Abstract
New packaging materials using biopolymers have been studied to substitute synthetic packaging materials that lead to environmental pollution. In this study, a new biodegradable packaging material was developed using the pectin extracted from Citrus junos pomace, which is considered a food processing byproduct. Rambutan peel extract (RPE), at different concentrations (0.25%, 0.5%, and 1.0%), was added as an active material, and the functional properties of the C. junos pectin (CJP) films were evaluated. The incorporation of RPE enhanced the extensibility of the CJP films and their light-blocking ability by decreasing light transmittance. As the concentration of RPE increased, antioxidant activities of the CJP films increased, along with an increase in total phenolic content. Subsequently, the CJP prepared in this study can be used as a low-cost active biodegradable film material, and RPE can be added as a natural antioxidant for the CJP films to confer antioxidant activity.
1. Introduction
There is increasing interest in the new packaging materials using biopolymers to preserve environment by substituting synthetic packaging materials that lead to environmental pollution [1]. Biopolymers can be obtained from food byproducts, and packaging materials using food byproducts can solve economic and environmental issues [2]. Among biopolymers, pectin is a polysaccharide constituting a plant cell wall with α-1,4-d-galacturonic acid structure [3]. Pectin can be used as a film material due to its non-toxicity, high solubility in water, and excellent film-forming ability [4].
Citrus junos (Yuza) is grown in Northeast Asian countries including Korea, where it is very popular, and it is consumed as juice, tea and dressing rather than raw fruit due to its unique flavor. As the demand for C. junos-processed products has increased, 1800 tons of C. junos pomace is produced annually in Korea [5]. C. junos pomace is either utilized as animal feed and fertilizer or discarded [6]; however, it contains various bioactive compounds including polyphenols, such as hesperidin and naringin [7]. C. junos pectin (CJP) is obtained from C. junos pomace. CJP mainly contains galacturonic acid (72%), while arabinose and galactose are the main neutral sugars [5]. Pectins extracted from C. junos pomace, which is a byproduct, can be used as a film base material in the food industry with pro-economic and -environmental potential. Nevertheless, CJP has not been studied as a film base material to date.
Rambutan (Nephelium lappaceum) is grown in tropical regions, such as Indonesia and Malaysia, and it is consumed as fresh fruit, juice, jelly, and jam [8]. Approximately 1.27 million metric tons of rambutan is cultivated annually, but large amounts of peels and seeds are discarded as byproducts during processing [9]. Some of the byproducts are utilized as animal feeds or fertilizers, however, most of them are discarded, causing environmental problems [10]. Moreover, rambutan peel accounts for 45% of its total weight, and rambutan peel extract (RPE) is reported to have higher radical scavenging activity and phenolic content than its leaves or seeds [11]. The antioxidant activity of RPE is attributed to large amounts of antioxidative compounds, such as geraniin, ellagic acid and corilagin [12]. Among them, geraniin is known to be the main compound of RPE and affects the antioxidant property of RPE [9].
In this study, an active film based on CJP and RPE that are discarded as food processing byproducts has been developed for the first time to substitute synthetic packaging materials. Therefore, the objective of this study was to develop a novel antioxidant packaging material based on the food processing byproducts that have not been studied. In particular, more characteristics of various antioxidant properties of the developed CJP films were tested than in previous studies to evaluate the functional properties of RPE. Moreover, the antioxidant films developed in this study with a low cost can be applied as novel packaging materials with antioxidant activity to retard lipid oxidation in food and preserve food quality.
2. Materials and Methods
2.1. Materials
C. junos pomace was generated after making C. junos tea, and it was obtained from Jeonnam Agricultural Research & Extension Services (Naju, Korea). Rambutan was obtained from Bbsusan (Chuncheon, Korea). Ascorbic acid, sodium carbonate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and glycerol were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
2.2. CJP Extraction
The CJP was extracted according to the extraction method described by Tamaki et al. [13], with minor modifications. The C. junos pomace was dried at 50 °C and ground. The ground pomace was mixed with 0.05 M HCl (1:30, w/v). The mixture was heated at 85 °C for 1 h and filtered using four layers of gauze. The filtrate was then cooled at 4 °C and adjusted to pH 4.5 using 0.5 M NaOH. The acidic solution was precipitated with 95% ethanol (1:3, v/v), and the precipitate was dried at 50 °C. The obtained CJP was ground and passed through a 180-mesh sieve. The CJP was kept at 4 °C and used for subsequent experiments. The yield was approximately 10%.
2.3. Preparation of RPE
The RPE was obtained according to the ethanol extraction method [14]. Rambutan peel was washed to remove impurities, and it was dried at 45 °C and ground. The ground rambutan peel was blended with 80% ethanol in a ratio of 1:10 (w/v) for 2 h and vacuum-filtered using Whatman filter paper No. 2. The filtrate was concentrated at 70 °C for 1 h using a vacuum evaporator to evaporate ethanol completely. The concentrated solution was then lyophilized and passed through a 180-mesh sieve. The yield was about 20%.
