Green Solid-State Chemical Reduction of Graphene Oxide Supported on a Paper Substrate
Abstract
:1. Introduction
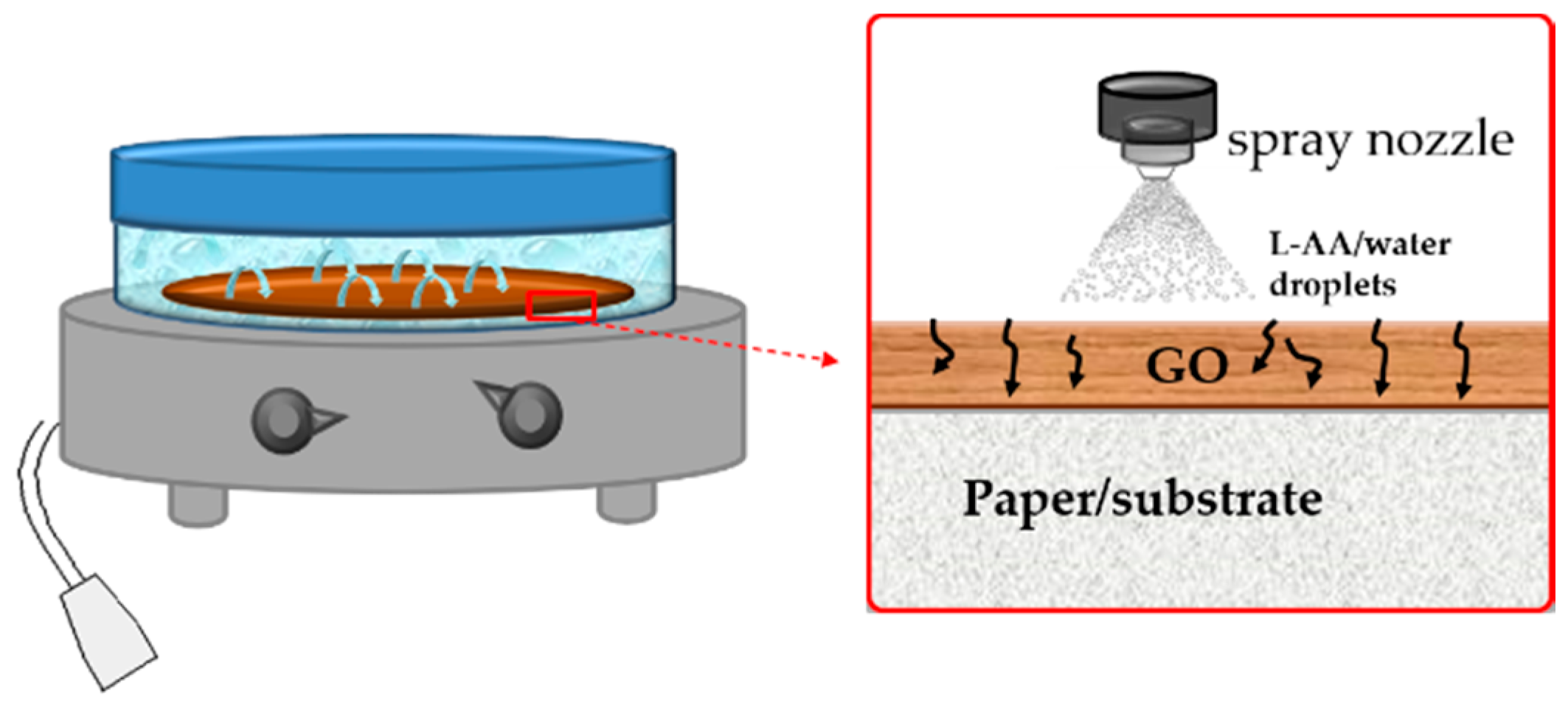
2. Materials and Methods
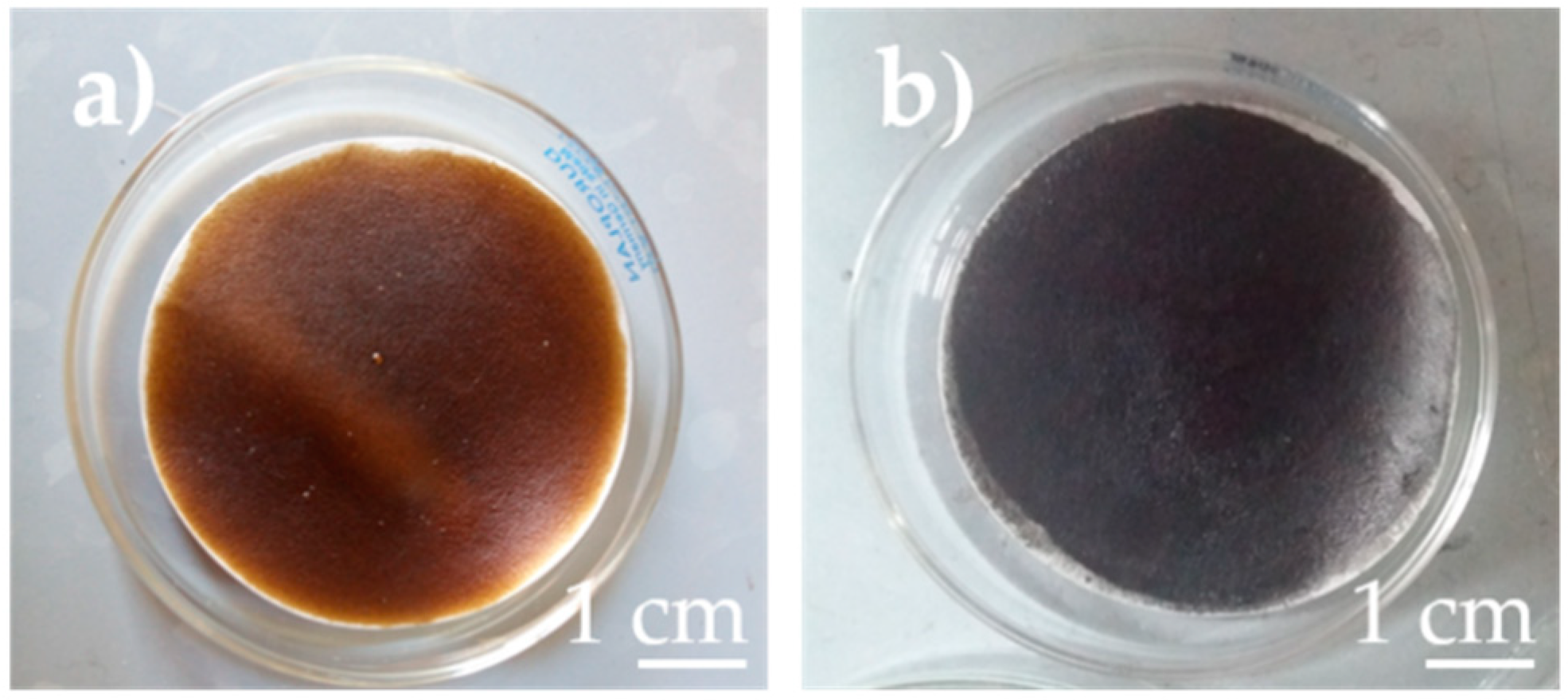
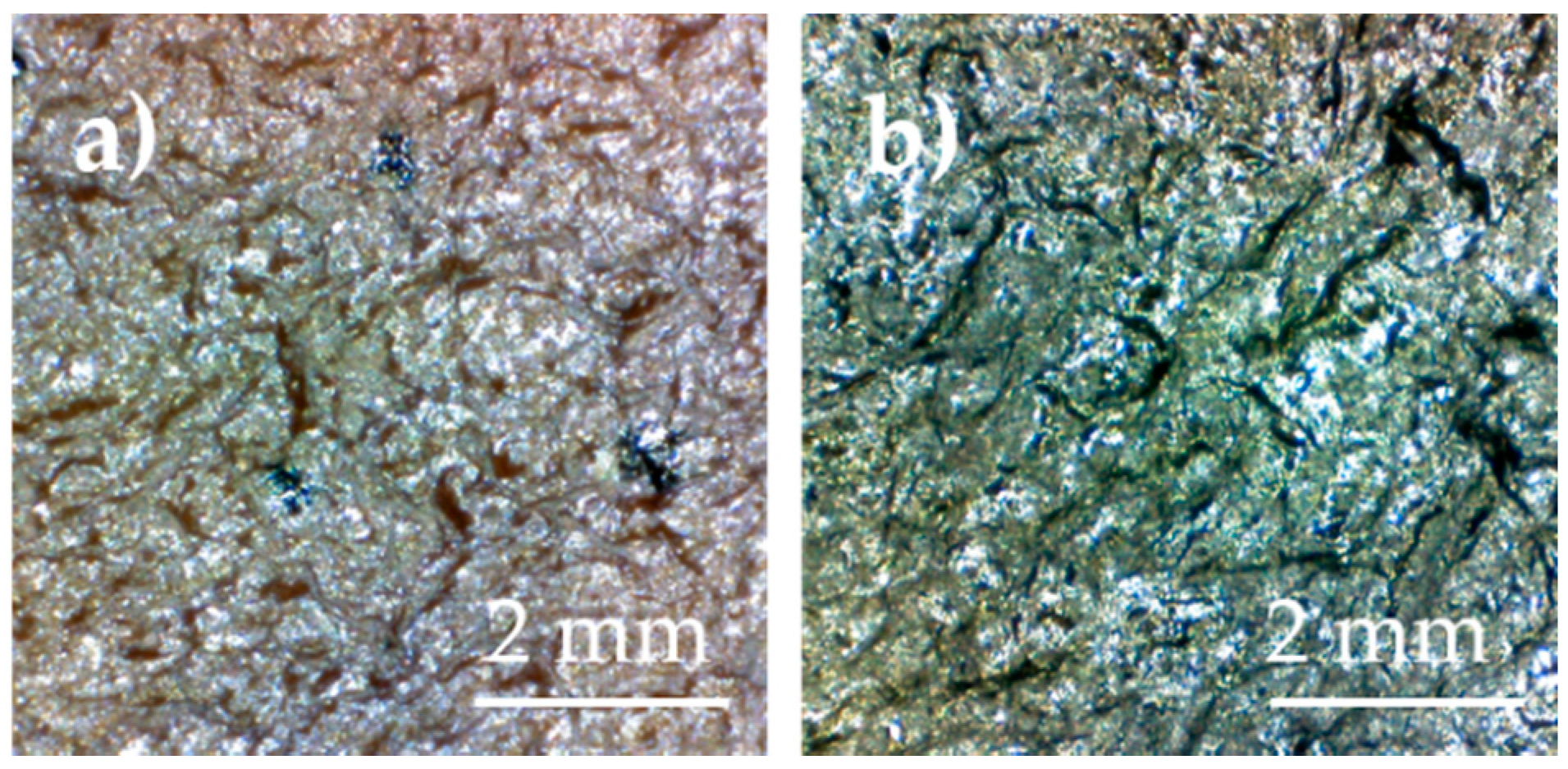
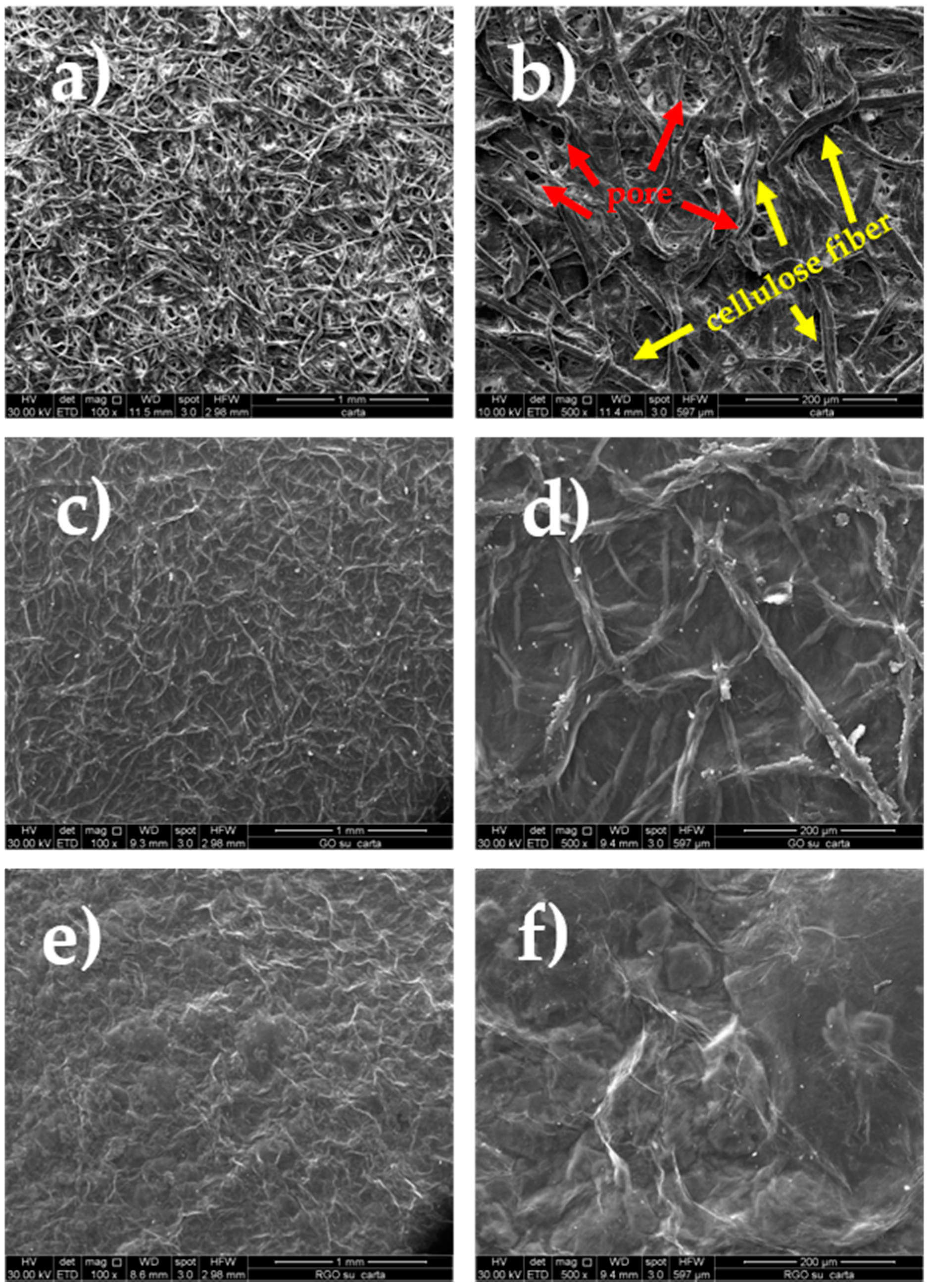
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shafraniuk, S. Graphene: Fundamentals, Devices, and Applications, 1st ed.; Jenny Stanford Publishing: Boka Raton, FL, USA, 2015; ISBN 9789814613477. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.S.; Dubonos, V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of grapheme. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Room-temperature quantum hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafhaeutl, C. On the combination of carbon with silicon and iron, and other metals, forming the different species of cast iron, steel, and malleable iron. Phil. Mag. 1840, 16, 570–590. [Google Scholar]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced grapheneoxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Roweley-Neale, S.J.; Randviir, E.P.; Abo Dena, A.S.; BanKs, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Li, F. Graphene Oxide: Physics and Application; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3662448281. [Google Scholar]
