Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained in a Zn-AlNiBi Galvanizing Bath
Abstract
1. Introduction
2. Experimental
2.1. Materials and Hot Dipping
2.2. Characterization Methods
2.3. Corrosion Testing Method
2.3.1. Neutral Salt Spray
2.3.2. Sulfur Dioxide Test in a Humid Atmosphere
2.3.3. Electrochemical Test
3. Results and Discussion
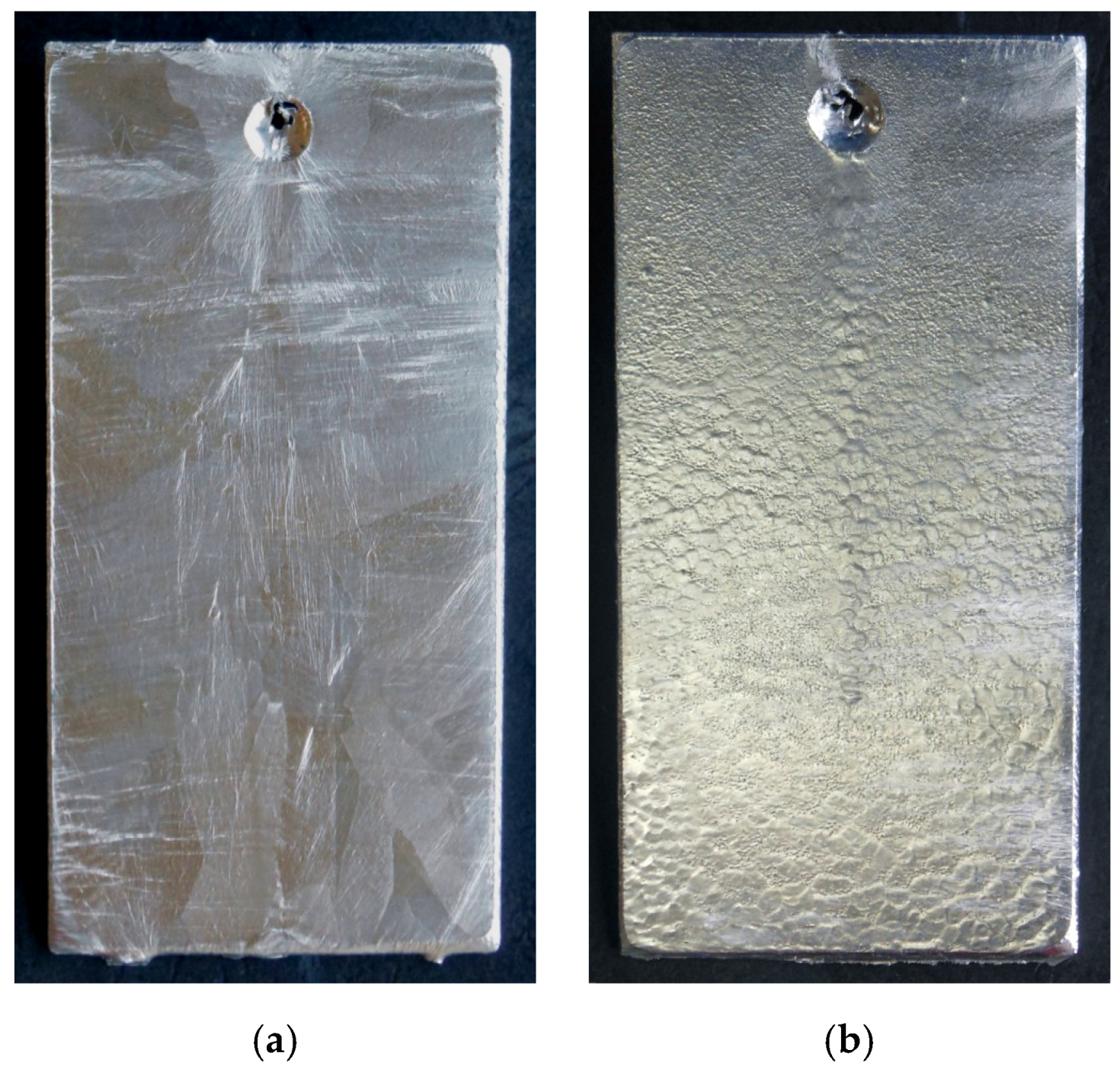
3.1. Appearance, Structure, and Thickness of Coating
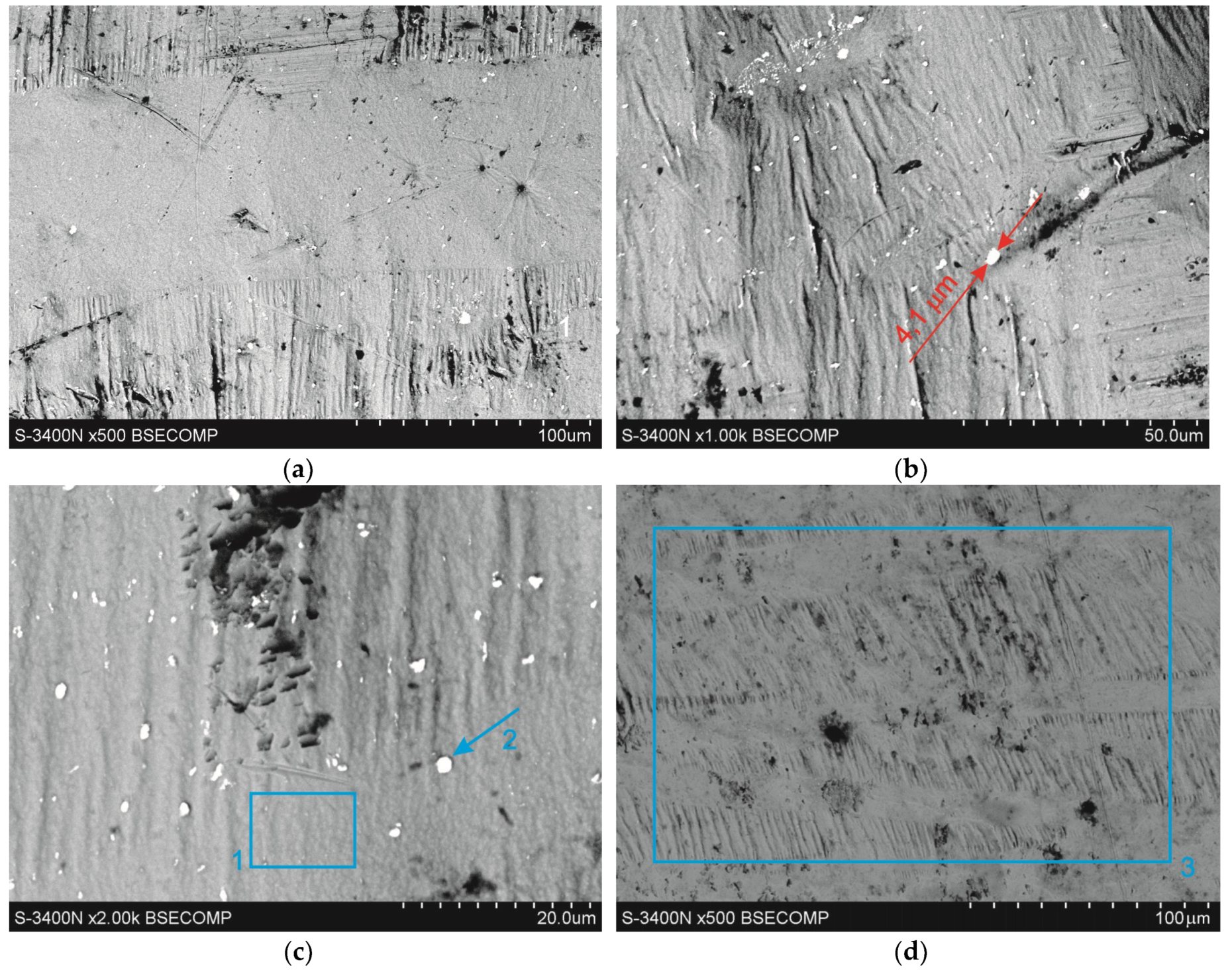
3.2. Surface Microstructure