2.4. HPLC Analysis of RPE
To analyze phenolic compounds of the RPE, high-performance liquid chromatography (HPLC) (Waters Co., Milford, MA, USA) was used. The standards (geraniin and ellagic acid) and RPE were dissolved in 80% methanol. Syringe filter (0.45 μm, Whatman, Clifton, NJ, USA) was used to filter the samples. After filtration, the samples (20 μL) were injected onto a C18 column (Waters Co.). As the mobile solvents, 0.5% formic acid (A) and acetonitrile (B) were used, and the absorbance at 280 nm was monitored. The gradient elution was performed as follows: 0 min, 100% A; 3 min, 95% A; 5 min, 85% A; 8 min, 75% A; 10 min, 70% A; 13 min, 45% A; 15 min, 40% A; 20 min, 20% A; 23 min, 65% A; 25 min, 100% A; 26 min, 100% A. The flow rate was 1.0 mL/min.
2.5. Preparation of CJP films
Based on the results of preliminary experiments, the concentrations of CJP and plasticizer were determined. Fructose, sorbitol, and glycerol were used to determine an optimal plasticizer. CJP (3%, w/v) and a selected plasticizer (0.3 g/g CJP) were dissolved in distilled water and heated at 85 °C for 20 min while stirring. After 20 min, RPE (0.25%, 0.5%, and 1.0%, w/v) was added and stirred for 10 min. To make a homogeneous film-forming solution, a homogenizer (IKA, Staufen, Germany) was used for 3 min at 8000 rpm, and the solution was filtered using two layers of gauze. The filtrate (25 mL) was poured onto the plate (12 × 15 cm) and dried at 25 °C for 15 h. The obtained film was conditioned (25 °C, 50% relative humidity (RH)) and used for subsequent experiments.
2.6. Mechanical Properties
Thickness, tensile strength (TS), elongation at break (EB), and Young’s modulus (YM) of the films (2.5 × 10.5 cm) were measured. Film thickness was measured using a thickness gauge (Mitutoyo, Tokyo, Japan), and its average value was obtained by measuring five spots randomly on the film. The TS, EB, and YM were measured using an Instron universal testing machine (Testometric Co., Lancashire, UK). All film sheets were stretched at 50 mm/min.
2.7. Water Vapor Permeability (WVP)
A piece (2 × 2 cm2) of the film was attached on the top of a polymethylacrylate cup, which contained distilled water (15 mL). The cup was stored in a room with constant temperature and humidity (25 °C, 50% RH) for 7 h. The cup weight was measured every hour to calculate the ratio of weight change [15].
2.8. Contact Angle Measurement
A contact angle analyzer (Phoenix 300 Plus, SEO Co., Suwon, Korea) was used to measure a contact angle, which indicates the water affinity of the film surface. A strip of the film (7 × 1 cm2) was placed on a glass slide, and a drop of water (5 µL) was placed on the strip surface. The contact angle (°) between the surface and the drop of water was measured.
2.9. Fourier Transform Infrared (FTIR) Analysis
The FTIR analysis was performed using a vacuum infrared spectrophotometer (Vertex 80 v, Billerica, MA, USA). The spectra were obtained in the wavenumber range of 400–4,000 cm−1.
2.10. Optical Properties
The color of the film was measured on a white standard plate (L* = 97.51, a* = −0.32, and b* = 2.14) using a colorimeter (CR-400M, Minolta, Tokyo, Japan), and an average value was obtained by measuring five spots randomly on the film. To measure the opacity of the film, the absorbance of the film (2 × 3 cm2) was measured at 600 nm [16]. To evaluate the UV- and visible light-blocking ability of the film, the transmittance was measured in the wavelength range between 800 and 200 nm using a spectrophotometer (UV-2450, Shimadzu Co., Kyoto, Japan) [16].
2.11. Antioxidant Activity
2.11.1. Total Phenolic Content (TPC)
A piece of the film (0.1 g) was immersed in 10 mL of distilled water for 2 h and centrifuged at 3000× g for 5 min. The supernatant was used as a film extract for determining antioxidant activities. Film extract (0.4 mL) and 10% Folin–Ciocalteu reagent (2 mL) were mixed, and 7% sodium carbonate (1.6 mL) was added and kept for 2 h. The absorbance was measured at 760 nm. TPC was expressed as a gallic acid equivalent (GAE, mg) per g film [17].
2.11.2. Radical Scavenging Activity
Potassium persulfate (2.45 mM) and ABTS (7 mM) were mixed to prepare an ABTS solution. After incubation for 16 h, the solution was diluted using distilled water until its absorbance was 0.7 at 734 nm. The film extract (60 µL) was mixed with the ABTS solution (2940 µL). After mixing, the mixture was kept at 25 °C for 10 min and the absorbance was measured at 734 nm. To estimate DPPH radical scavenging activity, 1 mM DPPH was diluted to 0.1 mM with methanol. The film extract (100 µL) was mixed with the final DPPH solution (3900 µL) and kept for 1 h. The absorbance was measured at 517 nm [18].
2.11.3. Reduction Power Assay
The film extract (2 mL), 0.2 M phosphate buffer (2 mL, pH 6.6), and 1% potassium ferricyanide (2 mL) were mixed. The mixture was kept at 50 °C for 20 min, and 10% trichloroacetic acid (2 mL) was added. After centrifugation, the supernatant (2 mL), distilled water (2 mL), and 0.1% ferric chloride (0.4 mL) were mixed and kept for 10 min. The absorbance was measured at 700 nm. The control contained all reagents except the film extract. Ascorbic acid was used as the standard, and the reduction power of the films was represented as µmol ascorbic acid per g film [19].
