Preparation of High Hardness Transparent Coating with Controllable Refractive Index by Sol-Gel Technology
Abstract
:1. Introduction
2. Experiment
2.1. Reagents
2.2. Preparation Technology
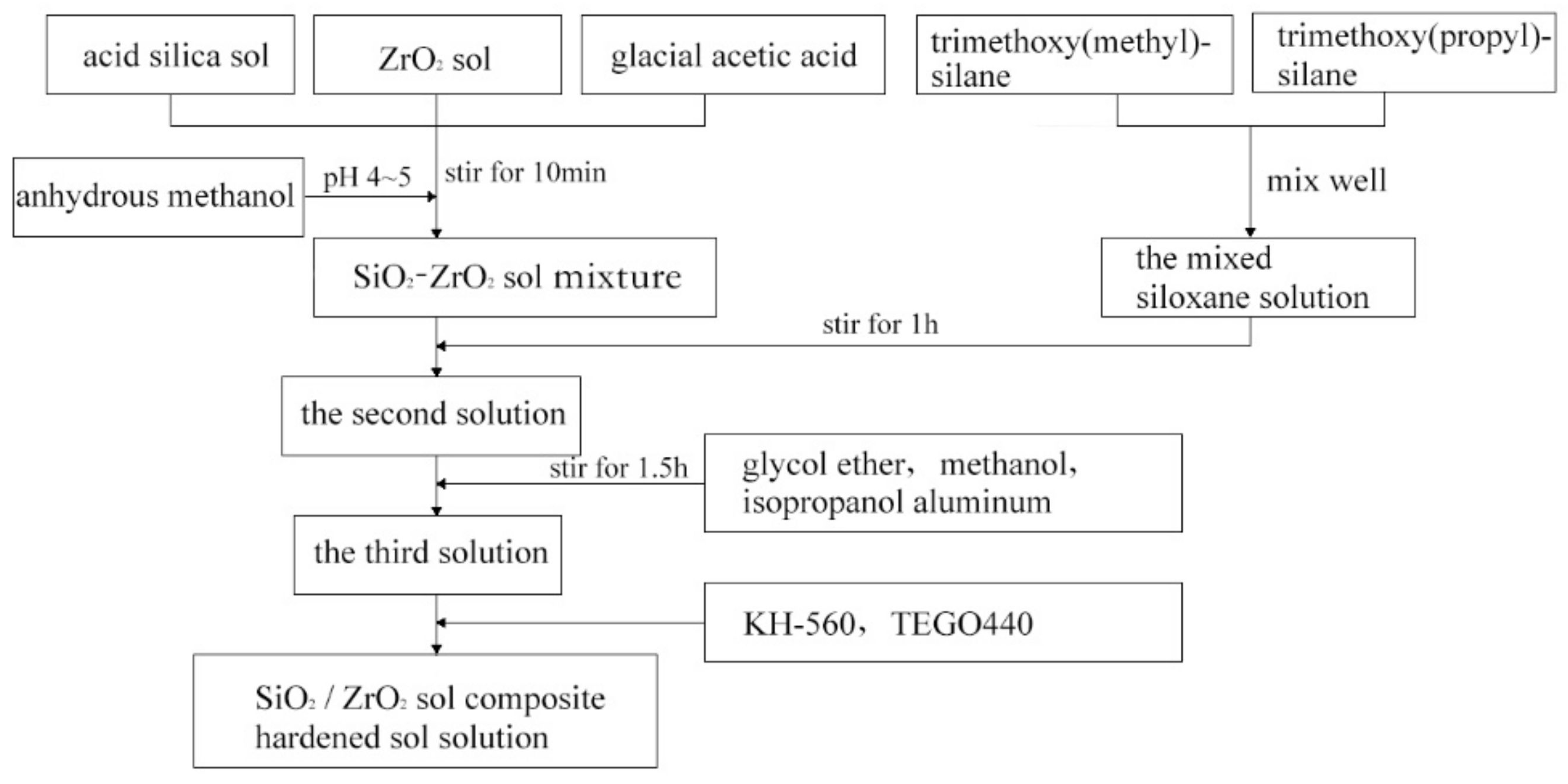
2.2.1. Preparation of SiO2/ZrO2 Sol Composite-Hardened Sol Solution
2.2.2. Preparation of SiO2/Al2O3 Sol Composite Organic–Inorganic-Hardened Sol Solution
2.2.3. Preparation of Controlled Refractive Index Composite-Hardened Sol Based on Aluminum Sol
2.2.4. Preparation of Transparent Coating with Controlled Refractive Index Based on Aluminum Sol
2.3. Characterization and Coating Performance Testing
2.3.1. Infrared Spectrum Analysis
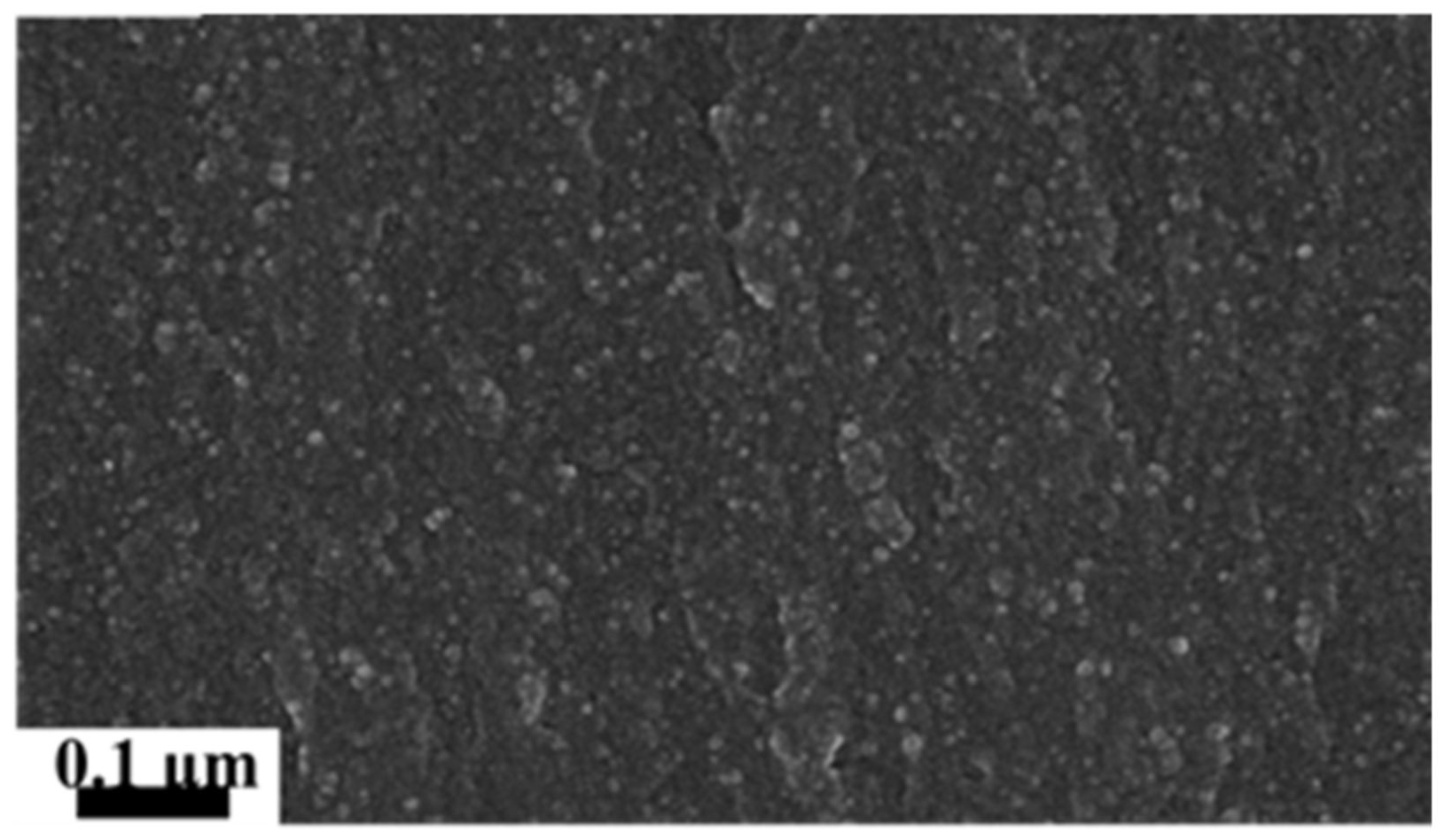
2.3.2. Scanning Electron Microscope (SEM) Analysis
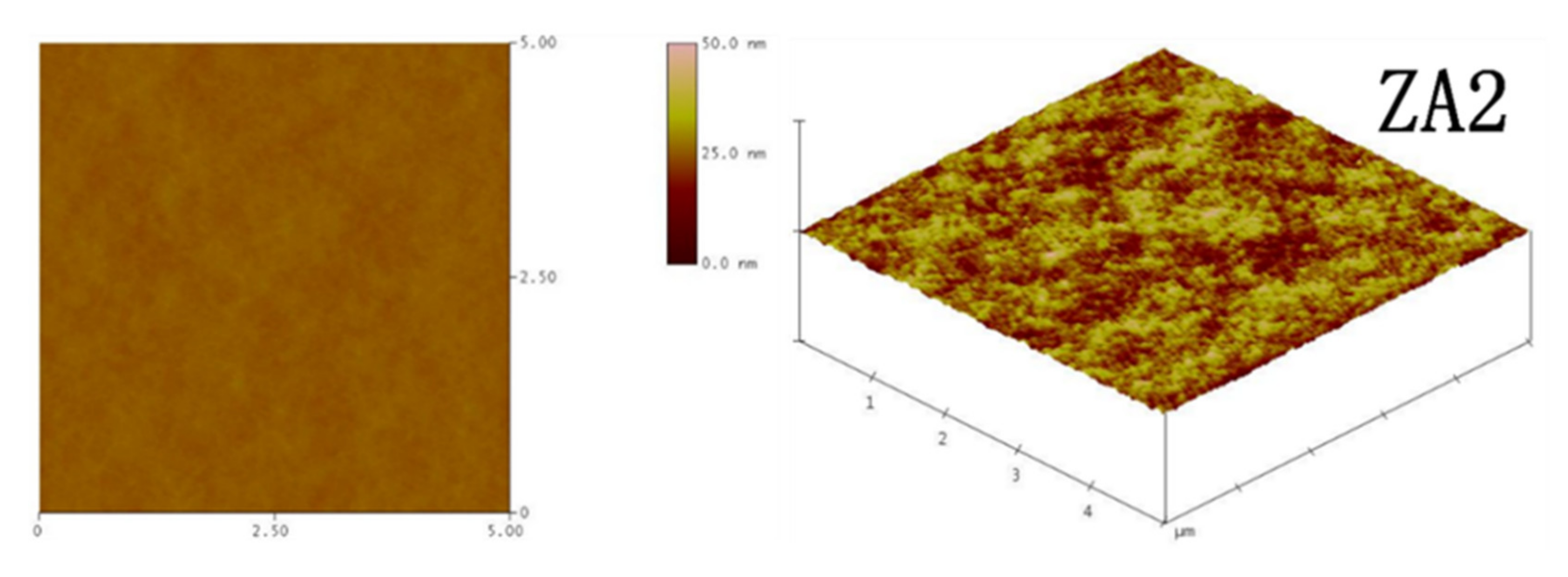
2.3.3. Atomic Force Microscope (AFM) Analysis
2.3.4. Particle Size Distribution
2.3.5. Transmittance Analysis
2.3.6. Hardness Analysis