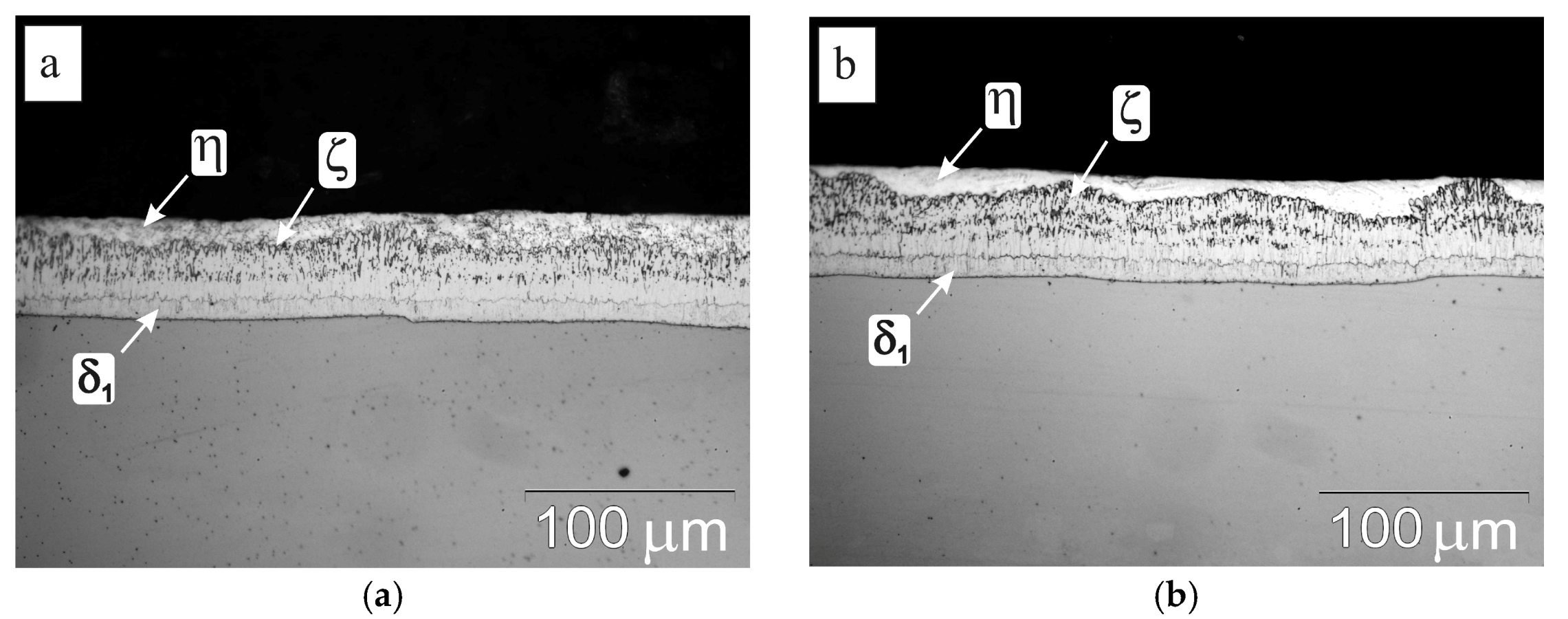
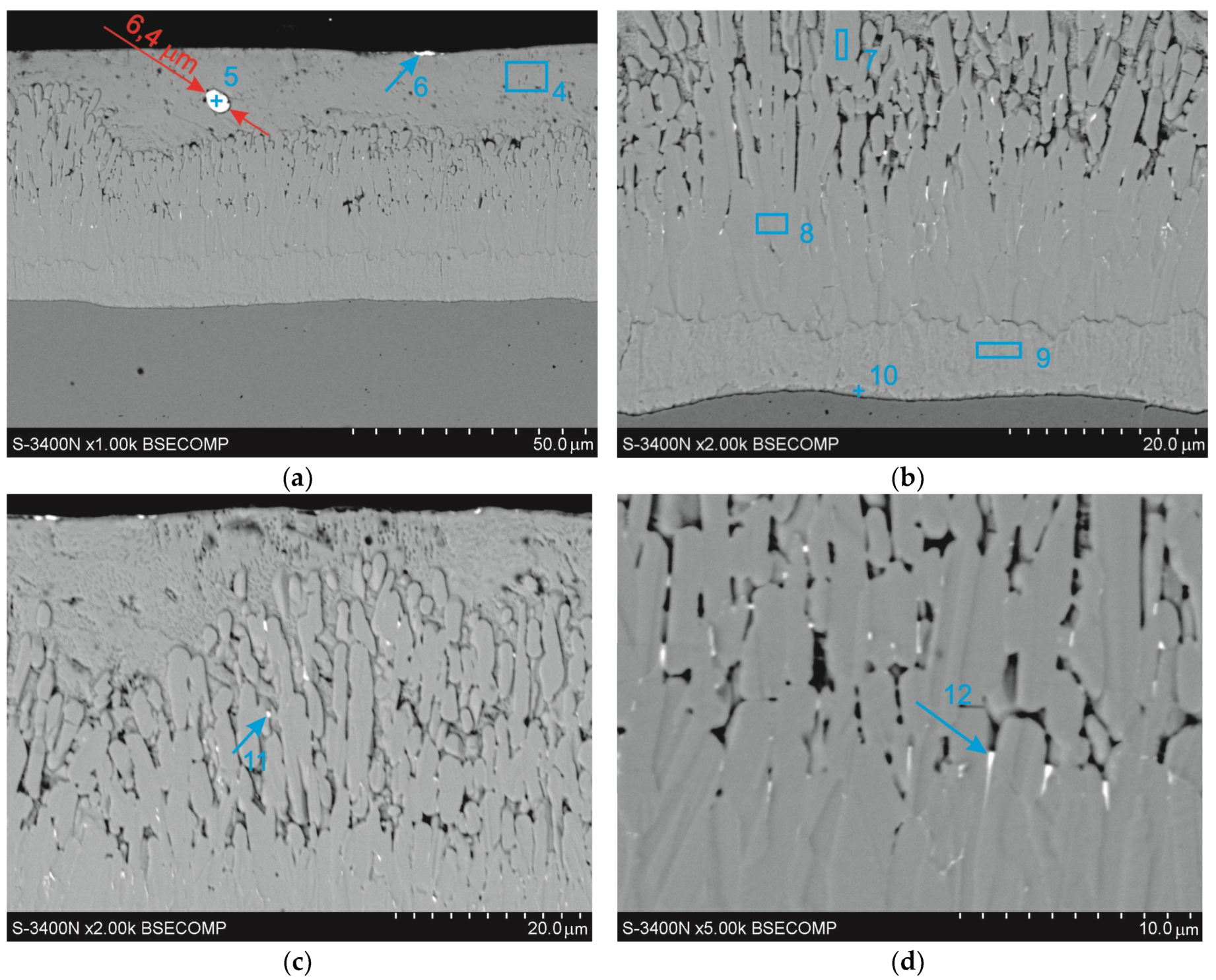
3.3. Cross-Sectional Microstructure of Coating
3.4. Effects of Corrosion Resistance Tests
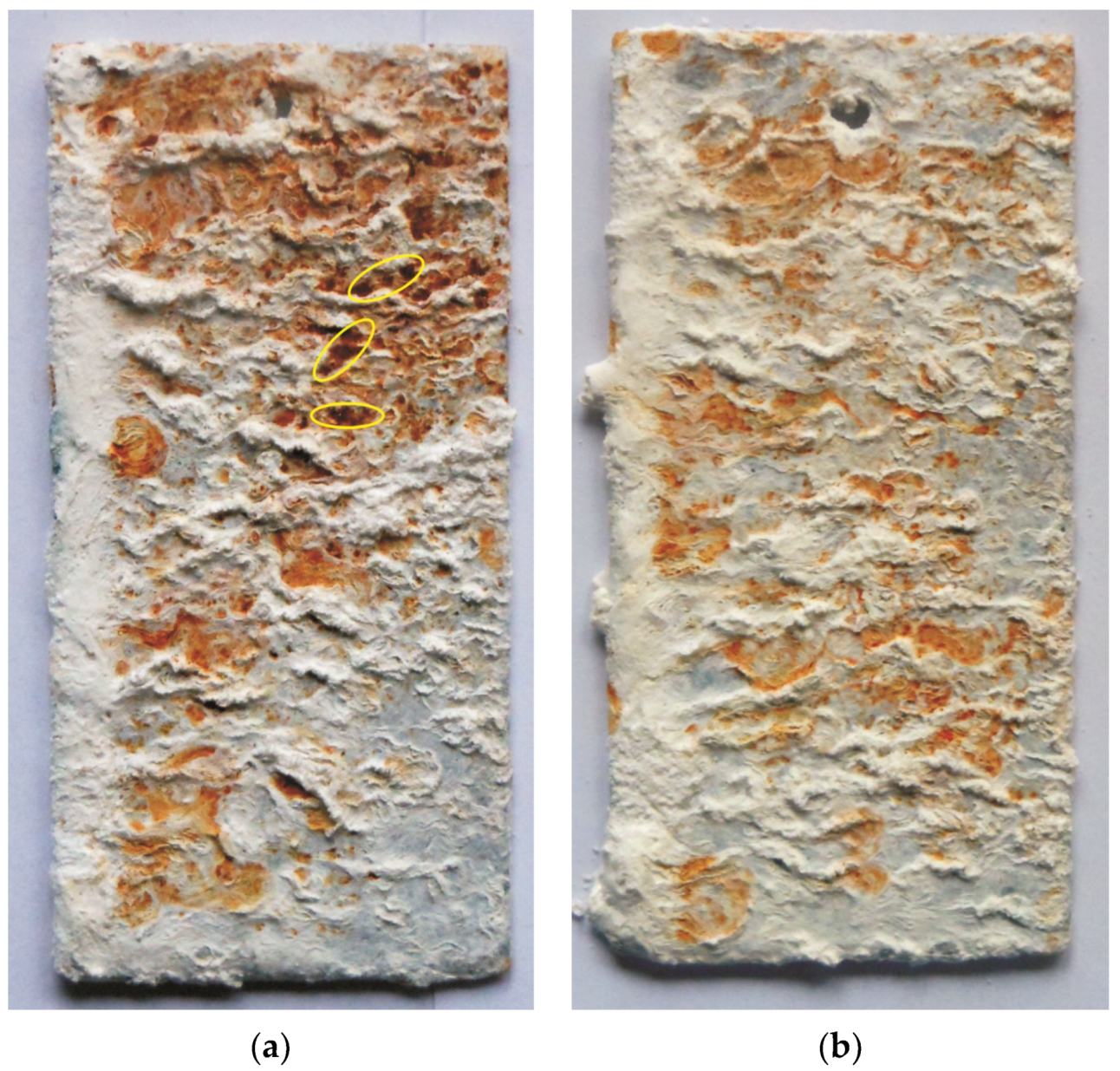
3.4.1. Corrosion Resistance Determined via the Neutral Salt Spray Test
