. Bull. 2016, 78, 46–52. [Google Scholar] [CrossRef]
- Saini, S.; Falco, C.Y.; Belgacem, M.N.; Bras, J. Surface cationized cellulose nanofibrils for the production of contact active antimicrobial surfaces. Carbohydr. Polym. 2016, 135, 239–247. [Google Scholar] [CrossRef]
- Pour, Z.S.; Makvandi, P.; Ghaemy, M. Performance properties and antibacterial activity of crosslinked films of quaternary ammonium modified starch and poly(vinyl alcohol). Int. J. Biol. Macromol. 2015, 80, 596–604. [Google Scholar] [CrossRef]
- Wang, B.B.; Yang, X.D.; Qiao, C.D.; Li, Y.; Li, T.D.; Xu, C.L. Effects of chitosan quaternary ammonium salt on the physicochemical properties of sodium carboxymethyl cellulose-based films. Carbohydr. Polym. 2018, 184, 37–46. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Nowzari, F.; Shabanpour, B.; Ojagh, S.M. Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bansal, N.; Yang, H.S. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (Trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Hasheminya, S.M.; Dehghannya, J. Novel ultrasound-assisted extraction of kefiran biomaterial, a prebiotic exopolysaccharide, and investigation of its physicochemical, antioxidant and antimicrobial properties. Mater. Chem. Phys. 2020, 243, 122645. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Lakra, A.K.; Domdi, L.; Tilwani, Y.M.; Arul, V. Physicochemical and functional characterization of mannan exopolysaccharide from Weissella confusa MD1 with bioactivities. Int. J. Biol. Macromol. 2020, 143, 797–805. [Google Scholar] [CrossRef]
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr. Opin. Food Sci. 2020, 40, 33–39. [Google Scholar] [CrossRef]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb. Pathog. 2019, 132, 10–19. [Google Scholar] [CrossRef]
- Wang, Y.C.; Mohan, C.O.; Guan, J.; Ravishankar, C.N.; Gunasekaran, S. Chitosan and gold nanoparticles-based thermal history indicators and frozen indicators for perishable and temperature-sensitive products. Food Control 2018, 85, 186–193. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Rosa, G.B.; Ferreira, A.L.A.; da Rosa, C.G.; Beling, P.C.; Xavier, L.O.; Hansen, C.M.; Ferrareze, J.P.; Nunes, M.R.; Barreto, P.L.M.; et al. Bioactive food packaging based on starch, citric pectin and functionalized with Acca sellowiana waste by-product: Characterization and application in the postharvest conservation of apple. Int. J. Biol. Macromol. 2020, 147, 295–303. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Rajasekharan, S.K.; Lee, J.; Ramalingam, S.; Lee, J. K-Carrageenan/lignin composite films: Biofilm inhibition, antioxidant activity, cytocompatibility, UV and water barrier properties. Mater. Today Commun. 2020, 24, 101346. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. Reinforcement of refined and semi-refined carrageenan film with nanocellulose. Polymers 2020, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Chiu, F.C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Luo, X.; Liu, L.; Dong, K.; Yang, R.; Lin, C.; Song, H.; Li, S.; Huang, Q. Formation mechanism of egg white protein/κ-Carrageenan composite film and its application to oil packaging. Food Hydrocoll. 2020, 105, 105780. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Active edible films based on semi-refined κ-carrageenan: Antioxidant and color properties and application in chicken breast packaging. Food Packag. Shelf 2020, 24, 100476. [Google Scholar] [CrossRef]