2.3.7. Refractive Index Test
2.3.8. Coating Wear Resistance Test
3. Results and Discussion
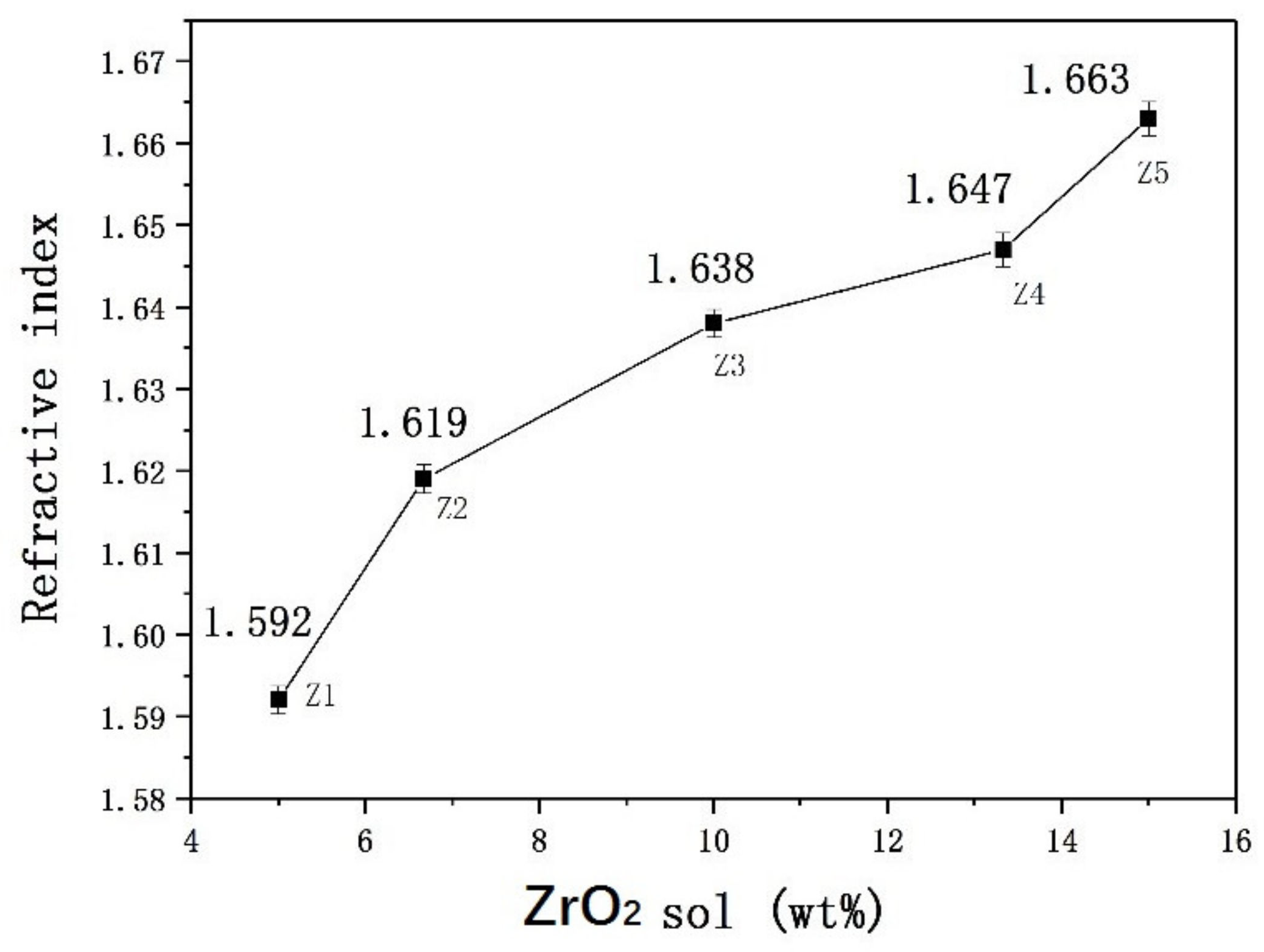
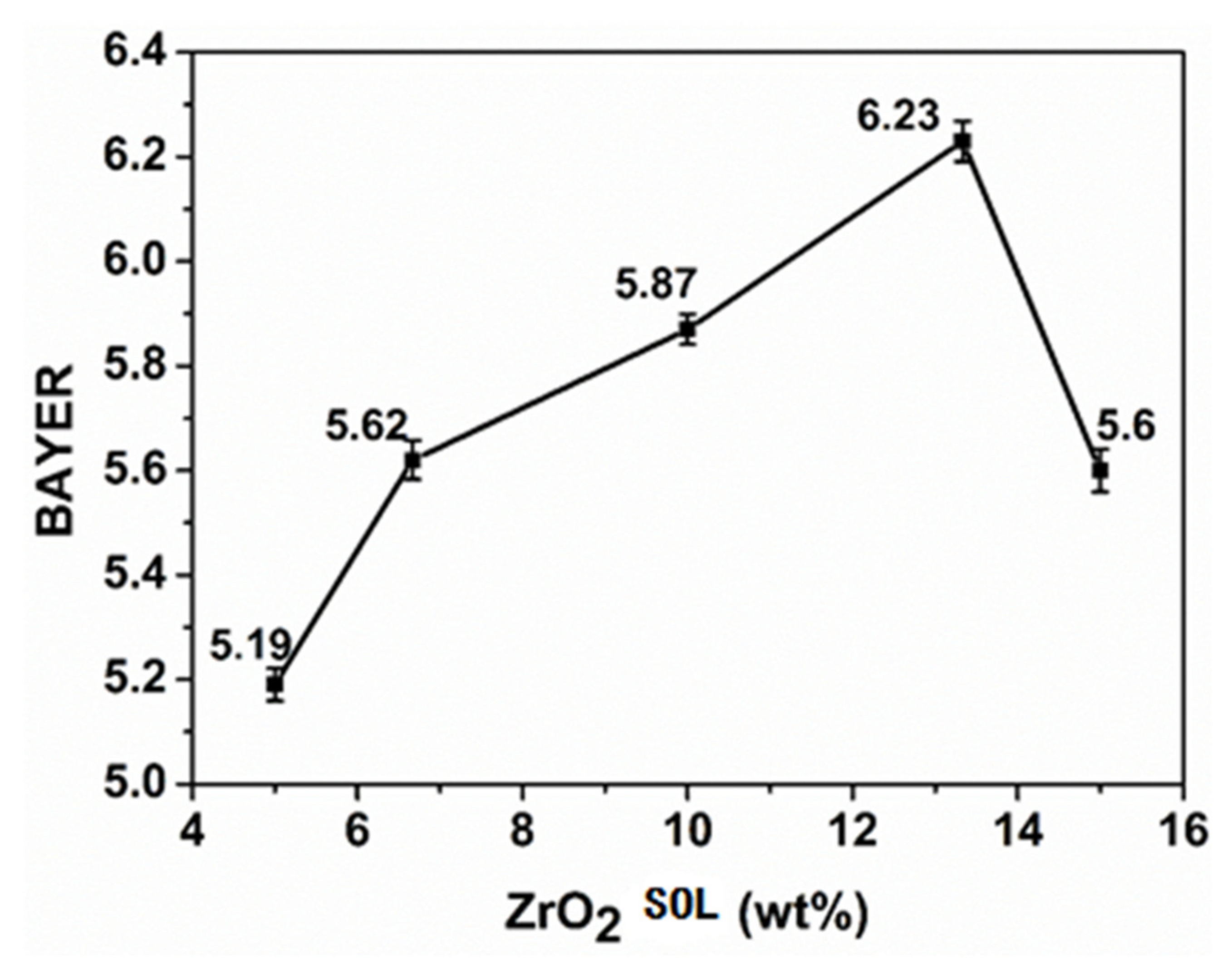
3.1. SiO2/ZrO2 Sol Composite Organic-Inorganic Transparent Coating
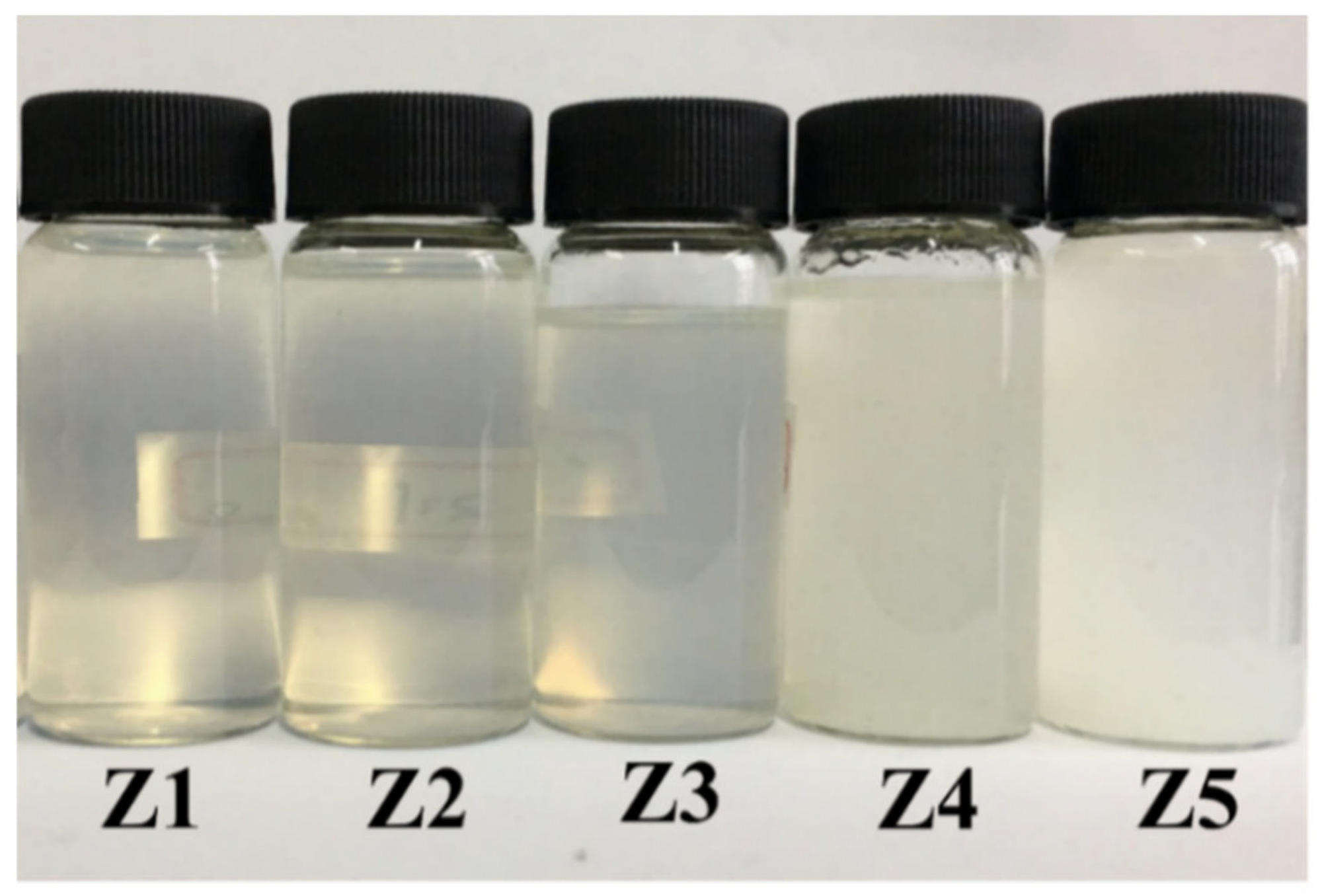
3.1.1. Properties of Composite-Hardening Sol Solution
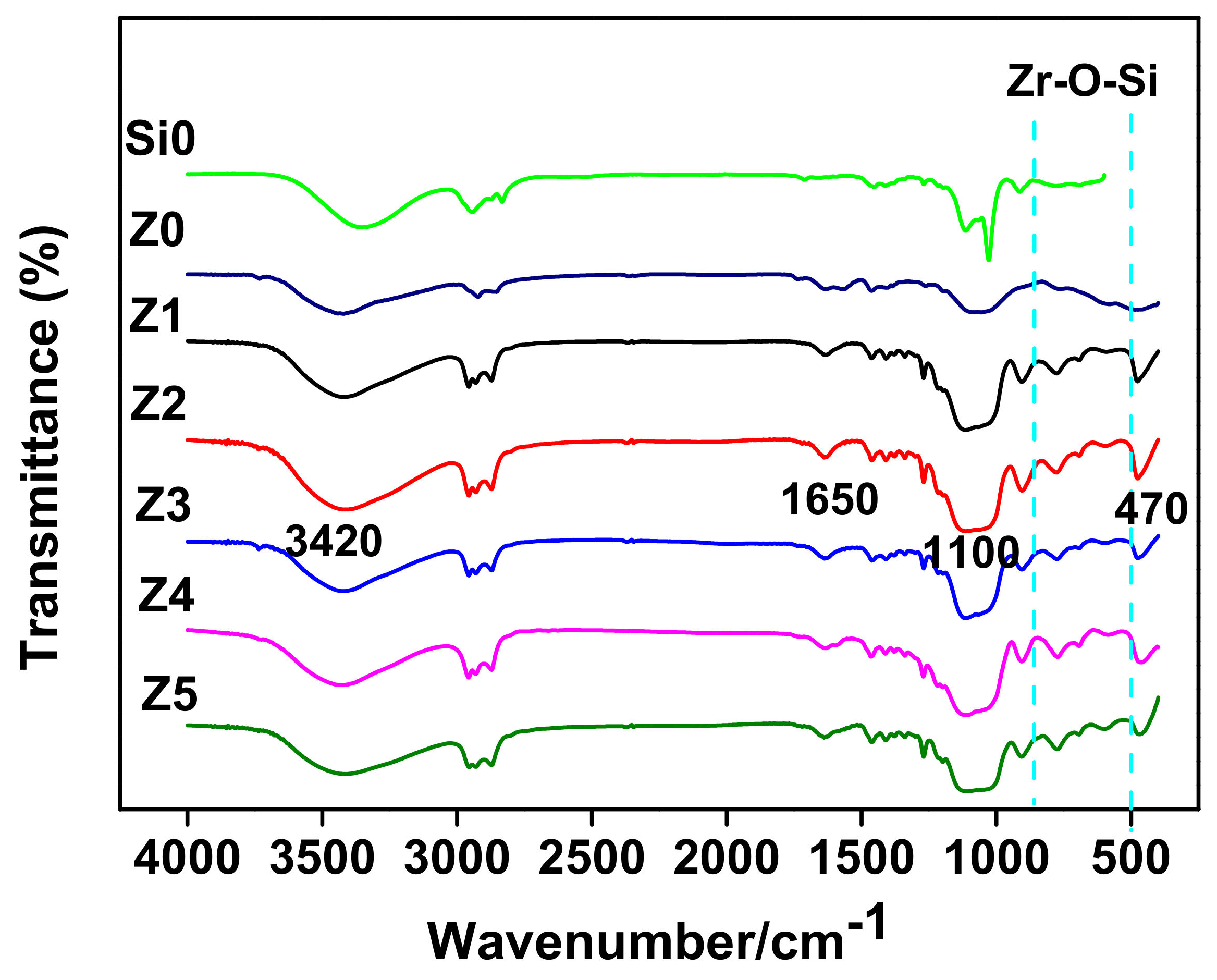
3.1.2. Structural Characterization
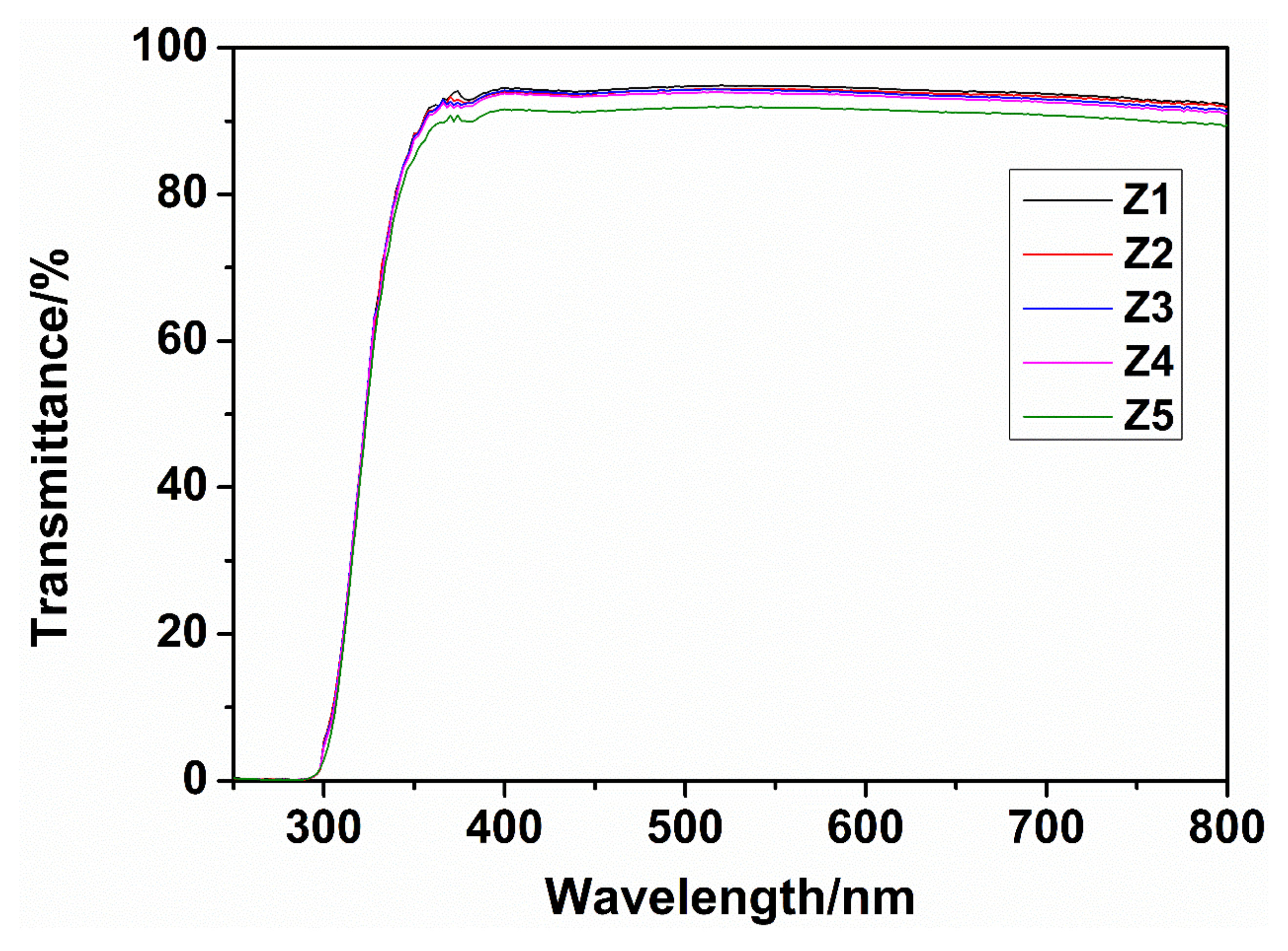
3.1.3. Performance Test
3.2. SiO2/Al2O3 Sol Composite Organic–Inorganic Transparent Coating
3.3. SiO2/ZrO2/Al2O3 Sol Composite Organic–Inorganic Transparent Coating
3.3.1. Structural Characterization
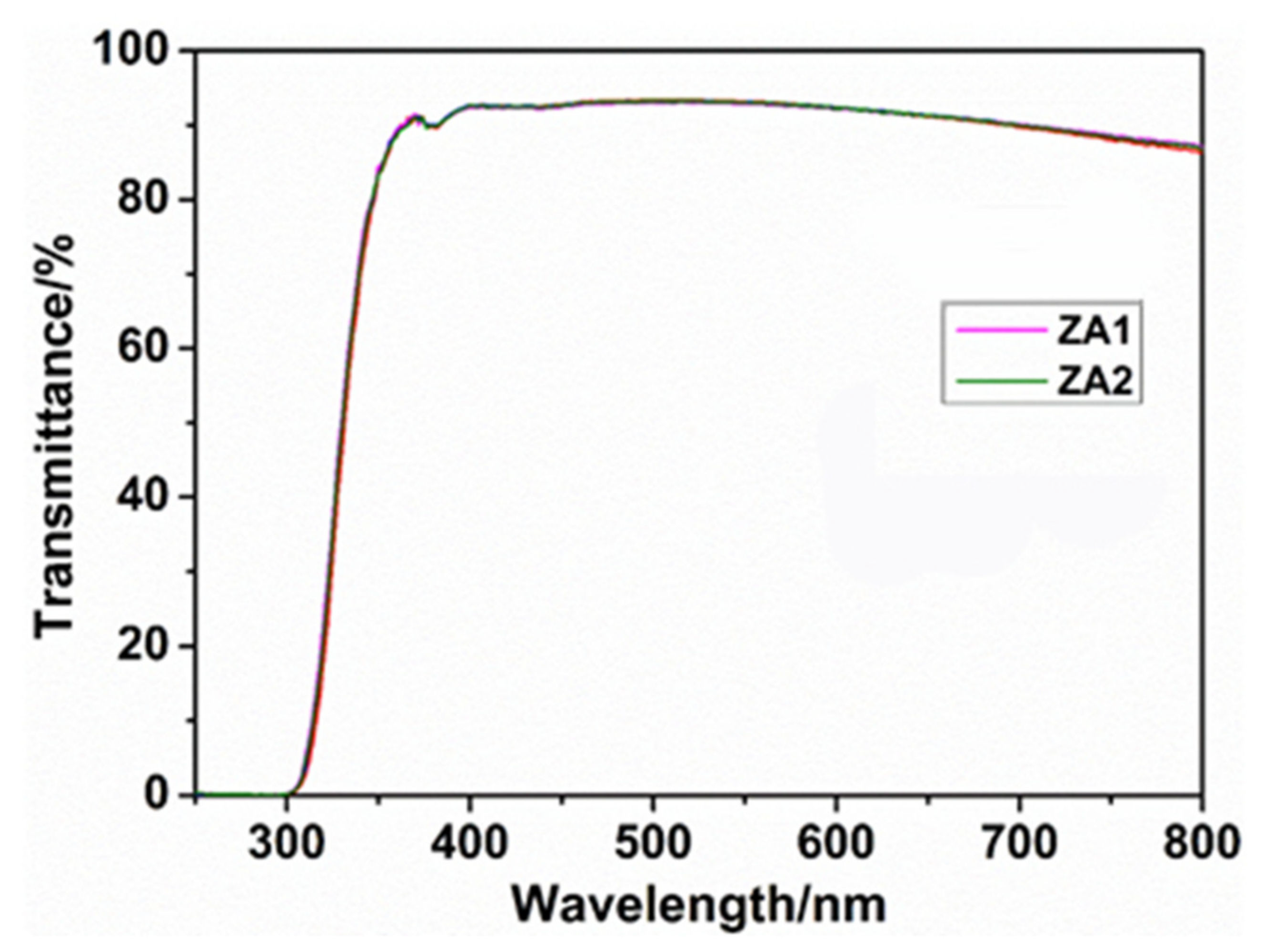
3.3.2. Performance Test
3.4. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jin, Y. Plastic optical elements win attention. Optoelectromechanical Inf. 2004, 2, 22–25. [Google Scholar]