- Longo, A.; Verrucchi, R.; Aversa, L.; Tatti, R.; Ambrosio, A.; Orabona, E.; Coscia, U.; Carotenuto, G.; Maddalena, P. Graphene oxide prepared by graphene nanoplatelets and reduced by laser treatment. Nanotecnology 2017, 28, 224002. [Google Scholar] [CrossRef]
- Sun, L.; Fugetsu, B. Mass production of graphene oxide from expanded graphite. Mater. Lett. 2013, 109, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Songfen, P.; Hui-Ming, C. The reduction of graphene oxide. Carbon 2012, 50, 3210. [Google Scholar]
- Seung, H.H. Thermal Reduction of Graphene Oxide—Physics and Applications of Graphene—Experiments; Mikhailov, S., Ed.; InTech: Rijeka, Yugoslavia, 2011. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Ji, L.C.; Xie, Y.Q.; Wang, T.; Shi, W.Z. Pulsed laser assisted reduction of graphene oxide. Carbon 2011, 49, 2431–2436. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Abdelsayed, V.; Khder, A.E.R.S.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A.A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Furst, A.; Berlo, R.C.; Hooton, S. Hydrazine as a reducing agent for organic compounds (catalytic hydrazine reductions). Chem. Rev. 1965, 65, 51–68. [Google Scholar] [CrossRef]