- Wahjuningsih, S.B.; Rohadi Susanti, S.; Setyanto, H.Y. The effect of k-carrageenan addition to the characteristics of jicama starch-based edible coating and its potential application on the grapevine. Int. J. Adv. Sci. Eng. Inf. Technol. 2019, 9, 405–410. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef]
- Puscaselu, R.; Gutt, G.; Amariei, S. The use of edible films based on sodium alginate in meat product packaging: An eco-friendly alternative to conventional plastic materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Pinto, C.T.; Pankowski, J.A.; Nano, F.E. The anti-microbial effect of food wrap containing beeswax products. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 145–148. [Google Scholar] [CrossRef][Green Version]
- De Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in foo—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef]
- Gazzotti, S.; Todisco, S.A.; Picozzi, C.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. Eugenol-grafted aliphatic polyesters: Towards inherently antimicrobial PLA-based materials exploiting OCAs chemistry. Eur. Polym. J. 2019, 114, 369–379. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Pereira, R.N.; Cerqueira, M.A.; Martins, J.R.; Teixeira, J.A.; Malcata, F.X.; Vicente, A.A. Bio-Based Nanocomposites for Food Packaging and Their Effect in Food Quality and Safety. In Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 271–306. [Google Scholar]
- Shao, X.; Sun, H.; Zhou, R.; Zhao, B.; Shi, J.; Jiang, R.; Dong, Y. Effect of bovine bone collagen and nano-TiO2 on the properties of hydroxypropyl methylcellulose films. Int. J. Biol. Macromol. 2020, 158, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Development of emulsion films based on bovine gelatin-nano chitin-nano ZnO for cake packaging. Food Sci. Nutr. 2020, 8, 1303–1312. [Google Scholar] [CrossRef]
- Saadat, S.; Pandey, G.; Tharmavaram, M.; Braganza, V.; Rawtani, D. Nano-interfacial decoration of Halloysite Nanotubes for the development of antimicrobial nanocomposites. Adv. Colloid Interface Sci. 2020, 275, 102063. [Google Scholar] [CrossRef]
- Oun, A.A.; Shankar, S.; Rhim, J.W. Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435–460. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Ghanbarzadeh, B.; Divband, B. Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration. Int. J. Food Microbiol. 2020, 312, 108375. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The synergistic effects of cinnamon essential oil and nano TiO2 on antimicrobial and functional properties of sago starch films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Dhital, R.; Kong, F.; Lin, M.; Mustapha, A. Antimicrobial effect and toxicity of cellulose nanofibril/silver nanoparticle nanocomposites prepared by an ultraviolet irradiation method. Colloids Surf. B Biointerfaces 2019, 180, 212–220. