Encapsulation of Grapefruit Essential Oil in Emulsion-Based Edible Film Prepared by Plum (Pruni Domesticae Semen) Seed Protein Isolate and Gum Acacia Conjugates
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Conjugates
2.2. Film-Forming Emulsion Properties
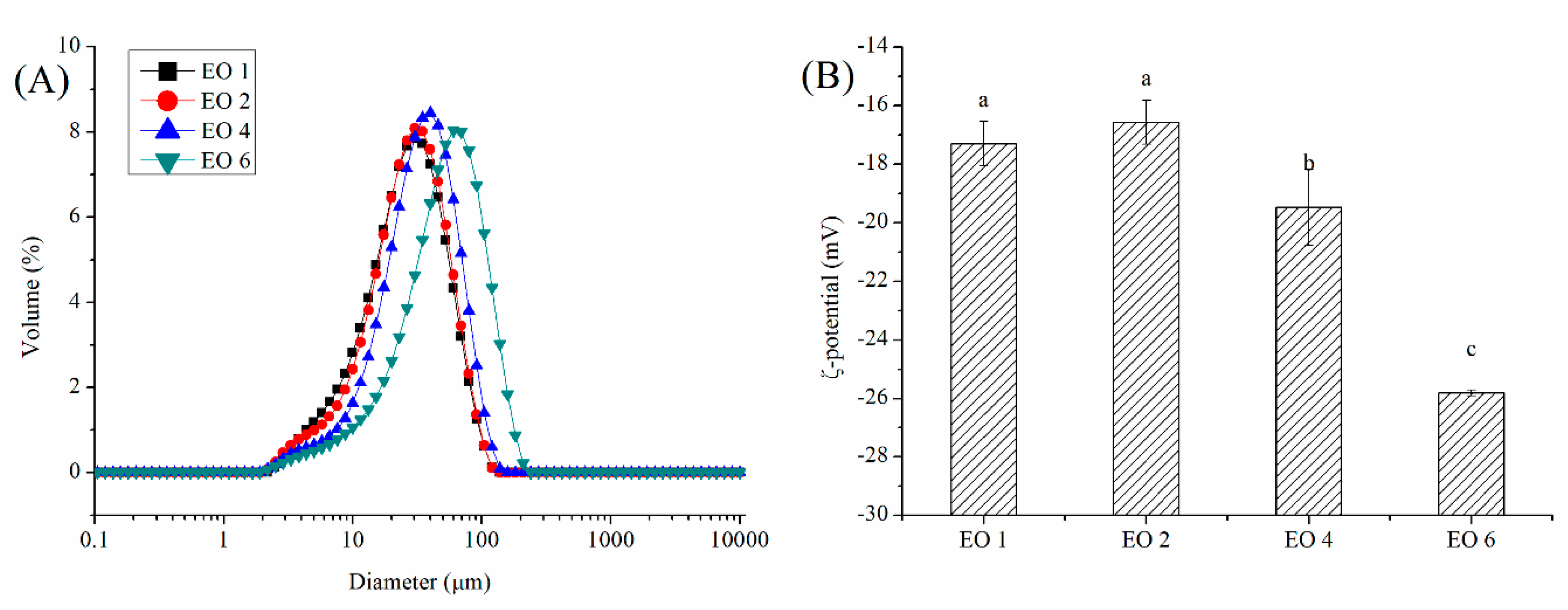
2.2.1. Size Distribution
2.2.2. ζ-Potential
2.2.3. Rheological Behavior
2.3. Film Physical Properties
2.3.1. Transparency, Whiteness Index, and Swelling Ability
2.3.2. Water Vapor Permeability (WVP), Contact Angle, and Mechanical Properties
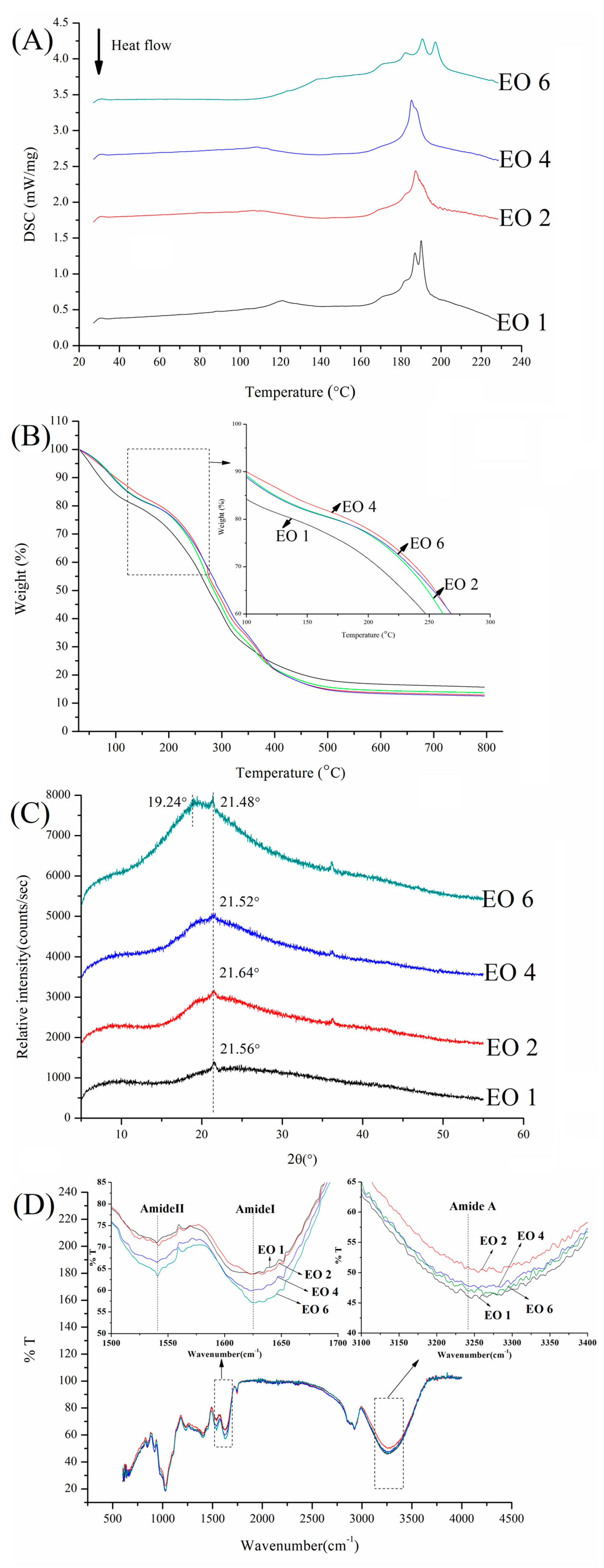
2.4. Thermal Properties of Films
2.5. X-Ray Diffractometry
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
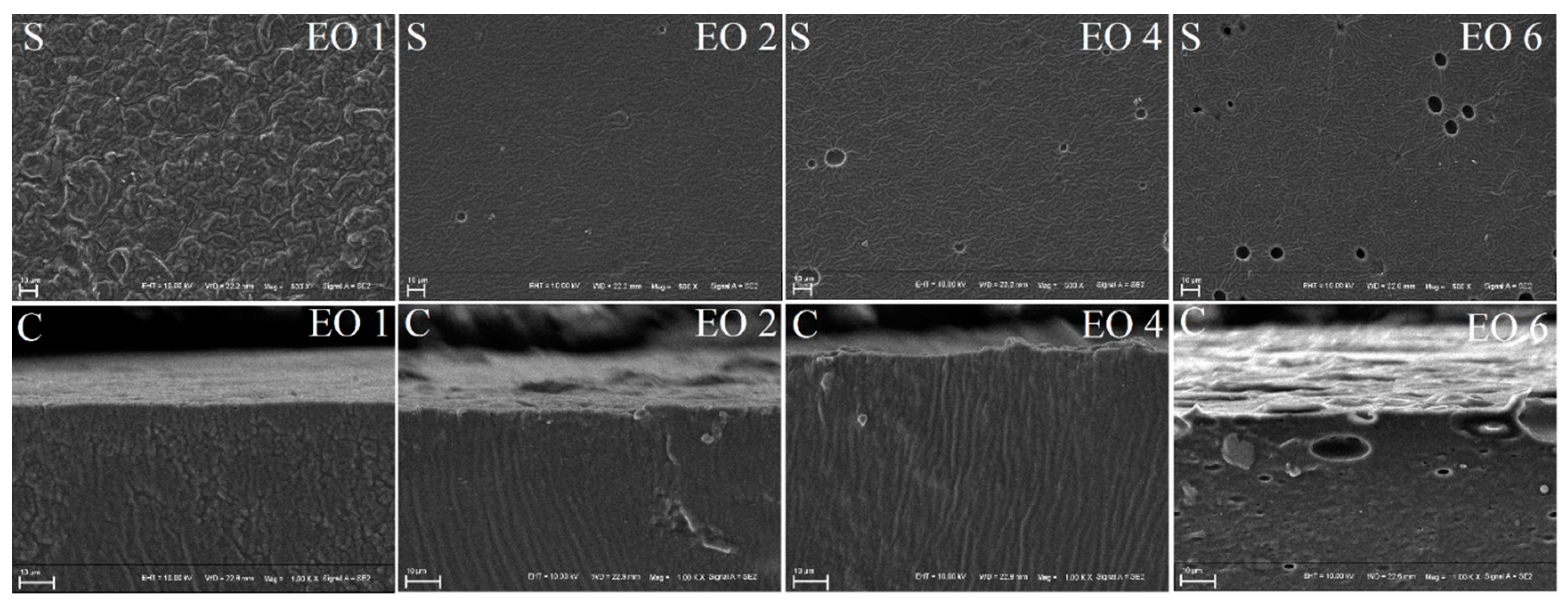
2.7. Microstructure
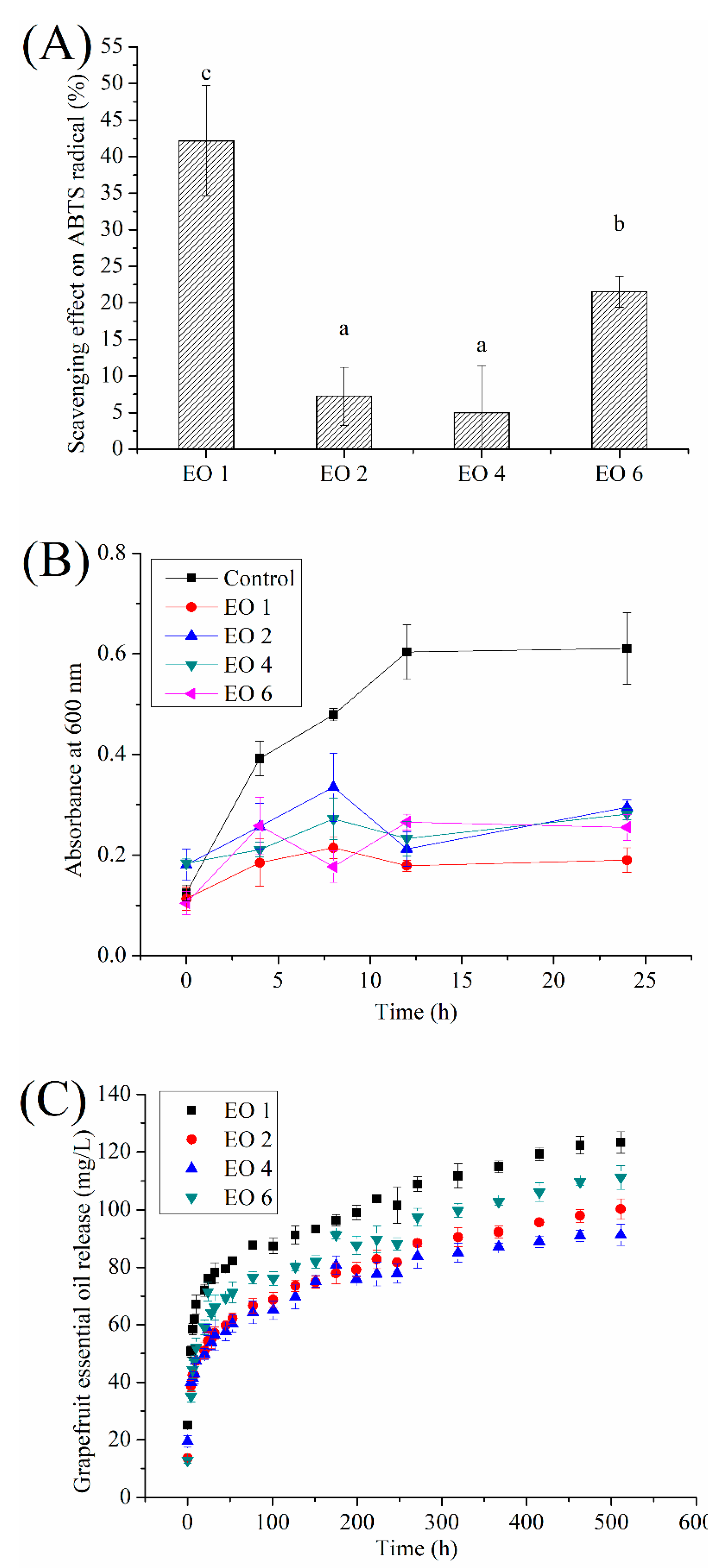
2.8. Antioxidant and Antimicrobial Activity
2.9. Release Kinetics of EO from Films
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Analysis of Grapefruit Essential Oil
3.3. Preparation of Plum Seed Protein Isolates (PSPI)—Gum Acacia (GA) Conjugates
3.4. Determination of Amino Acids, Emulsifying Properties, Surface Hydrophobicity, and Structure of Conjugates
3.5. Preparation of Film-Forming Emulsion
3.6. Characterization of Film-Forming Emulsion
3.6.1. Particle Size and ζ-Potentials
3.6.2. Rheological Behavior of Film-Forming Emulsions
3.7. Film Formation
3.8. Characterization of Films
3.8.1. Transparency, Whiteness Index (WI), Swelling Ability, Water Vapor Permeability (WVP) Measurements
3.8.2. Contact Angle-Sessile Drop Method
3.8.3. Mechanical Properties
3.8.4. Differential Scanning Calorimetry (DSC)
3.8.5. Thermal Gravimetric Analysis (TG)
3.8.6. X-ray
3.8.7. Fourier Transform Infrared Spectroscopy (FTIR)
3.8.8. Scanning Electron Microscopy (SEM)