- Bo, Z.; Zhu, W.; Tu, X.; Yang, Y.; Mao, S.; He, Y.; Chen, J.; Yan, J.; Cen, K. Instantaneous reduction of graphene oxide paper for supercapacitor electrodes with unimpeded liquid permeation. J. Phys. Chem. C 2014, 118, 13493–13502. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Moussa, S.; Hassan, H.; Aluri, H.S.; Collinson, M.M.; El-Shall, M.S. Photothermal deoxygenation of graphite oxide with laser excitation in solution and graphene-aided increase in water temperature. J. Phys. Chem. Lett. 2010, 1, 2804–2809. [Google Scholar] [CrossRef]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bao, Q.; Varghese, B.; Tang, L.A.; Tan, C.K.; Sow, C.H.; Loh, K.P. Microstructuring of graphene oxide nanosheets using direct laser writing. Adv. Mater. 2010, 22, 67–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Wei, S.; He, Y.; Xia, H.; Chen, Q.; Sun, H.-B.; Xiao, F.-S. Direct imprinting of microcircuits on graphene oxides film by femtosecond laser reduction. Nano Today 2010, 5, 15–20. [Google Scholar] [CrossRef]
- Sokolov, D.A.; Shepperd, K.R.; Orlando, T.M. Formation of graphene features from direct laser-induced reduction of graphite oxide. J. Phys. Chem. Lett. 2010, 1, 2633–2636. [Google Scholar] [CrossRef]