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Melente, A.E.; Holban, A.M.; Ficai, A.; Ditu, L.M.; Kamerzan, C.M.; Tihauan, B.M.; Nicoara, A.I.; Bezirtzoglou, E.; Chifiriuc, M.C.; et al. Novel hydrogels based on collagen and ZnO nanoparticles with antibacterial activity for improved wound dressings. Rom. Biotech. Lett. 2019, 24, 317–323. [Google Scholar] [CrossRef]
- Lungu, I.I.; Holban, A.M.; Ficai, A.; Grumezescu, A.M. Zinc oxide nanostrucures: New trends in antimicrobial therapy. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 503–514. [Google Scholar]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Ma, D.; Ahmed, S.; Qin, W.; Liu, Y. Effects of ultrasonication duration and graphene oxide and nano-zinc oxide contents on the properties of polyvinyl alcohol nanocomposites. Ultrason. Sonochem. 2019, 59, 104731. [Google Scholar] [CrossRef]
- Halder, S.; Schneller, T.; Waser, R. Enhanced stability of platinized silicon substrates using an unconventional adhesion layer deposited by CSD for high temperature dielectric thin film deposition. Appl. Phys. Mater. Sci. Process. 2007, 87, 705–708. [Google Scholar] [CrossRef]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zincexternal Link Disclaimer; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Aristizabal-Gil, M.V.; Santiago-Toro, S.; Sanchez, L.T.; Pinzon, M.I.; Gutierrez, J.A.; Villa, C.C. ZnO and ZnO/CaO nanoparticles in alginate films. Synthesis, mechanical characterization, barrier properties and release kinetics. LWT 2019, 112, 108217. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated Otolithes ruber fillets. Food Packag. Shelf 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Sharma, D.; Rajput, J.; Kaith, B.S.; Kaur, M.; Sharma, S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Suhr, K.I.; Nielsen, P.V. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 2003, 94, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef]
- Danila, E.; Kaya, D.A.; Patrascu, M.; Kaya, M.A.; Kumbakisaka, S. Comparative study of Lavandula Angustifolia essential oils obtained by microwave and classical hydrodistillation. Rev. Chim. (Bucharest) 2018, 69, 2240–2244. [Google Scholar] [CrossRef]
- Ullah, H.; Ali, S. Classification of anti-bacterial agents and their functions. In Antibacterial Agents; Kumavath, R.N., Ed.; Intech: London, UK, 2017; pp. 1–16. [Google Scholar]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13. [Google Scholar] [CrossRef]
- Yahya, N.A.; Attana, N.; Wahab, R.A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds. Food Bioprod. Process. 2018, 112, 69–85. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Davila-Ortiz, G.; Martinez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 5–13. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-products. Food Bioprocess Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.H.; Sastry, S. Extraction from Food and Natural Products by Moderate Electric Field: Mechanisms, Benefits, and Potential Industrial Applications. Compr. Rev. Food Sci. Food 2018, 17, 1040–1052. [Google Scholar] [CrossRef]