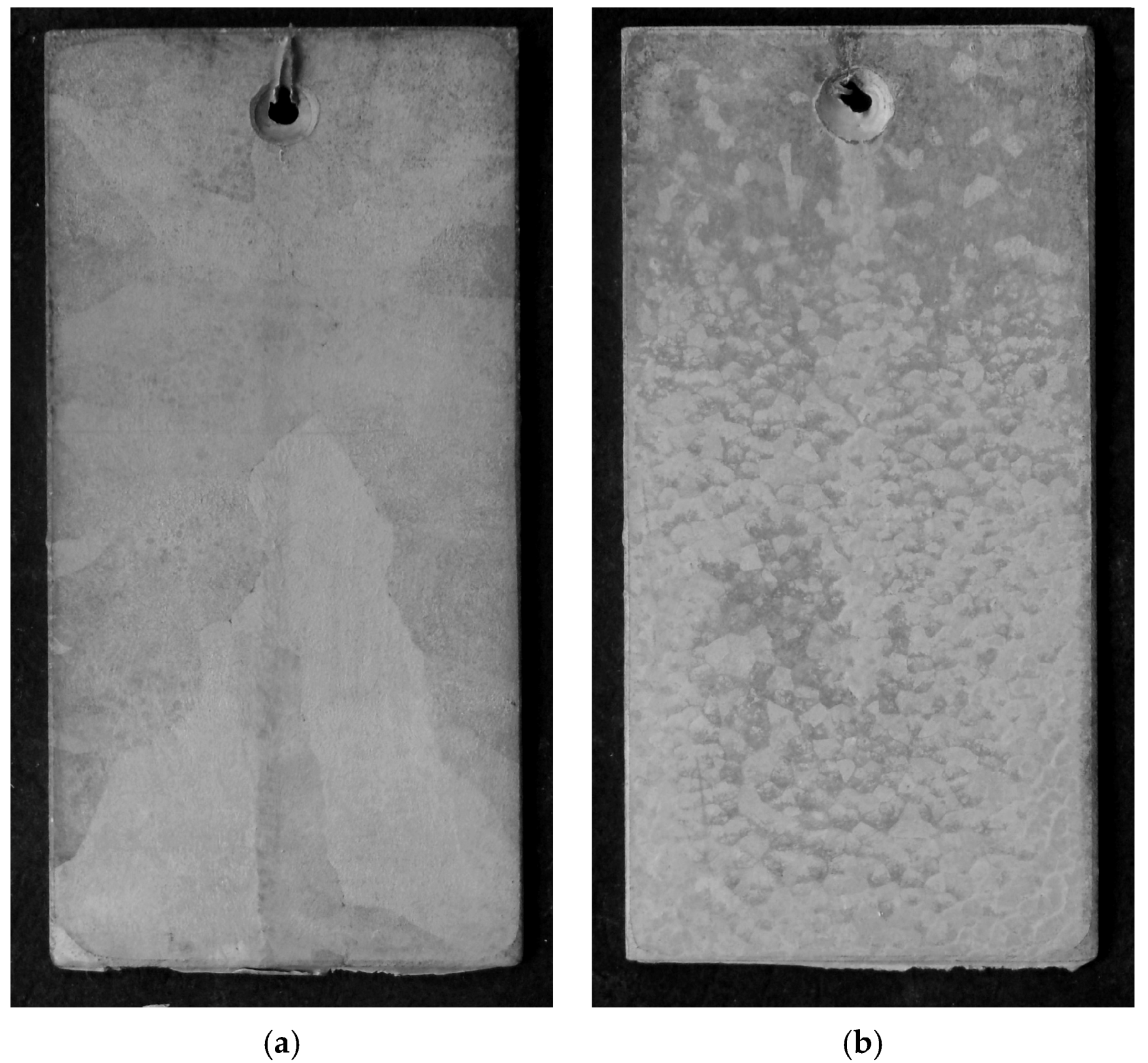
3.4.2. Corrosion Resistance Determined via the Sulfur Dioxide Test in a Humid Atmosphere
3.4.3. Corrosion Resistance Determined via an Electrochemical Test
4. Conclusions
- (1)
- In the microstructure of the coating obtained in the Zn-AlNiBi bath, the presence of large Bi precipitates in the outer layer on its surface and cross-section and small Bi precipitates in the ζ phase layer was found. No other bath alloying additives (Al and Ni) were found in the coating.