- Sokolov, D.A.; Rouleau, C.M.; Geohegan, D.B.; Orlando, T.M. Excimer laser reduction and patterning of graphite oxide. Carbon 2013, 53, 81–89. [Google Scholar] [CrossRef]
- Orabona, E.; Ambrosio, A.; Longo, A.; Carotenuto, G.; Nicolais, L.; Maddalena, P. Holographic patterning of graphene-oxide films by light-driven reduction. Opt. Lett. 2014, 39, 4263–4266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karrer, P. The Chemistry of Vitamins A and C. Chem. Rev. 1934, 14, 17–30. [Google Scholar] [CrossRef]
- Loeffler, H.J.; Ponting, J.D. Ascorbic Acid. Ind. Eng. Chem. Anal. Ed. 1942, 14, 846–849. [Google Scholar] [CrossRef]
- Austin, J.; Partridge, D.A. Vitamin C: Its Chemistry and Biochemistry; Davies, M.B., Ed.; Royal Society of Chemistry: London, UK, 1991. [Google Scholar]
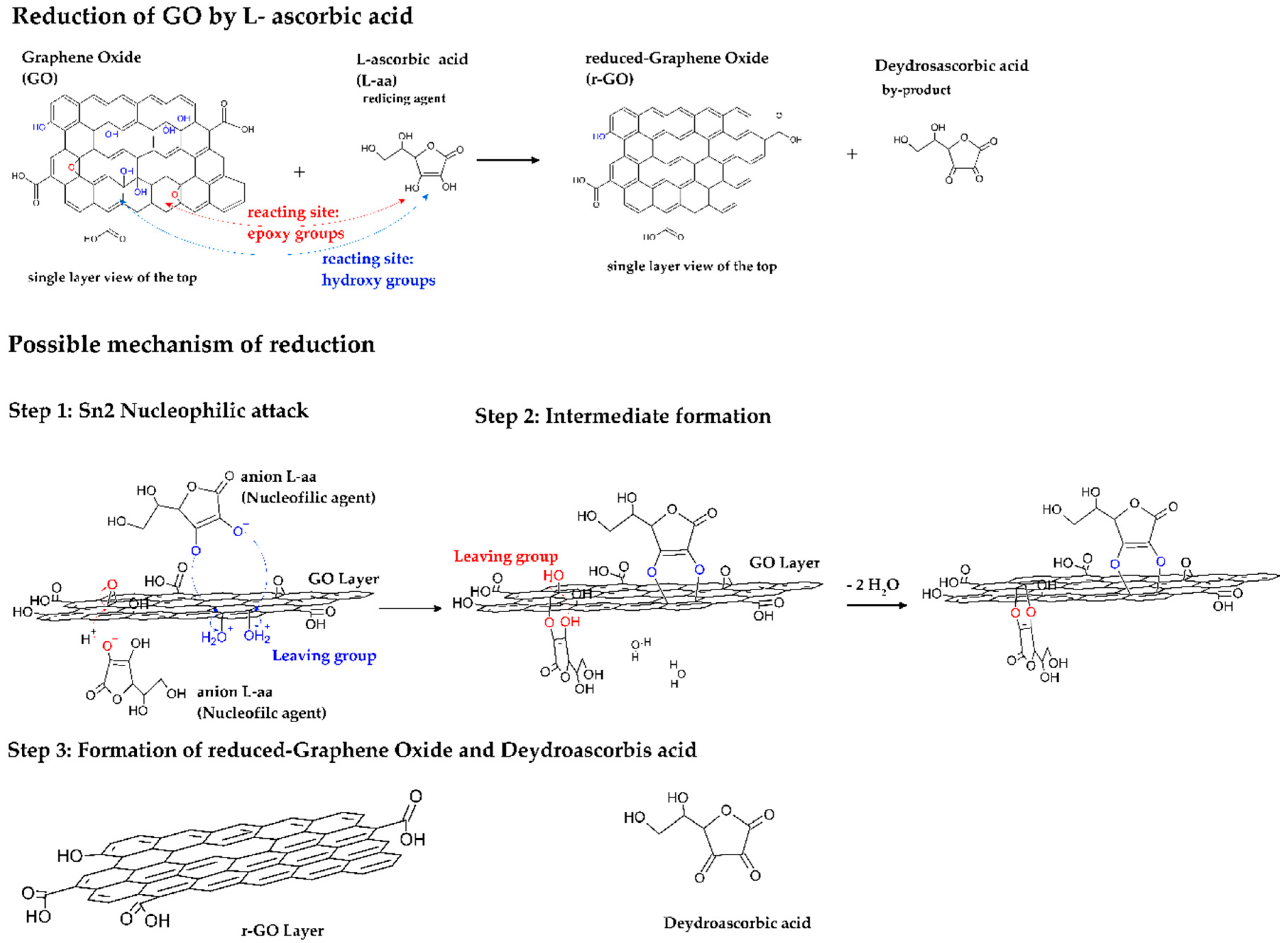
- Zhang, J.; Yang, H.; Sheng, G.; Cheng, P.; Zang, J.; Guo, S. Reduction of graphene oxide vial-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Wu, X.; Zhang, X.; Li, T. Environmentally friendly synthesis of graphene–silver composites with surface-enhanced Raman scattering and antibacterial activity via reduction with l-ascorbic acid/water vapor. Newj. Chem. 2015, 39, 5272–5281. [Google Scholar] [CrossRef]