- Xu, C.C.; Wang, B.; Pu, Y.Q.; Tao, J.S.; Zhang, T. Advances in extraction and analysis of phenolic compounds from plant materials. Chin. J. Nat. Med. 2017, 15, 721–731. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trend Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Welsch, T.T.; Gillock, E.T. Triclosan-resistant bacteria isolated from feedlot and residential soils. J. Environ. Sci. Health A 2011, 46, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Netikova, L.; Bogusch, P.; Heneberg, P. Czech Ethanol-Free Propolis Extract Displays Inhibitory Activity against a Broad Spectrum of Bacterial and Fungal Pathogens. J. Food Sci. 2013, 78, M1421–M1429. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Barba, F.J.; Dominguez, R.; Sant’Ana, A.S.; Khaneghah, A.M.; Gavahian, M.; Gomez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shavisi, N. A novel active food packaging film for shelf-life extension of minced beef meat. J. Food Saf. 2018, 38. [Google Scholar] [CrossRef]
- Rollini, M.; Mascheroni, E.; Capretti, G.; Coma, V.; Musatti, A.; Piergiovanni, L. Propolis and chitosan as antimicrobial and polyphenols retainer for the development of paper based active packaging materials. Food Packag. Shelf 2017, 14, 75–82. [Google Scholar] [CrossRef]
- Jafari, N.J.; Kargozari, M.; Ranjbar, R.; Rostami, H.; Hamedi, H. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J. Food Process. Pres. 2018, 42. [Google Scholar] [CrossRef]
- Formosa-Dague, C.; Duval, R.E.; Dague, E. Cell biology of microbes and pharmacology of antimicrobial drugs explored by Atomic Force Microscopy. Semin. Cell Dev. Biol. 2018, 73, 165–176. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. 2018, 58, 486–511. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.P.S.A.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Kwon, S.J.; Chang, Y.; Han, J. Oregano essential oil-based natural antimicrobial packaging film to inactivate Salmonella enterica and yeasts/molds in the atmosphere surrounding cherry tomatoes. Food Microbiol. 2017, 65, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Improving anti-listeria activity of cheese packaging via nanofiber containing nisin-loaded nanoparticles. LWT Food Sci. Technol. 2017, 81, 233–242. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Zhou, Y.; Miao, X.; Lan, X.; Luo, J.; Luo, T.; Zhong, Z.; Gao, X.; Mafang, Z.; Ji, J.; Wang, H.; et al. Angelica Essential Oil Loaded Electrospun Gelatin Nanofibers for Active Food Packaging Application. Polymers 2020, 12, 299. [Google Scholar] [CrossRef]