2.11.4. Ferrous-Ion Chelating (FIC) Ability
The film extract (1 mL) was mixed with 2 mM FeCl2·4H2O (0.1 mL) and methanol (3.7 mL). After 5 min, 5 mM ferrozine (0.2 mL) was added and kept for 10 min. The control contained all the aforementioned reagents except the film extract. The absorbance was measured at 562 nm [20].
2.12. Thermal Analysis
Thermogravimetric analysis (TGA) and derivative thermogravimetric analysis (DTGA) were performed using a TGA analyzer (Mettler Toledo, Columbus, OH, USA) to evaluate the effect of RPE on the thermal stability of the films. The film fragments (5.0 ± 0.3 mg) were heated at temperatures from 25 to 700 °C. The weight loss (%), decomposition temperature (°C), and the residue (%) were obtained.
2.13. Film Structure Analysis
2.13.1. Scanning Electron Microscopy (SEM)
To examine the microstructure, SEM image was analyzed using the UHR FR-SEM (SU-8230, Hitachi High-Technologies Co., Tokyo, Japan). The film was attached to the carbon tape and coated with platinum for 2 min. The SEM image was obtained at 5 kV with a magnification of 1000× for the surface and 3000× for the cross-section.
2.13.2. Atomic Force Microscopy (AFM)
An AFM-Raman spectrometer (INNOVA-LABRAM HR800, Horiba Jobin-Yvon Inc., Piscataway, NJ, USA) was used for surface roughness measurement of the films. The three-dimensional image (10 × 10 μm2) shows the roughness of the films, and Ra (mean roughness) and Rmax (maximum height) were evaluated as surface roughness parameters.
2.14. Statistical Analysis
Statistical analysis with Duncan’s multiple range test was performed using the SAS software (SAS Institute Inc., Cary, NC, USA) [17]. All values are represented as the means ± standard deviations; p < 0.05 indicates significant difference. All experiments were carried out at least five times.
3. Results and Discussion
3.1. High Performance Liquid Chromatography (HPLC) Analysis of Rambutan Peel Extract (RPE)
HPLC results show that the major peak of RPE corresponds to geraniin (Figure 1), indicating that the main phenolic compound present in RPE is geraniin. In addition, the third peak of RPE corresponds to ellagic acid. Previously, Thitilertdecha et al. [21] also reported that the main phenolic compound of RPE is geraniin and other phenolic compounds, such as ellagic acid and corilagin are contained. Furthermore, it has been reported that RPE contains polyphenolic compounds with antioxidant activity, among which geraniin is the main compound [12].
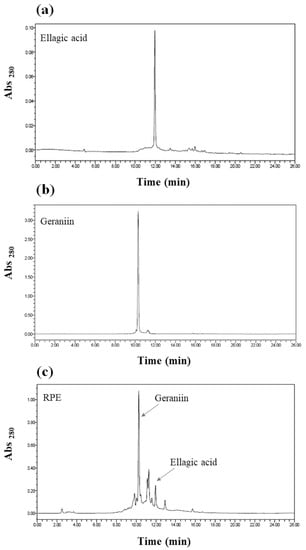
Figure 1.
HPLC results of rambutan peel extract (RPE): (a) ellagic acid; (b) geraniin; (c) RPE.
3.2. Mechanical Properties
The type of plasticizer influences the mechanical property of the films because plasticizers have different molecular weight, size, and hydrophilicity [22]. Sorbitol, fructose and glycerol were used to determine a suitable plasticizer for CJP films, and the effects of the plasticizer type are shown in Table 1. The film with fructose had the poorest mechanical properties, with TS, YM, and EB of 1.15 MPa, 36.81 MPa, and 6.40%, respectively, whereas the film with glycerol had greater TS (22.91 MPa), YM (223.93 MPa), and EB (10.42%) than the film with fructose or sorbitol. It is apparent that glycerol is effective in enhancing extensibility due to its small molecular weight [23]. Moreover, the films with glycerol absorb more moisture than the films with sorbitol, which increases the stretching ability of the film because of the plasticizing role of water molecules [24]. Therefore, glycerol was selected as the optimal plasticizer for CJP films.

Table 1.
Effect of plasticizer type on the mechanical properties of the C. junos pectin (CJP) film.
The type and concentration of active materials along with the interactions with polymers affect the mechanical property of films [25]. Table 2 shows the effect of RPE addition on the mechanical properties of CJP films. As the concentration of added RPE increased, the thickness of CJP films increased from 0.078 to 0.092 mm. An increase in film thickness is mainly due to the increased number of solids in the film matrix with the incorporation of RPE [26]. With increasing RPE content, TS and YM decreased and EB increased. The TS of the film without RPE was 22.91 MPa, whereas the TS of the film with 1.0% RPE was 10.22 MPa. Similarly, the YM of the film with 1.0% RPE was 70.96 MPa, compared to 223.93 MPa for the neat CJP film. Meanwhile, the EB of the film without RPE was 10.42%, whereas it increased to 14.50% for the film with 1% RPE. These results indicate decreases in the rigidity and stiffness and an increase in the stretching ability of the film with the addition of RPE. The addition of RPE formed a discontinuous film matrix, decreasing the rigidity of the film. Moreover, sugar molecules present in RPE act as a plasticizer, weakening the interactions between polymers and increasing the free volume, thereby decreasing the rigidity and enhancing the stretching ability of the films [27]. Similarly, pectin films containing fruit extracts also showed a decrease in TS due to the new interactions between polymer chains and the fruit extracts [28].