- Habte, A.T.; Ayele, D.W. Synthesis and characterization of reduced graphene oxide (rgo) started from graphene oxide (go) using the tour method with different Parameters. Adv. Mater. Sci. Eng. 2019, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.L.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 214, 6426–6432. [Google Scholar] [CrossRef]
- Chen, D.; Li, L.; Guo, L. an environment-friendly preparation of reduced graphene oxide nanosheets via amino acid. Nanotecnology 2011, 22, 325601. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kuila, T.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Dual role of glycine as a chemical functionalize and a reducing agent in the preparation of graphene: An environmentally friendly method. J. Mater. Chem. 2012, 22, 9696–9703. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Facile Synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. Acs Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef]
- Costa, M.N.; Veigas, B.; Jacob, J.M.; Santos, D.S.; Gomes, J.; Baptista, P.V.; Martins, R.; Inácio, J.; Fortunato, E. A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: Lab-on-paper. Nanotechnology 2014, 25, 094006. [Google Scholar] [CrossRef] [Green Version]
- Lua, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, stretchable sensors for wearable health monitoring: Sensing mechanisms, materials, fabrication strategies and features. Sensor 2018, 18, 645–652. [Google Scholar]
- Akyazi, T.; Basabe-Desmonts, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialization. Anal. Chim. Acta 2017, 1001, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhai, Y.; Zhang, Y. Bioinspired graphene oxide/polymer nanocomposite paper with high strength, toughness, and dielectric constant. Appl. Mater. Interfaces 2016, 8, 31264–31272. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Baek, S.; Lee, J. Highly sensitive bendable and foldable paper sensors based on reduced graphene oxide. Appl. Mater. Interfaces 2017, 9, 4658–4666. [Google Scholar] [CrossRef]
- Luong, N.D.; Pahimanolis, N.; Hippi, U.; Korhonen, J.T.; Ruokolainen, J.; Johansson, L.-S.; Namd, J.-D.; Seppälä, J. Graphene/cellulose nanocomposite paper with high electrical and mechanical performances. J. Mater. Chem. 2011, 21, 13991–13998. [Google Scholar] [CrossRef]
- Faniyi, I.O.; Fasakin, O.; Olofnjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl. Sci. 2019, 1, 1181. [Google Scholar] [CrossRef] [Green Version]