- Yang, Y.N.; Lu, K.Y.; Wang, P.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Development of bacterial cellulose/chitin multi-nano fi bers based smart films containing natural active microspheres and nanoparticles formed in situ. Carbohydr. Polym. 2020, 228, 115370. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical. mechanical, structural and antimicrobial attributes. Colloids Surf B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Baysal, G.; Dogan, F. Investigation and preparation of biodegradable starch-based nanofilms for potential use of curcumin and garlic in food packaging applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 1127–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Preparation and Characterization of Corn Starch Bio-Active Edible Packaging Films Based on Zein Incorporated with Orange-Peel Oil. Antioxidants 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Khan, M.A. Modified atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Yousefi, M.; Ehsani, A.; Jafari, S.M. Lipid-based nano delivery of antimicrobials to control food-borne bacteria. Adv. Colloid Interface Sci. 2019, 270, 263–277. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, G.J.E.; Chorianopoulos, N. Probiotic incorporation in edible films and coatings: Bioactive solution for functional foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef]
- Namratha, S.; Sreejit, V.; Preetha, R. Fabrication and evaluation of physicochemical properties of probiotic edible film based on pectin–alginate–casein composite. Int. J. Food Sci. Technol. 2020, 55, 1497–1505. [Google Scholar] [CrossRef]
- Settier-Ramírez, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Antilisterial properties of PVOH-based films embedded with Lactococcus lactis subsp. lactis. Food Hydrocoll. 2019, 87, 214–220. [Google Scholar] [CrossRef]
- Settier-Ramírez, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. PVOH/protein blend films embedded with lactic acid bacteria and their antilisterial activity in pasteurized milk. Int. J. Food Microbiol. 2020, 322, 108545. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, V.; Ficai, A.; Grumezescu, A.M.; Chifiriuc, M.C.; Iordache, F.; Andronescu, E. Highly Biocompatible Magnetite Nanoparticles Functionalized with Chitosan for Improving the Efficiency of Antibiotics. Univ. Politeh. Buchar. 2016, 78, 47–58. [Google Scholar]
- Danila, E.; Moldovan, Z.; Popa, M.; Chifiriuc, M.C.; Kaya, A.D.; Kaya, M.A. Chemical composition, antimicrobial and antibiofilm efficacy of limon and L. angustifolia EOs and of their mixtures against Staphylococcus epidermidis clinical strains. Ind. Crops Prod. 2018, 122, 483–492. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, W.; Luo, J.; Deng, D. Multifunctional nano-cellulose composite films with grape seed extracts and immobilized silver nanoparticles. Carbohydr. Polym. 2019, 205, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigos, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Dutta, J.; Dutta, P.K. Antimicrobial Activity of Chitin, Chitosan, and Their Oligosaccharides, Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications; CRC Press: Boca Raton, FL, USA, 2010; pp. 195–210. [Google Scholar]