Table 2.
Mechanical properties of the CJP films containing different concentrations of RPE.
3.3. Water Vapor Permeability
The WVP indicates the water barrier ability of the films. The permeability of films is affected by the solid content, solubility, and diffusivity of film components [29]. The WVPs of CJP films are shown in Table 3. The CJP film without RPE had the lowest WVP value. With the addition of RPE, WVP increased; however, there was no significant difference (p > 0.05) between the film with 0.5% RPE and the film with 1.0% RPE. Increasing the WVP value means increasing the permeability of the film. Viana et al. [29] reported that increased solid content affects the water barrier property of the films, resulting in increased WVP. The addition of RPE changes the film structure by the plasticizing effect and decreases the water barrier property. The plasticizing effect increases the stretching ability of the films and free volume, making it easy to evaporate water molecules; these changes are associated with decreased TS [27]. Similarly, Han and Song [30] reported that WVP increased alongside the amount of sage leaf extract because the bonds among the film components were weakened and the free volume of polymers was increased by the addition of the extract.

Table 3.
Water barrier properties of the CJP films containing different concentrations of RPE.
3.4. Contact Angle
A contact angle above 90° indicates that the surface is not wettable (hydrophobic), whereas an angle below 30° represents that the surface is completely wettable, (hydrophilic) and an angle between 30° and 90° indicates that the surface is partially wettable [31]. The contact angles of the CJP films are shown in Table 3. The CJP film without RPE had a contact angle of 39.35°, indicating that CJP film is partially wettable. Previously, Chaiwarit et al. [31] reported that the contact angle of pectin films was 34.27°. The hydrophilic property of pectin films is due to the hydration and water-binding property of pectins [32]. The contact angle of CJP films decreased with an increasing RPE concentration, suggesting that the film hydrophilicity increased. The contact angle of the CJP film with 1.0% RPE was 27°, implying that the film surface is completely wettable. Rambutan peel ethanol extract contains more soluble phenolic compounds than water or methanol extracts [33]. As the concentration of added RPE increased, the amount of soluble phenolic compounds increased, causing a decrease in the contact angle. Moreover, the increase in WVP value indicates that the hydrophilic components of CJP films are increased, and the decrease in contact angle value also suggests the increase in the hydrophilicity of CJP films. Similarly, the solubility of pectin films containing fruit extracts increased with increasing amount of extracts due to the hydrophilicity of pectin, glycerol, and fruit extracts [28].
3.5. Fourier-Transform Infrared Spectroscopy Analysis
The FTIR results showed the molecular interactions between the CJP and RPE (Figure 2). The spectra of CJP and RPE are shown in Figure 2A. In the CJP spectrum, the band at 3280 cm−1 corresponds to the –OH stretching vibration of pectin monomers [34], and the band at 2905 cm−1 is associated with methyl groups’ stretching vibration of pectin chains [35]. The band at 1740 cm−1 and the band at 1617 cm−1 are related to C=O stretching [36] and –COOH stretching vibration in pectin [35]. The band observed at 1230 cm−1 is related to carboxylic acids’ stretching [29]. The band at 1015 cm−1 corresponds to polygalacturonic acid, and the band at 820 cm−1 indicates the absorption of α–d–mannopyranose [37], while the band at 772 cm−1 indicates galactose absorption [38]. In the RPE spectrum, the band at 3267 cm−1 indicates –OH stretching and the band at 2925 cm−1 is related to C–H stretching [39]. The molecular interactions of CJP films containing RPE are shown in Figure 2B. The wavenumber of the films did not change much, but the intensity of the bands increased. The increase in the band intensity at 3280 cm−1 is mainly due to the increased hydrogen bonding between CJP and RPE [34].
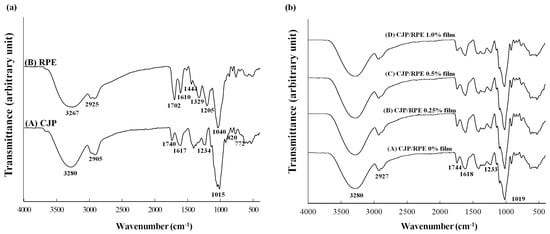
Figure 2.
FTIR of the CJP films containing different concentrations of RPE.
3.6. Optical Properties
The optical property of edible films affects appearance as well as consumer acceptance [34]. The optical properties of CJP films are shown in Table 4. The CJP film without RPE had the highest L* value and the lowest opacity, indicating a bright and transparent film. As the concentration of added RPE increased, the L* value decreased and opacity increased. These changes are mainly due to the phenolic components present in the RPE, making the films opaque [40]. In addition, redness and yellowness increased with an increasing amount of RPE. This can be explained by the orange-red color of rambutan peel [8]. Mendes et al. [26] reported that the color and transparency of pectin films was changed by the incorporation of spent coffee grounds, and they were affected by its amount, the interactions of phenolic compounds and the Maillard reaction during film preparation.

Table 4.