- (2)
- Corrosion tests carried out with a neutral salt spray, in a humid atmosphere containing sulfur dioxide, and potentiodynamic tests consistently confirm the reduction of corrosion resistance of coatings obtained in the Zn-AlNiBi bath in comparison with the corrosion resistance of coatings obtained in the bath of “pure” zinc.
- (3)
- The reason for the decrease in corrosion resistance may be the presence of Bi precipitates in the coating, which, having a positive standard potential, creates a corrosive cell with Zn, accelerating the corrosion process of the coating.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kania, H.; Liberski, P. Synergistic influence of the addition of Al, Ni and Pb to a zinc bath upon growth kinetics and structure of coatings. Solid State Phenom. 2014, 212, 115–120. [Google Scholar] [CrossRef]
- Kania, H.; Liberski, P. Synergistic influence of Al, Ni, Bi and Sn addition to a zinc bath upon growth kinetics and the structure of coatings. IOP Conf. Ser. Mater. Sci. Eng. 2012, 35. [Google Scholar] [CrossRef]
- Porter, F.C. Zinc Handbook: Properties Processing and Use in Design; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Reumont, G.; Perrot, P.; Foct, J. Thermodynamic study of the galvanizing process in a Zn-0.1%Ni bath. J. Mater. Sci. 1998, 33, 4759–4768. [Google Scholar] [CrossRef]
- Tang, N.Y. Alternative Description of dross formation when galvanizing steels in zinc-nickel baths. J. Phase Equilibria 1995, 16, 110–112. [Google Scholar] [CrossRef]
- Liberski, P. Antykorozyjne Metalowe Powłoki Zanurzeniowe; Politechnika Śląska: Gliwice, Poland, 2013. [Google Scholar]
- Lewis, G.P.; Pedersen, J.G. Optimizing the Nickel-Zinc Process for Hot Dip Galvanizing. In Proceedings of the 3rd Asian Pacific General Galvanizing Conference, Queensland, Australia, 8 September 1996. [Google Scholar]
- Fasoyino, F.A.; Weinberg, F. Spangle formation in galvanized sheet steel coatings. Met. Trans. B Process Met. 1990, 21, 549–558. [Google Scholar] [CrossRef]
- Strutzenberger, J.; Faderl, J. Solidification and spangle formation of hot-dip-galvanized zinc coatings. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1998, 29, 631–646. [Google Scholar] [CrossRef]
- Krepski, R.P. The influence of lead in after-fabrication hot dip galvanizing. In Proceedings of the 14th International Galvanizing Conference, Zinc Development Association, London, UK, 6–12 June 1986. [Google Scholar]
- Beguin, P.; Bosschaerts, M.; Dhaussy, D.; Pankert, R.; Gilles, M. Galveco a solution for galvanizing reactive steel. In Proceedings of the 19th International Galvanizing Conference, EGGA Berlin/Heidelberg, Germany, 1–8 March 2000. [Google Scholar]
- Reumont, G.; Perrot, P. Fundamental study of lead additions in industrial zinc. In Proceedings of the 18th International Galvanizing Conference, EGGA Birmingham, UK, 8–11 June 1997. [Google Scholar]
- Gagne, M. Hot-dip galvanizing with zinc-bismuth alloys. Metallurgy 1999, 53, 269–271. [Google Scholar]
- Tatarek, A.; Saternus, M. Badanie zjawisk rozpuszczania dyfuzyjnego stali reaktywnych w kąpieli cynkowej z dodatkiem bizmutu. Ochr. Przed Korozją 2018, 7, 186–190. (In Polish) [Google Scholar] [CrossRef]
- Mendala, J. The Possibility of the LME phenomenon in elements subjected to metallization in Zn bath with Bi addition. Solid State Phenom. 2015, 226, 167–172. [Google Scholar] [CrossRef]
- Deutscher Ausschuß für Stahlbau. DASt-Richtlinie 022—Guideline for Hot-Dip-Zinc-Coating of Prefabricated Load-Bearing Steel Components; Deutscher Ausschuß für Stahlbau: Düsseldorf, Germany, 2009. [Google Scholar]