3.9. Antioxidant and Antimicrobial Activity of Films
3.10. Essential Oil Release Kinetic from Films
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EBEF | emulsion-based edible films |
| PSPI | plum seed protein isolate |
| GA | gum acacia |
| EO | grapefruit essential oil |
References
- Lacroix, M.; Cooksey, K. 18—Edible films and coatings from animal origin proteins. Innov. Food Packag. 2005, 301–317. [Google Scholar] [CrossRef]
- Lin, H.-C.; Wang, B.-J.; Weng, Y.-M. Development and characterization of sodium caseinate edible films cross-linked with genipin. LWT 2020, 118, 108813. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible protein films: Properties enhancement. Int. Food Res. J. 2009, 16, 1–9. [Google Scholar]
- Debeaufort, F.; Quezada-Gallo, J.A. Lipid hydrophobicity and physical state effects on the properties of bilary edible films. J. Membr. Sci. 2000, 180, 37–46. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Fabra, M.J.; Perez-Masia, R.; Talens, P.; Chiralt, A. Influence of the homogenization conditions and lipid self-association on properties of sodium caseinate based films containing oleic and stearic acids. Food Hydrocoll. 2011, 25, 1112–1121. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E. Whey protein-maltodextrin conjugates as emulsifying agents: An alternative to gum arabic. Food Hydrocoll. 2007, 21, 607–616. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.; Peng, Q.; Shan, Y.; Xue, F. Physicochemical properties of peanut protein isolate-glucomannan conjugates prepared by ultrasonic treatment. Ultrason. Sonochem. 2014, 21, 1722–1727. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Xue, F.; Wu, Z.; Tong, J.; Zheng, J.; Li, C. Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Biosci. Biotechnol. Biochem. 2017, 81, 1891–1898. [Google Scholar] [CrossRef]