- Buerger, M.J. X-Ray Crystallography; Wiley: New York, NY, USA, 1942. [Google Scholar]
- Cullity, B.D. Elements of X-Ray Diffraction, Reading; Addison-Wesley: Boston, MA, USA, 1956. [Google Scholar]
- Sharma, R.; Chadha, N.; Saini, P. Determination of defect density, crystallite size and number of graphene layers in graphene analogues using X-ray diffraction and Raman spectroscopy. Indian J. Pure Appl. Phys. 2017, 55, 625–629. [Google Scholar]
- Dimotrov, A.S.; Kralchevsky, P.A.; Nicolov, A.D.; Noshi, H.; Matsumoto, M. Contact angle measurements with sessile drops and bubbles. J. Colloid Interface Sci. 1991, 145, 279–282. [Google Scholar] [CrossRef]
- Kim, J.; Khoh, W.-H.; Weea, B.-H.; Hong, J.-D. Fabrication of flexible reduced graphene oxide–TiO2 freestanding films for supercapacitor application. Rsc Adv. 2015, 5, 9904–9911. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Lee, H.D.; Shin, J.E.; Yoo BM Park, H.B. Water and ion sorption, diffusion, and transport in graphene oxide membranes revisited. J. Membr. Sci. 2017, 544, 425–435. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-Friendly Method to Produce Graphene That Employs Vitamin C and Amino Acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]



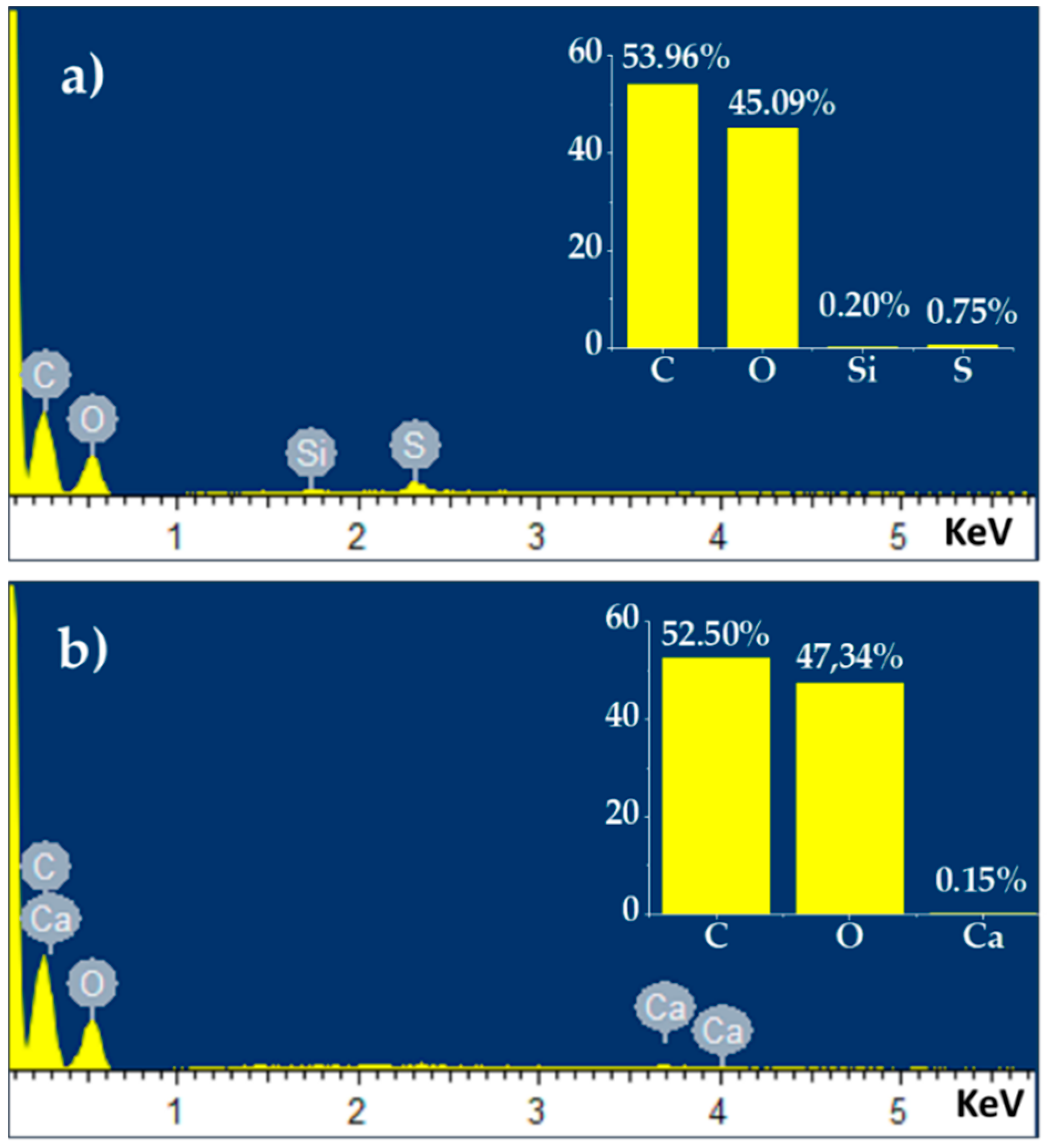