- Ding, L.; Li, X.; Hu, L.; Zhang, Y.; Jiang, Y.; Mao, Z.; Xu, H.; Wang, B.; Feng, X.; Sui, X. A naked-eye detection polyvinyl alcohol/cellulose-based pH sensor for intelligent packaging. Carbohydr. Polym. 2020, 233, 115859. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Tajik, H.; Moradi, M.; Molaei, R. Intelligent pH-sensitive indicator based on starch-cellulose and alizarin dye to track freshness of rainbow trout fillet. Int. J. Biol. Macromol. 2019, 132, 157–165. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Lim, L.T. Colorimetric array indicator for NH3 and CO2 detection. Sens. Actuators B Chem. 2018, 255, 3216–3226. [Google Scholar] [CrossRef]
- Safarik, I.; Pospiskova, K.; Horska, K.; Safarikova, M. Potential of magnetically responsive (nano)biocomposites. Soft. Matter. 2012, 8, 5407–5413. [Google Scholar] [CrossRef]
- Ardila-Diaz, L.D.; Oliveira, T.V.; Soares, N.F.F. Development and Evaluation of the Chromatic Behavior of an Intelligent Packaging Material Based on Cellulose Acetate Incorporated with Polydiacetylene for an Efficient Packaging. Biosensors 2020, 10, 59. [Google Scholar] [CrossRef]
- Lopez-Carballo, G.; Muriel-Galet, V.; Hernandez-Munoz, P.; Gavara, R. Chromatic Sensor to Determine Oxygen Presence for Applications in Intelligent Packaging. Sensors 2019, 19, 4684. [Google Scholar] [CrossRef]
- Mohan, C.O.; Gunasekaran, S.; Ravishankar, C.N. Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. Npj Sci. Food. 2019, 2, 3. [Google Scholar] [CrossRef]
- Lee, S.J.; Rahman, A.T.M.M. Intelligent Packaging for Food Products. Innov. Food Packag. 2014, 171–209. [Google Scholar] [CrossRef]
- Galagan, Y.; Su, W.F. Fadable ink for time-temperature control of food freshness: Novel new time-temperature indicator. Food Res. Int. 2008, 41, 653–657. [Google Scholar] [CrossRef]
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Taoukis, P.S.; Labuza, T.P. Applicability of Time-Temperature Indicators as Shelf-Life Monitors of Food-Products. J. Food Sci. 1989, 54, 783–788. [Google Scholar] [CrossRef]
- Fang, Z.X.; Zhao, Y.Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Wang, S.D.; Liu, X.H.; Yang, M.; Zhang, Y.; Xiang, K.Y.; Tang, R. Review of Time Temperature Indicators as Quality Monitors in Food Packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Brizio, A.P.D.R.; Prentice, C. Use of smart photochromic indicator for dynamic monitoring of the shelf life of chilled chicken based products. Meat Sci. 2014, 96, 1219–1226. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonca, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Stratakos, A.C. Fresh and Processed Meat and Meat Products. Contemp. Food Eng. 2012, 223–259. [Google Scholar]
- Arvanitoyannis, I.S.; Stratakos, A.C. Application of Modified Atmosphere Packaging and Active/Smart Technologies to Red Meat and Poultry: A Review. Food Bioprocess. Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- Umuhumuza, L.C.; Sun, X.L. Rapid detection of pork meat freshness by using L-cysteine-modified gold electrode. Eur. Food Res. Technol. 2011, 232, 425–431. [Google Scholar] [CrossRef]