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res. Int. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Li, C.; Zhu, B.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Physicochemical Properties of Dry-heated Peanut Protein Isolate Conjugated with Dextran or Gum Arabic. J. Am. Oil Chem. Soc. 2013, 90, 1801–1807. [Google Scholar] [CrossRef]
- Li, C.; Zhu, W.; Xue, H.; Chen, Z.; Chen, Y.; Wang, X. Physical and structural properties of peanut protein isolate-gum Arabic films prepared by various glycation time. Food Hydrocoll. 2015, 43, 322–328. [Google Scholar] [CrossRef]
- Zhou, C.; Qian, L.; Ma, H.; Yu, X.; Zhang, Y.; Qu, W.; Zhang, X.; Xia, W. Enhancement of amygdalin activated with β-D-glucosidase on HepG2 cells proliferation and apoptosis. Carbohydr. Polym. 2012, 90, 516. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, F.; Abdenour, A.; Bruno, M.; Silvia, P.; Alessandra, P.; Danilo, F.; Drifa, Y.-G.; Fahmi, E.M.; Khodir, M.; Mohamed, C. Chemical composition and in vitro antimicrobial, insecticidal and antioxidant activities of the essential oils of Mentha pulegium L. and Mentha rotundifolia (L.) Huds growing in Algeria. Ind. Crops Prod. 2016, 88, 96–105. [Google Scholar] [CrossRef]
- Cristóbal-Luna, J.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G. Grapefruit and its biomedical, antigenotoxic and chemopreventive properties. Food Chem. Toxicol. 2017, 112, 224–234. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Shi, J.; Huang, X.; Peng, Q.; Xue, F. Encapsulation of tomato oleoresin using soy protein isolate-gum aracia conjugates as emulsifier and coating materials. Food Hydrocoll. 2015, 45, 301–308. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Kato, A. Industrial Applications of Maillard-Type Protein-Polysaccharide Conjugates. Food Sci. Technol. Res. 2002, 8, 193–199. [Google Scholar] [CrossRef]