- Zhang, B.; Ten, M. Optical properties and application of optical transparent polymer materials. J. Zhanjiang Norm. Univ. 2008, 29, 52–54. [Google Scholar]
- Blythe, A.R.; Vinson, J.R. Polymeric materials for devices in optical fibre systems. Polym. Adv. Technol. 2000, 11, 601–611. [Google Scholar] [CrossRef]
- Dunkers, J.P.; Cicerone, M.T.; Washburn, N.R. Collinear optical coherence and confocal fluorescence microscopies for tissue engineering. Opt. Express 2003, 11, 3074–3079. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Chen, W.; Liaw, D.; Lu, H. Synthesis and properties of new polyimide–silica hybrid films through both intrachain and interchain bonding. Polymer 2003, 44, 7079–7087. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Tai, N.; Chen, S.; Chueh, Y. Controlled growth of carbon nanotube–graphene hybrid materials for flexible and transparent conductors and electron field emitters. Nanoscale 2012, 4, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, B.; Innocenzi, P. Hybrid materials for optics and photonics. Chem. Soc. Rev. 2011, 40, 886–906. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, C.; Tang, C. Progress in preparation of Cu-Zn-Sn sulfur solar cells by Sol-Gel method. Power Technol. 2019, 43, 1054–1056. [Google Scholar]
- Xiao, T.; Qian, G.; Jin, J.; Liu, L.; Bai, X.; Jin, D.; Huang, Q. A review of SiO2 matrix composite phase change materials based on solution-gel preparation process. Mater. Dev. China 2019, 38, 1113–1120. [Google Scholar]
- Wang, B.; Wilkes, G.L. Novel Hybrid Inorganic-Organic Abrasion-Resistant Coatings Prepared by a Sol-Gel Process. J. Macromol. Sci. Part A 1994, 31, 249–260. [Google Scholar] [CrossRef]
- Watling, J.; Lewis, D. Condensation of Glycidoxypropyltrimethoxysilane to Afford Siloxane Pre-polymers Useful in the Preparation of Hardcoating Resins. J. Sol-Gel Sci. Technol. 2003, 28, 167–174. [Google Scholar] [CrossRef]