- Fuertes, G.; Soto, I.; Carrasco, R.; Vargas, M.; Sabattin, J.; Lagos, C. Intelligent Packaging Systems: Sensors and Nanosensors to Monitor Food Quality and Safety. J. Sens. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yang, Y.J.; Kim, J.S.; Choi, D.S.; Park, S.H.; Jin, S.Y.; Park, J.S. Non-destructive monitoring of apple ripeness using an aldehyde sensitive colorimetric sensor. Food Chem. 2018, 267, 149–156. [Google Scholar] [CrossRef] [PubMed]
| Food | Natural Active Agents | References |
|---|---|---|
| Meat | Antioxidant agents: Ascorbic Acid, Phenols and Polyphenols, Ferulic Acid, a-Tocopherol, Phycocyanin Antimicrobial agents: Thymol, Cinnamaldehyde, Gallic Acid, Curcumin, Carvacrol, Essential Oils Antifungal agents: Curcumin, essential oils | [105,106,107] |
| Fruits and vegetables | Curcumin, rosemary extract, oregano essential oil, Asian spice essential oil | [106,107,108,109] |
| Bread | Carvacrol | [106,107,108] |
| Cheese | Antimicrobial agents: Ginger Essential Oil, Moringa Essential Oil, Lisin | [106,110,111] |
| Polymer Support | Bioactive Agent/activity | References |
|---|---|---|
| Native Bioactive Polymers as Packaging Materials | ||
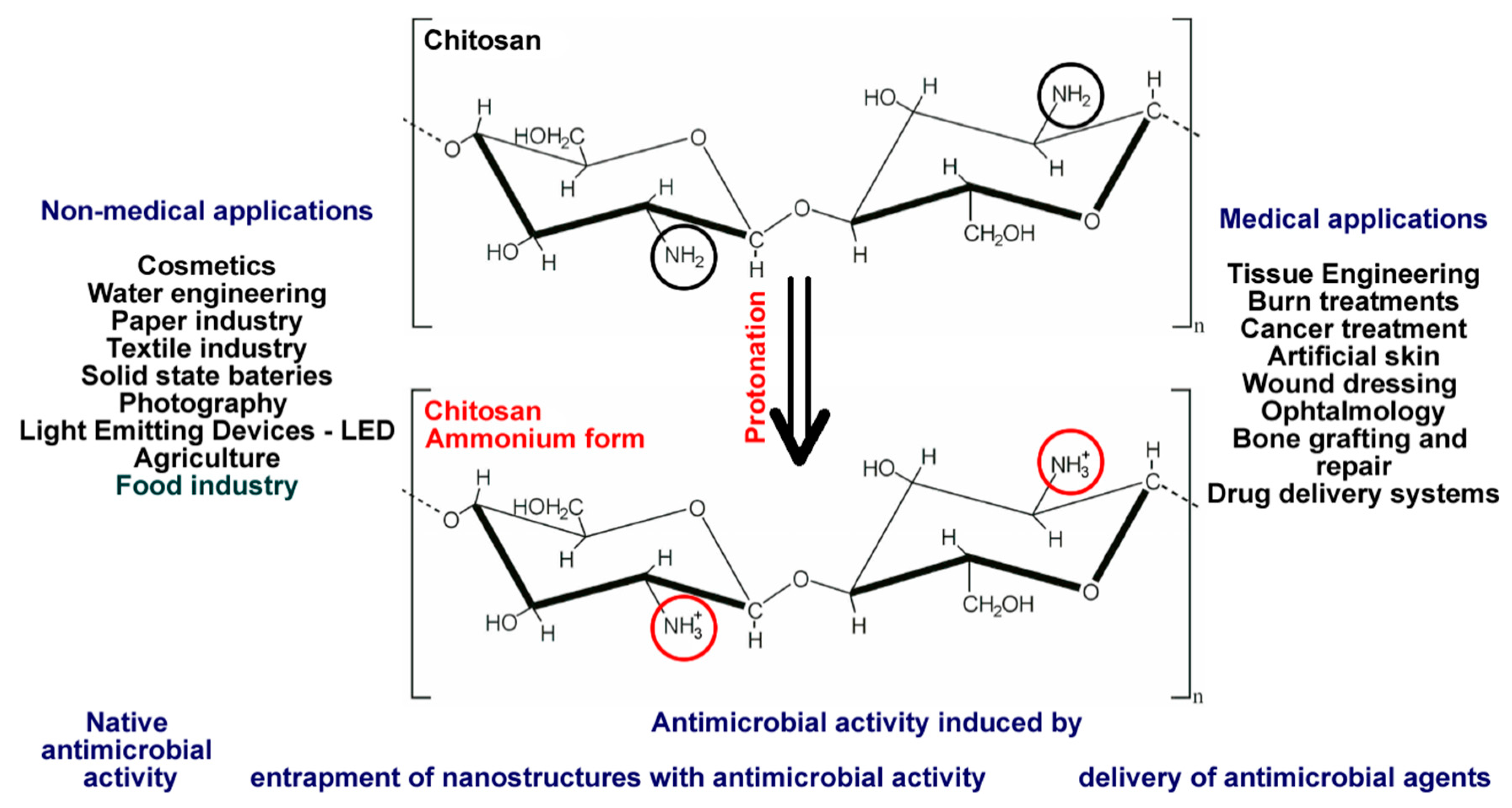
| Chitosan | Chitosan/Antimicrobial activity | [19,128] |
| Chitosan, ammonium salt | Chitosan, ammonium salt/Antimicrobial activity | [33] |
| Chitosan or quaternized chitosan/other polymers | Chitosan or chitosan, ammonium salt/Antimicrobial activity | [33,37,38] |
| Nanostructured Polymers as Packaging Materials | ||
| Chitosan/gelatine | AgNPs/antimicrobial activity | [75] |
| Cellulose nanofibril | AgNPs/antimicrobial | [73] |
| Hydroxypropyl methylcellulose (HPMC)/Bovine collagen | TiO2/antibacterial activity | [65] |
| cellulose, sodium alginate | CuO/antioxidant and antimicrobial activity | [66] |
| Bovine Gelatine | ZnO/antifungal activity | [67] |
| Bionanocomposite films of agar | ZnO/antifungal activity | [79] |
| Polyvinyl alcohol (PVA) | Graphene oxide (GO), and zinc oxide nanoparticles (ZnO NPs)/antibacterial activity against foodborne pathogenic and spoilage bacteria | [80] |
| Drug delivery systems as packaging materials | ||
| Gelatin | Angelica Essential Oil/antioxidant activity and inhibitory effect against both Gram-negative and Gram-positive bacteria | [112] |
| Chitosan/gelatin | Tarragon essential oil/Antioxidant, Antibacterial activity | [105] |
| Bacterial cellulose nanofiber (BCNF)/chitin nanofiber (CNF) | Curcumin/Antibacterial and Antioxidant activity | [113] |
| Chitosan-whey protein/zein | Cinnamon oil/antibacterial activity | [114,115] |
| Lipid nanostructures/? | Antibiotic loaded lipid-based nanostructures/antimicrobial | [119] |
| Biodegradable starch-based nanofilms | Garlic extracts/antibacterial activity against salmonella and S. aureus bacteria | [116] |
| Corn Starch Bio-Active Edible Packaging Films based on Zein | Orange-Peel Oil/antioxidant activity | [117] |
| Chitosan/PEO | Natural extracts and essential oils with antioxidant, antibacterial, and antifungal activity | [106] |
| Bioactive packaging materials with multiple mechanisms of activity | ||