- Atarés, L.; De Jesús, C.; Talens, P.; Chiralt, A. Characterization of SPI-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 99, 384–391. [Google Scholar] [CrossRef]
- Atarés, L.; Bonilla, J.; Chiralt, A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Chang, C.; Lam, R.S.H.; Nickerson, M.T. Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Res. Int. 2015, 67, 418–425. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.H.; Yin, S.W.; Yang, X.Q.; Wang, Q.; Liu, F.; Wei, Z.H. Characterization of gelatin-based edible films incorporated with olive oil. Food Res. Int. 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Liquid and vapour water transfer through whey protein/lipid emulsion films. J. Sci. Food Agric. 2010, 90, 1673–1680. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Zhang, L. Rheological behavior of Aeromonas gum in aqueous solutions. Food Hydrocoll. 2006, 20, 723–729. [Google Scholar] [CrossRef]
- Peressini, D.; Bravin, B.; Lapasin, R.; Rizzotti, C.; Sensidoni, A. Starch–methylcellulose based edible films: Rheological properties of film-forming dispersions. J. Food Eng. 2003, 59, 25–32. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Sun, W.W.; Yu, S.J.; Yang, X.Q.; Wang, J.M.; Zhang, J.B.; Zhang, Y.; Zheng, E.L. Study on the rheological properties of heat-induced whey protein isolate–dextran conjugate gel. Food Res. Int. 2011, 44, 3259–3263. [Google Scholar] [CrossRef]