Optical properties of CJP films containing different concentrations of RPE.
UV-visible light transmittance, which represents the light-blocking ability of CJP films, is shown in Figure 3. The film without RPE showed the highest transmittance in the range of all wavelengths, whereas the films containing RPE decreased the light transmittance as the concentration of RPE increased, improving the ability to block light. In particular, the CJP films containing RPE blocked UV light completely. The incorporation of RPE in the intermolecular spaces of the CJP film matrix improved light-blocking ability due to the phenolic compounds present in the RPE. The pectin films containing fruit extracts also had better light blocking ability than the film without the extracts, because the phenolic compounds of the extracts acted as UV light absorbers [28].
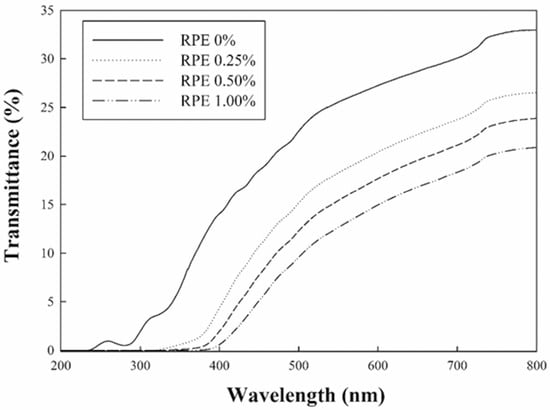
Figure 3.
UV-visible light transmittance of the CJP films containing different concentrations of RPE.
3.7. Antioxidant Properties
Fruit extracts contain many bioactive compounds and, because their antioxidant mechanisms are different, various antioxidant assays should be applied [41]. Rambutan peel has been regarded as a source of natural antioxidants. It contains flavonoids and tannins, which are known to be related to the excellent antioxidant activity of RPE [10]. The antioxidant activities of the CJP films are shown in Figure 4. The CJP film without RPE had low TPC, but the TPC increased with increasing RPE content (Figure 4A). The CJP film without RPE had 5.08 mg GAE/g film, whereas the CJP film with 1% RPE had 53.00 mg GAE/g film. RPE mainly contains phenolic acids and ellagitannins, such as geraniin, corilagin, and ellagic acid [8]. Therefore, the addition of RPE increases the number of phenolic compounds in the films, resulting in increased TPC. The TPC also affects the radical scavenging activities of CJP films. As the concentration of added RPE increased, ABTS and DPPH radical scavenging activities, as well as TPC, increased. Both ABTS and DPPH radical scavenging assays showed little activity for the neat CJP film, whereas the ABTS and DPPH radical scavenging activities of the CJP film containing 1.0% RPE were 98.20% and 90.87%, respectively. However, ABTS radical scavenging activity was not significantly different (p > 0.05) between the film with 0.5% RPE and the film with 1.0% RPE. It has been reported that RPE has higher DPPH radical scavenging activity than rambutan leaves, seeds and pulp [11]. The reducing power and FIC ability of CJP films also tended to increase with the addition of RPE. Rambutan peel has been reported to have strong reducing power, similar to that of vitamin C [33]. The CJP film without RPE had 1.48 mg ascorbic acid/g film, while the film with 1.0% RPE had 41.64 mg ascorbic acid/g film. Pectins obtained from C. junos could be chelating agents because they have chelating ability [42]. The CJP film without RPE had some FIC ability (35.14%) due to the chelating ability of CJP. The FIC ability of CJP films increased as the concentration of added RPE increased. The FIC ability (76.39%) of the CJP film containing 1.0% RPE was more than that (35.14%) of the film without RPE.
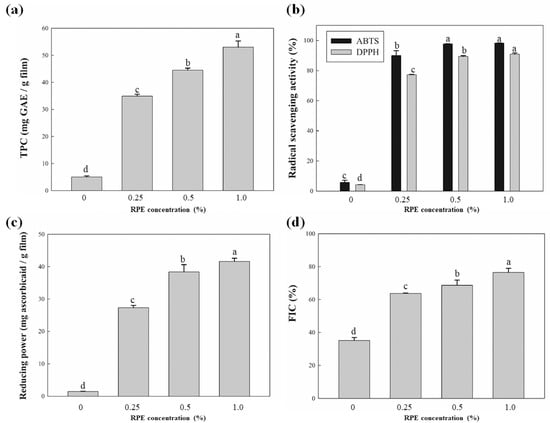
Figure 4.
Antioxidant activities of the CJP films containing different concentrations of RPE: (a) TPC; (b) radical scavenging activities; (c) reducing power; (d) FIC.
There are also some antioxidant activities of CJP films without RPE due to the effects of hesperidin and naringin present in the C. junos pomace (Figure 4). As the RPE concentration increased, the antioxidant activities of CJP films tended to increase. Rambutan peel ethanol extract contains high levels of natural antioxidants, such as geraniin, corilagin and ellagic acid [12]. Among them, geraniin is the main compound (Figure 1). The enhanced antioxidant activities of CJP films are due to the antioxidant activity of geraniin. Biopolymer composite films containing essential oils also show a tendency to increase radical scavenging activity, reducing power and FIC ability as the amount of essential oils increase, and it is related to the antioxidant activity of polyphenolic compounds present in the essential oils [43]. Han and Song [30] reported that pectin films with sage leaf extract had enhanced FIC activity as well as radical scavenging activities, owing to ferulic acid and the rosmarinic acid present in the sage leaf extract. Moreover, it should also be noted that the antioxidant activities of CJP films containing RPE were better than other citrus films containing clove bud essential oil or sage leaf extract in the literature [30,34], owing to the antioxidant activities of C. junos pomace and RPE. Overall, the antioxidant activities of CJP films were better than other citrus pectin films in the previous studies and oxidative deterioration in food can be prevented while maintaining food quality [44].