- Kania, H.; Saternus, M.; Kudláček, J. Structural aspects of decreasing the corrosion resistance of zinc coating obtained in baths with Al, Ni, and Pb additives. Materials 2020, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Pryor, M.J. Bimetallic corrosion. In Corrosion; Shreir, L.L., Ed.; George Newnes Ltd.: London, UK, 1963; Volume 1, p. 69. [Google Scholar]
- Seré, P.R.; Culcasi, J.D.; Elsner, C.I.; Sarli, A.R. Relationship between texture and corrosion resistance in hot-dip galvanized steel sheets. Surf. Coat. Technol. 1999, 122, 143–149. [Google Scholar] [CrossRef]
- Pyun, S.; Bae, J.; Park, S.; Kim, J.; Lee, Z. The anodic behavior of hot-galavnized zinc layer in alkaline solution. Corros. Sci. 1994, 36, 827–835. [Google Scholar] [CrossRef]
- Liu, L.M. Nonferrous Metals. Extr. Metall. 2005, 3, 47. [Google Scholar]
- Radu, T.; Ciocan, A.; Potecasu, F.; Balint, L. Obtaining and characterizing Zn-Al-Bi coatings on steel band. In Proceedings of the 21st International Conference on Metallurgy and Materials, Brno, Czech Republic, 23–25 May 2012. [Google Scholar]
- Wang, J.H.; Wang, X.M.; Liu, C.M.; Su, X.P.; Wu, C.J.; Liu, Y.; Tu, H. Effect of bismuth on the microstructure and corrosion resistance of galvanized coating. Adv. Mater. Res. 2011, 391, 1183–1188. [Google Scholar] [CrossRef]
- ISO 9227:2017, Corrosion Tests in Artificial Atmospheres—Salt Spray Tests; ISO: Geneva, Switzerland, 2017.
- PN-EN ISO 6988:2000, Metallic and Other Non-Organic Coatings—Sulfur Dioxide Test with General Condensation of Moisture; Polish Committee for Standardization: Warszawa, Poland, 2000.
- Wołczyński, W.; Guzik, E.; Janczak-Rusch, J.; Kopyciński, D.; Golczewski, J.; Mo Lee, H.; Kloch, J. Morphological characteristics of multi-layer/substrate systems. Mater. Charact. 2006, 56, 274–280. [Google Scholar] [CrossRef]
- EN ISO 1461:2009, Hot Dip Galvanized Coatings on Fabricated Iron and Steel Articles—Specifications and Test Methods; ISO: Geneva, Switzerland, 2009.
- Massalski, T.B.; Okamoto, H. Binary Alloy Phase Diagrams, 2nd ed.; ASM Internationa: Materials Park, OH, USA, 1990. [Google Scholar]
- Vizdal, J.; Braga, M.H.; Kroupa, A.; Richter, K.W.; Soares, D.; Malheiros, L.F.; Ferreira, J. Phase field simulations in miscibility gaps. Calphad 2007, 31, 438–448. [Google Scholar] [CrossRef]
- Kubaschewski, O. Iron-Binary Phase Diagrams; Springer: Berlin/Heidelberg, Germany, 1982; p. 172. [Google Scholar]
- Liberski, P.; Tatarek, A.; Kania, H.; Podolski, P. Coating growth on silicon-containing iron alloys in hot dip galvanizing process. In Proceedings of the 22nd International Galvanizing Conference Intergalva 2009, EGGA, Madrid, Spain, 8–12 June 2009; pp. 181–187. [Google Scholar]
- Waseda, Y.; Suzuki, S. Characterization of Corrosion Products on Steel Surfaces; Series: Advances in Materials Research; Springer: Berlin/Heidelberg, Germany, 2006; Volume 7. [Google Scholar]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Springer: New York, NY, USA, 2013. [Google Scholar]
- Kania, H.; Sipa, J. Microstructure characterization and corrosion resistance of zinc coating obtained on high-strength grade 10.9 bolts using a new thermal diffusion process. Materials 2019, 12, 1400. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.W.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 2003. [Google Scholar]
- Kuz, M. Handbook of Environmental Degradation of Materials; William Andrew Publishing, Inc.: Norwich, UK, 2005. [Google Scholar]
- Dalledone, E.; Barbosa, M.A.; Wolynec, S. Zinc-55% aluminum-1.6% silicon coating compared with zinc coating. Mater. Perform. 1995, 34, 24–28. [Google Scholar]