- Binsi, P.K.; Ravishankar, C.N.; Gopal, T.K.S. Development and Characterization of an Edible Composite Film Based on Chitosan and Virgin Coconut Oil with Improved Moisture Sorption Properties. J. Food Sci. 2013, 78, E526–E534. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; Talens, P.; Chiralt, A. Tensile properties and water vapor permeability of sodium caseinate films containing oleic acid-beeswax mixtures. J. Food Eng. 2008, 85, 393–400. [Google Scholar] [CrossRef]
- Han, J.H.; Seo, G.H.; Park, I.M.; Kim, G.N.; Lee, D.S. Physical and Mechanical Properties of Pea Starch Edible Films Containing Beeswax Emulsions. J. Food Sci. 2010, 71, E290–E296. [Google Scholar] [CrossRef]
- Valenzuela, C.; Abugoch, L.; Tapia, C. Quinoa protein–chitosan–sunflower oil edible film: Mechanical, barrier and structural properties. LWT Food Sci. Technol. 2013, 50, 531–537. [Google Scholar] [CrossRef]
- Wang, K.; Wu, K.; Xiao, M.; Kuang, Y.; Corke, H.; Ni, X.; Jiang, F. Structural characterization and properties of konjac glucomannan and zein blend films. Int. J. Biol. Macromol. 2017, 105. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Huang, Z.; Yuan, X.Y.; Wang, X.Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of Plasticized Edible Films from Opuntia ficus-indica Mucilage: A Comparative Study of Various Polyol Plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenol. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Sun, H.; Li, S.; Chen, S.; Wang, C.; Liu, D.; Li, X. Antibacterial and antioxidant activities of sodium starch octenylsuccinate-based Pickering emulsion films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2020, 159, 696–703. [Google Scholar] [CrossRef]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- De Souza, A.G.; dos Santos, N.M.A.; da Silva Torin, R.F.; dos Santos Rosa, D. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bustos, C.R.O.; Alberti, R.F.O.; Matiacevich, S.B. Edible antimicrobial films based on microencapsulated lemongrass oil. J. Food Sci. Technol. 2016, 53, 832. [Google Scholar] [CrossRef] [PubMed]