3.8. TGA Analysis
The TGA and DTG curves show the thermal degradation of CJP films (Figure 5). Degradation of the CJP films occurred in three stages. The first decomposition occurred at 76–84 °C due to the elimination of water molecules. The second decomposition occurred at 203–207 °C, where pectin and glycerol break down. The third decomposition occurred by the oxidation of polymers and RPE at 322–328 °C. Similarly, the pectin films containing nanoparticles were also decomposed in three stages, where there was the evaporation of water molecules in the first stage, decomposition of polymer and plasticizer in the second stage, and oxidation of polymer and nanoparticles in the third stage [35]. The DTG results show that with increasing RPE content, the peak shifted to a higher temperature, thereby enhancing the thermal stability of the CJP films. The first, second, and third stage decomposition temperatures were 76.30, 203.03, and 322.38 °C, respectively, for the CJP film without RPE, whereas those for the film with 1.0% RPE were 84.94, 207.02, and 328.69 °C, respectively. The thermal stability of CJP films containing RPE increased because new bonds between pectin and RPE were formed. Lei et al. [45] reported that the thermal stability of pectin films increased as the amount of tea polyphenol added increased because strong interactions were formed by the incorporation of tea polyphenol to the polymer matrix. It can be explained that phenolic compounds added to biopolymer composite films increased the thermal stability of films [43]. After thermal decomposition, the amount of residue gradually increased to 22.78%, 23.58%, 25.08%, and 26.65% with increasing RPE concentration, and the increase in the residue is due to the increase in the number of aromatic rings contained in the extract [30].
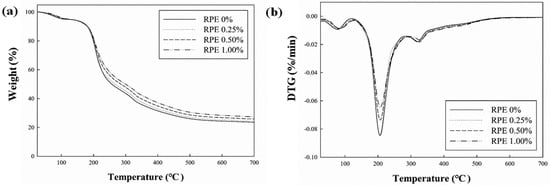
Figure 5.
Thermogravimetric analysis (TGA) (a) and derivative TGA (DTGA) (b) of the CJP films containing different concentrations of RPE.
3.9. Film Structure
3.9.1. SEM Analysis
The SEM images of the CJP films show the change in the microstructure of the films depending on RPE concentration (Figure 6). The CJP film had a smooth and uniform surface without pores and cracks. However, the film surface became rough and uneven, and white aggregates were observed with the addition of RPE. These white aggregates were generated by the sedimentation of RPE particles during film drying [26]. Viana et al. [29] reported that pectin films with guava puree or mango puree had a rough and irregular surface compared to the neat film because of the complex property of purees. The cross-sectional image of CJP films containing RPE also showed irregular shape and increased number of pores with increasing RPE content. The most heterogeneous structure was observed in the CJP film containing 1.0% RPE. The added extract to polymer film network is usually dispersed unevenly, forming pores and creating a discontinuous film structure [45]. Gao et al. [44] also reported that pectin–chitosan composite films had a smooth cross-sectional structure, whereas the composite film with tea polyphenol had a porous structure resulting from the aggregation of tea polyphenols. The structural change affects the mechanical property of the films, and heterogeneous film structure is associated with a decrease in TS.
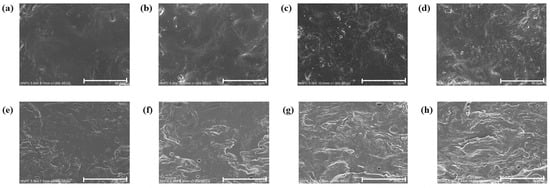
Figure 6.
SEM images of the CJP films containing different concentrations of RPE: (a–d): surface of the films; (e–h): cross-section of the films; (a,e) CJP film; (b,f) CJP/0.25% RPE film; (c,g) CJP/0.5% RPE film; (d–h) CJP/1.0% RPE film.
3.9.2. AFM
The AFM results indicate the roughness of the film surface. The three-dimensional AFM images show the change in the surface roughness of the CJP films containing RPE (Figure 7). The Ra value, which corresponds to roughness, increased as the concentration of RPE increased. The film without RPE had an even film surface, and the Ra value (23.3) of the film was the smallest. On the other hand, the film containing 1.0% RPE had the roughest film surface, and the Ra value (58.1) increased more than twice of that of the neat film. Similarly, the Rmax value also increased with RPE content. The Rmax of the neat film was 283 nm, whereas that of the film with 1.0% RPE was 767 nm. The increases in both parameters indicate the increase in the roughness of the film surface. The insoluble particles and aggregates of RPE in the film matrix create an uneven film surface. In particular, the insoluble particles of RPE migrate to the film surface during film drying, resulting in a rough surface [46]. The surface roughness of the film is also affected by the formation of new bonds and disordered structures due to the incorporation of additives [47]. Chaichi et al. [48] reported that the added nanocellulose aggregates made the surface of pectin films uneven, and the agglomerated nanocellulose particles caused the decrease in the TS of the films.