- Gilberts, J.; Tinnemans, A.H.A.; Hogerheide, M.P.; Koster, T.P.M. UV Curable Hard Transparent Hybrid Coating Materials on Polycarbonate Prepared by the Sol-Gel Method. J. Sol-Gel Sci. Technol. 1998, 11, 153–159. [Google Scholar] [CrossRef]
- Wouters, M.E.L.; Wolfs, D.P.; van der Linde, M.C.; Hovens, J.H.P.; Tinnemans, A.H.A. Transparent UV curable antistatic hybrid coatings on polycarbonate prepared by the sol–gel method. Prog. Org. Coat. 2004, 51, 312–319. [Google Scholar] [CrossRef]
- He, T.; Gao, C. Two-component silicone coatings and their enhancement on the surface of plexiglass. Organosilicon Mater. 2006, 6, 288–291. [Google Scholar]
- Lee, S.; Shin, H.J.; Yoon, S.M.; Yi, D.K.; Choi, J.; Paik, U. Refractive index engineering of transparent ZrO2-polydimethyldiloxane nanocomposites. J. Mater. Chem. 2008, 18, 1751–1755. [Google Scholar] [CrossRef]
- Das, A.; Das, G.; Kabiraj, D.; Basak, D. High conductivity along with high visible light transparency in Al implanted sol-gel ZnO thin film with an elevated figure of merit value as a transparent conducting layer. J. Alloy. Compd. 2020, 835, 155221. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, Y. Preparation Method of Hard Liquid for Lens. CN104629500A, 20 May 2015. [Google Scholar]
- Schubert, E.F. Low-refractive-index materials: A new class of optical thin-coating materials. Phys. Status Solidi 2007, 244, 3002–3008. [Google Scholar] [CrossRef]
- Rodič, P.; Iskra, J.; Milošev, I. A hybrid organic-inorganic sol-gel coating for protecting aluminium alloy 7075-T6 against corrosion in Harrison’s solution. J. Sol-Gel Sci. Technol. 2014, 70, 90–103. [Google Scholar] [CrossRef]








| Sample | Mixed Components | Mass Ratio | A3 Content% |
|---|---|---|---|
| ZA1 | Z3/A3 | 1/3:1 | 75 |
| ZA2 | Z3/A3 | 1/2:1 | 66.67 |
| ZA3 | Z3/A3 | 1:1 | 50 |
| Sample | SiO2/ZrO2 Sol Mass Ratio | ZrO2 Sol Content wt% | Sol State | COATING-FORMING Property | Storage Time/d |