- Lidia Herrera, M.; Bustos, R.O.; Matiacevich, S.B.; Alarcón-Moyano, J.K. Alginate edible films containing microencapsulated lemongrass oil or citral: Effect of encapsulating agent and storage time on physical and antimicrobial properties. J. Food Sci. Technol. 2017, 54, 2878–2889. [Google Scholar]
- Xue, F.; Zhu, C.; Liu, F.; Wang, S.; Liu, H.; Li, C. Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate. J. Sci. food Agric. 2018, 98, 5690–5699. [Google Scholar] [CrossRef]
- Silva, E.K.; Gomes, M.T.M.S.; Hubinger, M.D.; Cunha, R.L.; Meireles, M.A.A. Ultrasound-assisted formation of annatto seed oil emulsions stabilized by biopolymers. Food Hydrocoll. 2015, 47, 1–13. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy;protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Zúñiga, R.N.; Osorio, F.; Pedreschi, F. Physical properties of emulsion-based hydroxypropyl methylcellulose/whey protein isolate (HPMC/WPI) edible films. Carbohydr. Polym. 2015, 123, 27–38. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Yikling, C.; Jookheng, G.; Yauyan, L. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009, 116, 13–18. [Google Scholar]


| Sample | Transparency (%/mm) | Whiteness Index WI | Swelling (%) | WVP mg/(cm2∙d) | Contact Angle (°) | TS (MPa) | EB (%) | Appearance |
|---|---|---|---|---|---|---|---|---|
| EO 1 | 1.36 ± 0.06 d | 69.99 ± 0.68 d | 599.57 ± 15.07 d | 90.98 ± 1.80 c | 104.94 ± 1.20 a | 1.88 ± 0.09 b | 26.55 ± 4.02 a |  |
| EO 2 | 0.85 ± 0.01 c | 64.34 ± 0.42 c | 567.75 ± 11.07 c | 87.22 ± 1.06 b | 108.14 ± 0.81 b | 2.78 ± 0.17 c | 30.43 ± 2.99 a |  |
| EO 4 | 0.71 ± 0.02 a | 59.47 ± 0.44 b | 466.51 ± 42.21 b | 78.95±2.22 a | 114.96 ± 2.01 c | 3.45 ± 0.24 d | 45.30 ± 1ss.76 c |  |