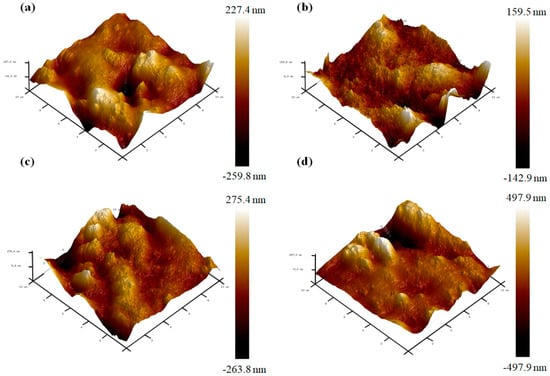
Figure 7.
AFM images of the CJP films containing different concentrations of RPE: (a) CJP film; (b) CJP/0.25% RPE film; (c) CJP/0.5% RPE film; (d) CJP/1.0% RPE film.
4. Conclusions
New antioxidant films were developed using C. junos pomace and rambutan peel, which are discarded as food processing byproducts, and their physicochemical properties were evaluated. The added RPE enhanced the stretching ability and light-blocking ability of the films. In particular, CJP films containing RPE completely blocked UV light. As the RPE concentration increased, the antioxidant activities such as radical scavenging activities, reducing power and FIC increased. Moreover, the antioxidant properties of the developed CJP films in this study were better than those of other citrus pectin films, owing to the functional properties of RPE. Subsequently, our results indicate that the CJP film is applicable as an antioxidant biodegradable film that retards lipid oxidation in foods, and further studies are needed to apply in a real food system in the future.
Author Contributions
Conceptualization, E.-J.G.; data curation, E.-J.G.; funding acquisition, K.B.S.; investigation, E.-J.G.; methodology, E.-J.G.; project administration, K.B.S.; resources, K.B.S.; supervision, K.B.S.; writing—original draft, E.-J.G.; writing—review and editing, K.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Jeonnam Agricultural Research & Extension Services for providing Citrus junos pomace samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meerasri, J.; Sothornvit, R. Characterization of bioactive film from pectin incorporated with gamma-aminobutyric acid. Int. J. Biol. Macromol. 2020, 147, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I.Á. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Norcino, L.B.; Mendes, J.F.; Natarelli, C.V.L.; Manrich, A.; Oliveira, J.E.; Mattoso, L.H.C. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Lim, J.; Yoo, J.; Ko, S.; Lee, S. Extraction and characterization of pectin from Yuza (Citrus junos) pomace: A comparison of conventional-chemical and combined physical–enzymatic extractions. Food Hydrocoll. 2012, 29, 160–165. [Google Scholar] [CrossRef]
- Tao, B.; Ye, F.; Li, H.; Hu, Q.; Xue, S.; Zhao, G. Phenolic profile and in vitro antioxidant capacity of insoluble dietary fiber powders from citrus (Citrus junos Sieb. ex Tanaka) pomace as affected by ultrafine grinding. J. Agric. Food Chem. 2014, 62, 7166–7173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Mahmood, K.; Fazilah, A.; Yang, T.A.; Sulaiman, S.; Kamilah, H. Valorization of rambutan (Nephelium lappaceum) by-products: Food and non-food perspectives. Int. Food Res. J. 2018, 25, 890–902. [Google Scholar]
- Phuong, N.N.M.; Le, T.T.; Van Camp, J.; Raes, K. Evaluation of antimicrobial activity of rambutan (Nephelium lappaceum L.) peel extracts. Int. J. Food. Microbiol. 2020, 321, 108539. [Google Scholar] [CrossRef]
- Fila, W.O.; Johnson, J.T.; Edem, P.N.; Odey, M.O.; Ekam, V.S.; Ujong, U.P.; Eteng, O.E. Comparative anti-nutrients assessment of pulp, seed and rind of rambutan (Nephelium Lappaceum). Ann. Biol. Res. 2012, 3, 5151–5156. [Google Scholar]
- Palanisamy, U.; Cheng, H.M.; Masilamani, T.; Subramaniam, T.; Ling, L.T.; Radhakrishnan, A.K. Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants. Food Chem. 2008, 109, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Flores-Gallegos, A.C.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J.A. Rambutan (Nephelium lappaceum L.): Nutritional and functional properties. Trends Food Sci. Technol. 2019, 85, 201–210. [Google Scholar] [CrossRef]
- Tamaki, Y.; Konishi, T.; Fukuta, M.; Tako, M. Isolation and structural characterization of pectin from endocarp of Citrus depressa. Food Chem. 2008, 107, 352–361. [Google Scholar] [CrossRef]
- Samuagam, L.; Sia, C.M.; Akowuah, G.A.; Okechukwu, P.N.; Yim, H.S. In vivo antioxidant potentials of rambutan, mangosteen, and langsat peel extracts and effects on liver enzymes in experimental rats. Food Sci. Biotechnol. 2015, 24, 191–198. [Google Scholar] [CrossRef]
- Ju, A.; Song, K.B. Incorporation of yellow onion peel extract into the funoran-based biodegradable films as an antioxidant packaging material. Int. J. Food Sci. Technol. 2020, 55, 1671–1678. [Google Scholar] [CrossRef]
- Baek, S.K.; Song, K.B. Characterization of active biodegradable films based on proso millet starch and curcumin. Starch-Stärke 2019, 71, 1800174. [Google Scholar] [CrossRef]
- Ju, A.; Song, K.B. Active biodegradable films based on water soluble polysaccharides from white jelly mushroom (Tremella fuciformis) containing roasted peanut skin extract. LWT Food Sci. Technol. 2020, 126, 109293. [Google Scholar] [CrossRef]