| Nano-cellulose composite films | Grape seed extracts and immobilized silver nanoparticles/antimicrobial activity against E. coli and S. aureus | [126] |
| Cellulose acetate based nano-biocomposite films | AgNPs-organoclay and/or thymol/combined antimicrobial/antioxidant properties | [127] |
| Sago starch films | Cinnamon essential oil and nano-TiO2 on antimicrobial and functional properties | [72] |
| Support | Active Agent | Mechanism and Use | Ref. |
|---|---|---|---|
| Polyvinyl Alcohol/Cellulose | acidochromic dye | pH-induced color change for shrimp freshness monitoring | [129] |
| Starch-Cellulose | alizarin | pH sensitive indicator for monitoring the freshness of rainbow trout fillet | [130] |
| Ethylcellulose | pH sensitive dyes (dimethyl yellow, methyl red, chlorophenol red, methyl orange, phenol red, thymol blue, m-cresol purple) at acidic (hydrochloric acid) and alkaline (tetrabutylammonium hydroxide) pH | A colorimetric array based on the before presented pH sensitive dyes was developed and according to the color change of the 16 spots, assessments related to the freshness of the foods can be achieved | [131] |
| Cellulose Acetate | polydiacetylene | Color change from blue to red can be achieved as a consequence of extreme processing/storage conditions (pH and temperature) | [133] |
| Ethylene-Vinyl Alcohol copolymer | methylene blue | Oxygen sensor based on methylene blue ⇔ its leuco form | [134] |
| Chitosan | gold nanoparticles | Color change occurs as a consequence of the growth and aggregation or agglomeration of AuNPs due to their localized surface plasmon resonance | [135] |
| VITSAB indicator * | lipolytic enzyme solution /lipids | Temperature induced activation of the enzyme which catalyses the color change from green to red because a pH change occurred with the generation of fatty acids | [140,141] |
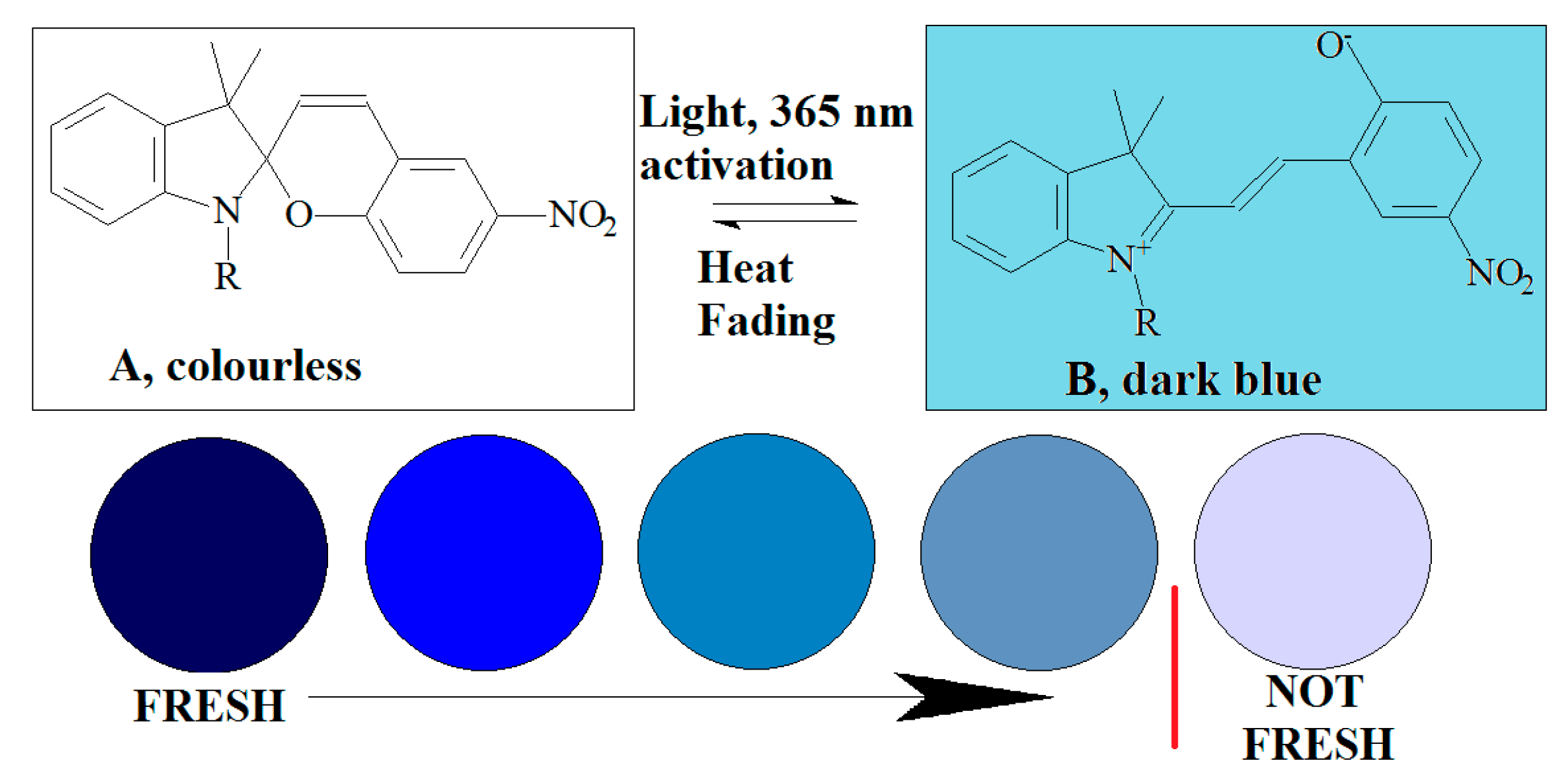
| OnVuTM label * | Photochromic | UV activation of the photochromic indicator which is discolored similarly with the degradation of the chilled boneless chicken breast | [142] |
| Paper | Methyl red | The color change (from yellow to orange and red) is a consequence of the Cannizzaro reaction of the evolved aldehydes in both liquid or gaseous form, in alkaline pH. | [148] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Andronescu, E. Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches. Coatings 2020, 10, 806. https://doi.org/10.3390/coatings10090806
Motelica L, Ficai D, Oprea OC, Ficai A, Andronescu E. Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches. Coatings. 2020; 10(9):806. https://doi.org/10.3390/coatings10090806
Chicago/Turabian StyleMotelica, Ludmila, Denisa Ficai, Ovidiu Cristian Oprea, Anton Ficai, and Ecaterina Andronescu. 2020. "Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches" Coatings 10, no. 9: 806. https://doi.org/10.3390/coatings10090806
APA StyleMotelica, L., Ficai, D., Oprea, O. C., Ficai, A., & Andronescu, E. (2020). Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches. Coatings, 10(9), 806. https://doi.org/10.3390/coatings10090806