| EO 6 | 0.78 ± 0.01 b | 56.52 ± 0.64 a | 388.77 ± 30.47 a | 80.22±1.83 a | 110.85 ± 3.11 bc | 1.54 ± 0.11 a | 39.40 ± 2.21 b |  |
| Sample | Peppas Model | ||
| Regression Coefficient (r2) | Kp | n | |
| EO 1 | 0.9771 | 0.3230 | 0.1643 |
| EO 2 | 0.9928 | 0.2581 | 0.1986 |
| EO 4 | 0.9835 | 0.2753 | 0.1777 |
| EO 6 | 0.9671 | 0.2689 | 0.1938 |
| Sample | Weibull Model | ||
| Regression Coefficient (r2) | a | b | |
| EO 1 | 0.9352 | 0.3100 | 0.3027 |
| EO 2 | 0.9589 | 0.2384 | 0.3347 |
| EO 4 | 0.9696 | 0.2721 | 0.2878 |
| EO 6 | 0.9504 | 0.2340 | 0.3455 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Pei, J.; Xiong, X.; Xue, F. Encapsulation of Grapefruit Essential Oil in Emulsion-Based Edible Film Prepared by Plum (Pruni Domesticae Semen) Seed Protein Isolate and Gum Acacia Conjugates. Coatings 2020, 10, 784. https://doi.org/10.3390/coatings10080784
Li C, Pei J, Xiong X, Xue F. Encapsulation of Grapefruit Essential Oil in Emulsion-Based Edible Film Prepared by Plum (Pruni Domesticae Semen) Seed Protein Isolate and Gum Acacia Conjugates. Coatings. 2020; 10(8):784. https://doi.org/10.3390/coatings10080784
Chicago/Turabian StyleLi, Chen, Jiliu Pei, Xiaohui Xiong, and Feng Xue. 2020. "Encapsulation of Grapefruit Essential Oil in Emulsion-Based Edible Film Prepared by Plum (Pruni Domesticae Semen) Seed Protein Isolate and Gum Acacia Conjugates" Coatings 10, no. 8: 784. https://doi.org/10.3390/coatings10080784
APA StyleLi, C., Pei, J., Xiong, X., & Xue, F. (2020). Encapsulation of Grapefruit Essential Oil in Emulsion-Based Edible Film Prepared by Plum (Pruni Domesticae Semen) Seed Protein Isolate and Gum Acacia Conjugates. Coatings, 10(8), 784. https://doi.org/10.3390/coatings10080784