| (hkl) | Position (2θ) | Area (Counts) | FWHM (2θ) | d (nm) | L(hkl) (nm) | n (Counts) | |
|---|---|---|---|---|---|---|---|
| GO | (002) | 10.62 | 2699.59 | 0.66 | 0.832 | 11.72 | 15 |
| GO | (002) | 11.53 | 876.68 | 0.39 | 0.767 | 19.95 | 27 |
| r-GO | (002) | 25.39 | 123.05 | 0.28 | 0.354 | 27.21 | 79 |
| (100) | 43.02 | 54.36 | 0.37 | - | 41.03 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, A.; Palomba, M.; Carotenuto, G. Green Solid-State Chemical Reduction of Graphene Oxide Supported on a Paper Substrate. Coatings 2020, 10, 693. https://doi.org/10.3390/coatings10070693
Longo A, Palomba M, Carotenuto G. Green Solid-State Chemical Reduction of Graphene Oxide Supported on a Paper Substrate. Coatings. 2020; 10(7):693. https://doi.org/10.3390/coatings10070693
Chicago/Turabian StyleLongo, Angela, Mariano Palomba, and Gianfranco Carotenuto. 2020. "Green Solid-State Chemical Reduction of Graphene Oxide Supported on a Paper Substrate" Coatings 10, no. 7: 693. https://doi.org/10.3390/coatings10070693
APA StyleLongo, A., Palomba, M., & Carotenuto, G. (2020). Green Solid-State Chemical Reduction of Graphene Oxide Supported on a Paper Substrate. Coatings, 10(7), 693. https://doi.org/10.3390/coatings10070693