- Baek, S.K.; Kim, S.; Song, K.B. Cowpea starch films containing maqui berry extract and their application in salmon packaging. Food Packag. Shelf Life 2019, 22, 100394. [Google Scholar] [CrossRef]
- Pires, C.; Ramos, C.; Teixeira, B.; Batista, I.; Nunes, M.L.; Marques, A. Hake proteins edible films incorporated with essential oils: Physical, mechanical, antioxidant and antibacterial properties. Food Hydrocoll. 2013, 30, 224–231. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Thitilertdecha, N.; Chaiwut, P.; Saewan, N. In vitro antioxidant potential of Nephelium lappaceum L. rind extracts and geraniin on human epidermal keratinocytes. Biocatal. Agric. Biotechnol. 2020, 23, 101482. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizer effect on mechanical properties of β-lactoglobulin films. J. Food Eng. 2001, 50, 149–155. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.; Song, K.B. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015, 46, 208–215. [Google Scholar] [CrossRef]
- Abdorreza, M.N.; Cheng, L.H.; Karim, A.A. Effects of plasticizers on thermal properties and heat sealability of sago starch films. Food Hydrocoll. 2011, 25, 56–60. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.N.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Neto, A.S.; Pinheiro, A.C.M.; Mattoso, L.H.C.; Martins, M.A. Development and physical-chemical properties of pectin film reinforced with spent coffee grounds by continuous casting. Carbohydr. Polym. 2019, 210, 92–99. [Google Scholar] [CrossRef]
- Otoni, C.G.; De Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Mattoso, L.H. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Eça, K.S.; Machado, M.T.; Hubinger, M.D.; Menegalli, F.C. Development of active films from pectin and fruit extracts: Light protection, antioxidant capacity, and compounds stability. J. Food Sci. 2015, 80, C2389–C2396. [Google Scholar] [CrossRef]
- Viana, R.M.; Sá, N.M.; Barros, M.O.; de Fátima Borges, M.; Azeredo, H.M. Nanofibrillated bacterial cellulose and pectin edible films added with fruit purees. Carbohydr. Polym. 2018, 196, 27–32. [Google Scholar] [CrossRef]
- Han, H.S.; Song, K.B. Antioxidant activities of mandarin (Citrus unshiu) peel pectin films containing sage (Salvia officinalis) leaf extract. Int. J. Food Sci. Technol. 2020, 1–9. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Masavang, S.; Mahe, J.; Sommano, S.; Ruksiriwanich, W.; Brachais, C.H.; Jantrawut, P. Mango (cv. Nam Dokmai) peel as a source of pectin and its potential use as a film-forming polymer. Food Hydrocoll. 2020, 102, 105611. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Hatakeyama, H.; Hatakeyama, T. Influence of pectin modification on water binding properties. Food Hydrocoll. 2012, 27, 494–502. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Zhuang, Y. Preparation of free, soluble conjugate, and insoluble-bound phenolic compounds from peels of rambutan (Nephelium lappaceum) and evaluation of antioxidant activities in vitro. J. Food Sci. 2012, 77, C198–C204. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Shankar, S.; Tanomrod, N.; Rawdkuen, S.; Rhim, J.W. Preparation of pectin/silver nanoparticles composite films with UV-light barrier and properties. Int. J. Biol. Macromol. 2016, 92, 842–849. [Google Scholar] [CrossRef]
- Sartori, T.; Feltre, G.; Do Amaral Sobral, P.J.; Da Cunha, R.L.; Megegalli, F.C. Properties of films produced from blends of pectin and gluten. Food Packag. Shelf Life 2018, 18, 221–229. [Google Scholar] [CrossRef]
- Younis, H.G.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Angulo, Y. Fabrication of silver nanoplates using Nephelium lappaceum (Rambutan) peel: A sustainable approach. J. Mol. Liq. 2015, 211, 476–480. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Tanaka, M.; Takamizu, A.; Hoshino, M.; Sasaki, M.; Goto, M. Extraction of dietary fiber from Citrus junos peel with subcritical water. Food Bioprod. Process. 2012, 90, 180–186. [Google Scholar] [CrossRef]
- Akhter, R.; Masoodi, F.A.; Wani, T.A.; Rather, S.A. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef]
- Gao, H.X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Ju, A.; Baek, S.K.; Kim, S.; Song, K.B. Development of an antioxidative packaging film based on khorasan wheat starch containing moringa leaf extract. Food Sci. Biotechnol. 2019, 28, 1057–1063. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.H.; Song, K.B. Development of a sword bean (Canavalia gladiata) starch film containing goji berry extract. Food Bioprocess Technol. 2020, 13, 911–921. [Google Scholar] [CrossRef]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).