|---|---|---|---|---|---|
| Z1 | 1:1/3 | 5 | Transparency | Well | ≥120 |
| Z2 | 1:1/2 | 6.67 | Transparency | Well | ≥120 |
| Z3 | 1:1 | 10 | Transparency | Well | ≥120 |
| Z4 | 1:2 | 13.33 | Emulsion | Small white | 15 |
| Z5 | 1:3 | 15 | Emulsion | White | 3 |
| Sample | Z1 | Z2 | Z3 | Z4 | Z5 |
|---|---|---|---|---|---|
| Hardness/H | 2 | 3 | 4 | 6 | 5 |
| Sample | SiO2/Al2O3 Sol Mass Ratio | Al2O3 Sol Content wt% | Sol State | COATING-FORMING Property | Storage Time/d |
|---|---|---|---|---|---|
| A1 | 1:1/3 | 5 | Transparency | Well | ≥120 |
| A2 | 1:1/2 | 6.67 | Transparency | Well | ≥120 |
| A3 | 1:1 | 10 | Transparency | Well | ≥120 |
| A4 | 1:2 | 13.33 | Transparency | Well | ≥60 |
| A5 | 1:3 | 15 | Half-Transparency | Well | 30 |
| Sample | A3 | ZA1 | ZA2 | Z3 |
|---|---|---|---|---|
| A3 wt/% | 100 | 75 | 66.67 | 0 |
| Refractive index | 1.521 | 1.58 | 1.591 | 1.638 |
| Hardness/H | 4 | 5 | 5 | 4 |
| Bayer Ratio | 6.76 | 7.86 | 7.12 | 5.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, Y.; Bai, T. Preparation of High Hardness Transparent Coating with Controllable Refractive Index by Sol-Gel Technology. Coatings 2020, 10, 690. https://doi.org/10.3390/coatings10070690
Liu X, Wang Y, Bai T. Preparation of High Hardness Transparent Coating with Controllable Refractive Index by Sol-Gel Technology. Coatings. 2020; 10(7):690. https://doi.org/10.3390/coatings10070690
Chicago/Turabian StyleLiu, Xiaolin, Yansong Wang, and Tao Bai. 2020. "Preparation of High Hardness Transparent Coating with Controllable Refractive Index by Sol-Gel Technology" Coatings 10, no. 7: 690. https://doi.org/10.3390/coatings10070690
APA StyleLiu, X., Wang, Y., & Bai, T. (2020). Preparation of High Hardness Transparent Coating with Controllable Refractive Index by Sol-Gel Technology. Coatings, 10(7), 690. https://doi.org/10.3390/coatings10070690