- Radu, T.; Vlad, M. Zinc coatings on steel substrate attained by different elements added. Teh. J. 2011, 18, 133–139. [Google Scholar]
- Barranco, V.; Feliu, S.; Feliu, S. EIS study of the corrosion behaviour ofzinc-based coatings on steel in quiescent 3% NaCl solution. Part 1: Directly exposed coatings. Corros. Sci. 2004, 46, 2203–2220. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press/Taylor and Francis Group LLC: Boca Raton, FL, USA, 2010. [Google Scholar]
- Bockris, J.; Reddy, A.K.N. Modern Electrochemistry; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1998. [Google Scholar]




| Bath | Content [wt.%] | ||||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Ni | Pb | Bi | Sn | Zn and Others | |
| Zn-AlNiBi | 0.0053 ± 0.0016 | 0.029 ± 0.010 | 0.054 ± 0.0011 | 0.002 ± 0.0002 | 0.0623 ± 0.0012 | 0.0009 ± 0.0001 | residue |
| “pure”zinc [17] | 0.0002 ± 0.0001 | 0.031 ± 0.002 | 0.00010 ± 0.00006 | 0.0013 ± 0.0005 | 0.0003 ± 0.0001 | 0.0007 ± 0.0001 | residue |
| Grade | Content [wt.%] | |||||
|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Fe and Others | |
| G235JRG2 | 0.13860 ± 0.00607 | 0.02101 ± 0.00070 | 0.74366 ± 0.00278 | 0.00869 ± 0.00034 | 0.00881 ± 0.00043 | residue |
| Analyzing Points | Element Contents/Error (+/−1 Sigma) | |||||||
|---|---|---|---|---|---|---|---|---|
| Zn-K | Bi-M | |||||||
| wt.% | wt.% Error | at.% | at.% Error | wt.% | wt.% Error | at.% | at.% Error | |
| point 1 | 100 | ±1.8 | 100 | ±1.8 | – | – | – | – |
| point 2 | 23.3 | ±1.4 | 49.3 | ±2.7 | 76.7 | ±0.7 | 50.7 | ±0.5 |
| point 3 | 100 | ±1.6 | 100 | ±1.6 | – | – | – | – |
| Analyzing Points | Element Contents/Error (+/− 1 Sigma) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn-K | Fe-K | Bi-M | ||||||||||
| wt.% | wt.% Error | at.% | at.% Error | wt.% | wt.% Error | at.% | at.% Error | wt.% | wt.% Error | at.% | at.% Error | |
| point 4 | 100 | ±2.4 | 100 | ±2.4 | – | – | – | – | – | – | – | – |
| point 5 | 12.8 | ±1.2 | 31.9 | ±1.6 | – | – | – | – | 87.2 | ±0.8 | 68.1 | ±0.6 |
| point 6 | 37.7 | ±1.2 | 65.9 | ±2.2 | – | – | – | – | 62.3 | ±0.5 | 34.1 | ±0.3 |
| point 7 | 94.6 | ±2.1 | 93.7 | ±2.1 | 5.4 | ±0.4 | 6.3 | ±0.5 | – | – | – | – |
| point 8 | 93.9 | ±2.0 | 92.9 | ±1.9 | 6.1 | ±0.2 | 7.1 | ±0.3 | – | – | – | – |
| point 9 | 90.7 | ±2.0 | 89.3 | ±2.0 | 9.3 | ±0.5 | 10.7 | ±0.5 | – | – | – | – |
| point 10 | 77.6 | ±1.6 | 74.7 | ±1.5 | 22.4 | ±0.5 | 25.3 | ±0.6 | – | – | – | – |
| point 11 | 84.7 | ±1.9 | 91.5 | ±2.0 | 3.6 | ±0.4 | 4.6 | ±0.5 | 11.7 | ±0.4 | 4.0 | ±0.1 |
| point 12 | 80.5 | ±1.6 | 89.1 | ±1.9 | 4.4 | ±0.4 | 5.7 | ±0.5 | 15.1 | ±0.4 | 5.2 | ±0.2 |
| Coating | jcorr (mA/cm2) | Ecorr (mV vs. NHE) |
|---|---|---|
| Zn-AlNiBi | −14.26 | −779.67 |
| “pure” zinc | −10.18 | −766.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, H.; Saternus, M.; Kudláček, J.; Svoboda, J. Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained in a Zn-AlNiBi Galvanizing Bath. Coatings 2020, 10, 758. https://doi.org/10.3390/coatings10080758
Kania H, Saternus M, Kudláček J, Svoboda J. Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained in a Zn-AlNiBi Galvanizing Bath. Coatings. 2020; 10(8):758. https://doi.org/10.3390/coatings10080758
Chicago/Turabian StyleKania, Henryk, Mariola Saternus, Jan Kudláček, and Jakub Svoboda. 2020. "Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained in a Zn-AlNiBi Galvanizing Bath" Coatings 10, no. 8: 758. https://doi.org/10.3390/coatings10080758
APA StyleKania, H., Saternus, M., Kudláček, J., & Svoboda, J. (2020). Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained in a Zn-AlNiBi Galvanizing Bath. Coatings, 10(8), 758. https://doi.org/10.3390/coatings10080758







