Correlations for Easy Calculation of the Critical Coalescence Concentration (CCC) of Simple Frothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Frothers
2.2. Materials
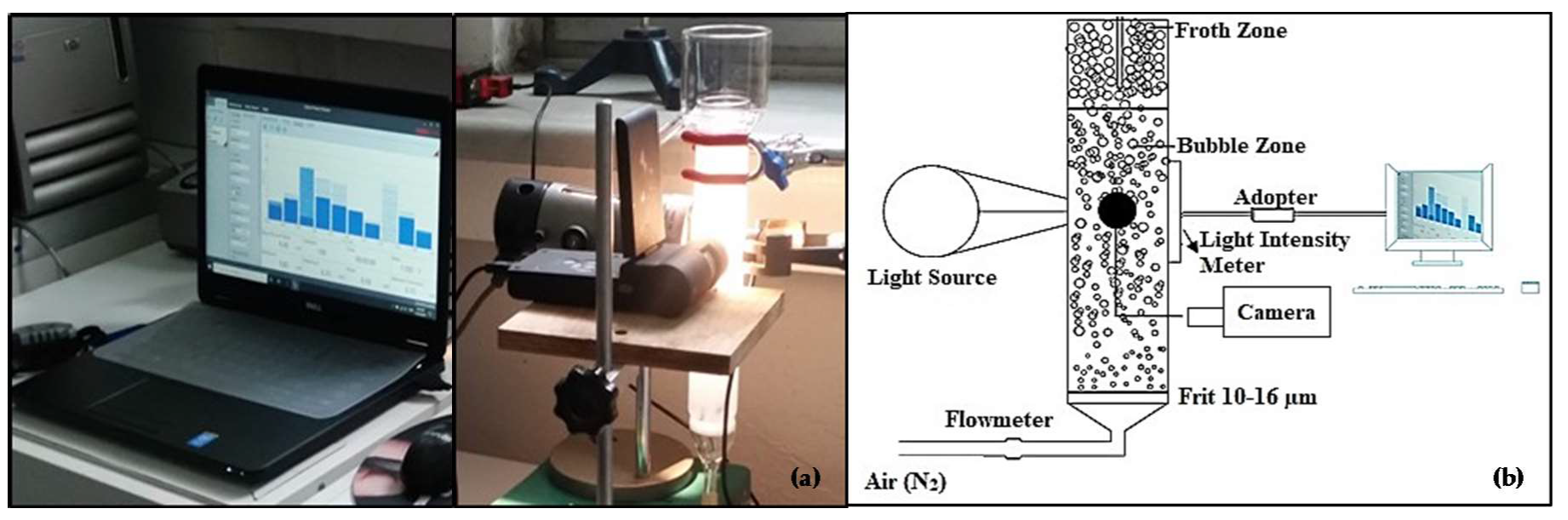
2.3. Experimental Conditions
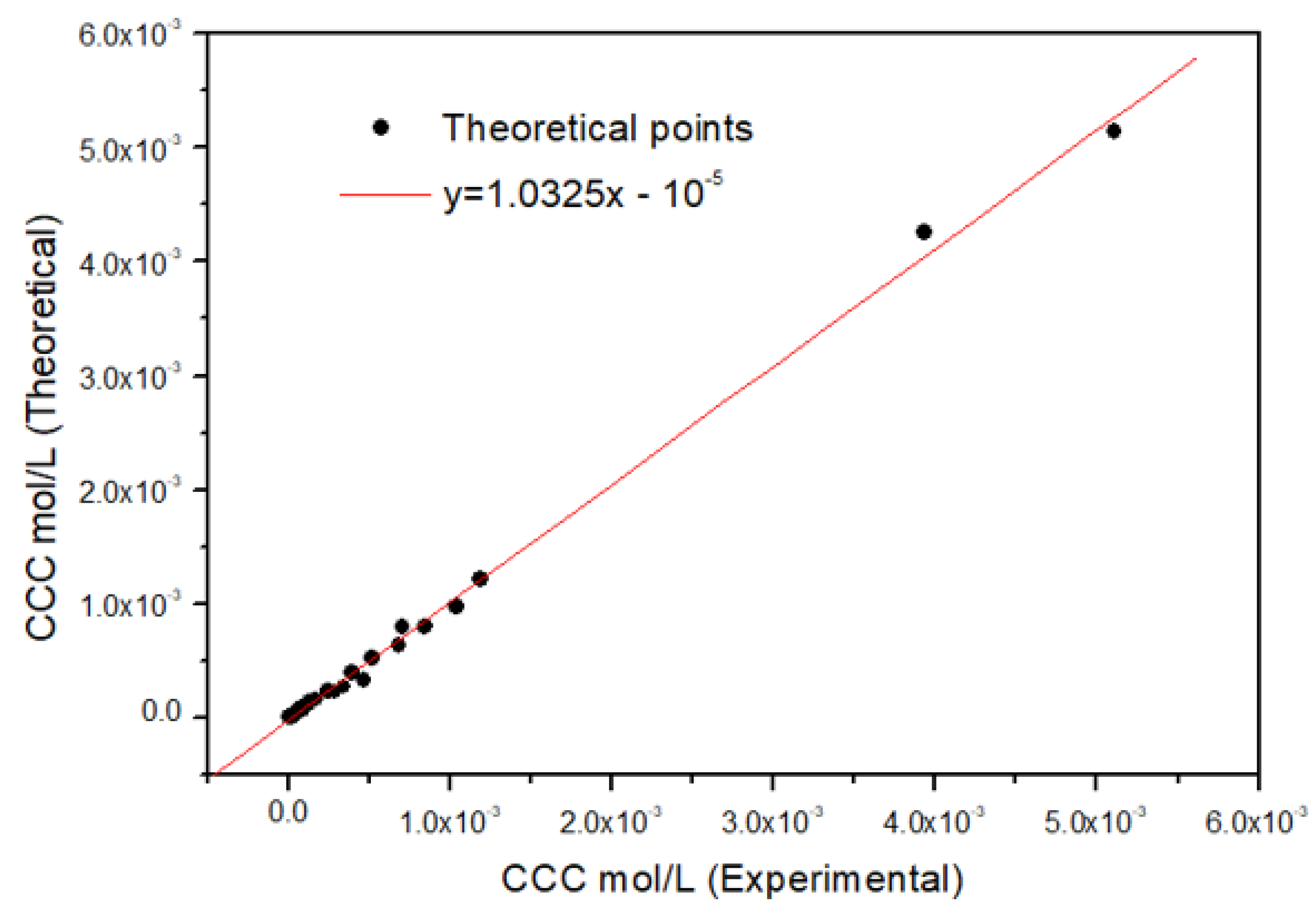
3. Results and Discussion
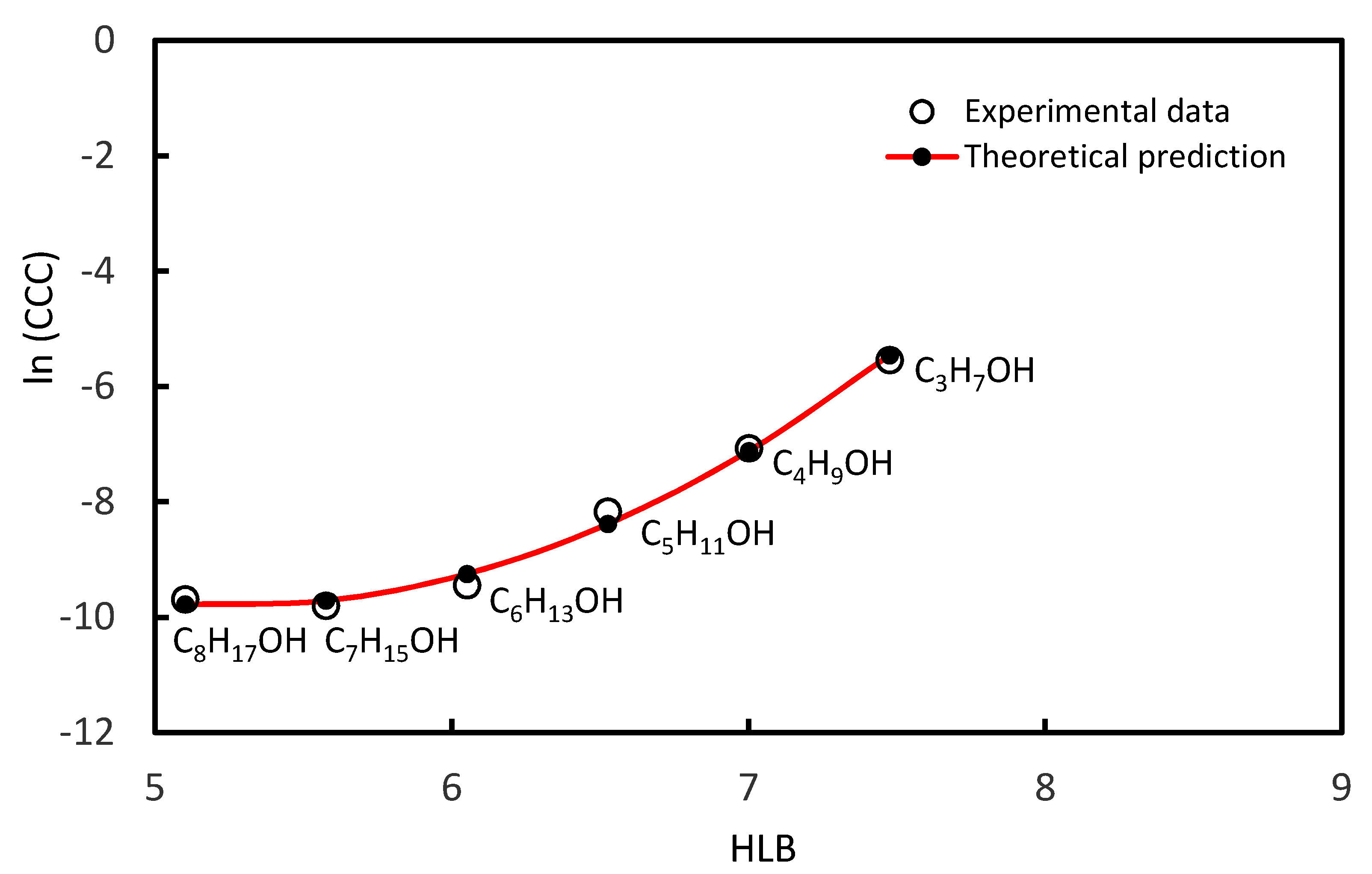
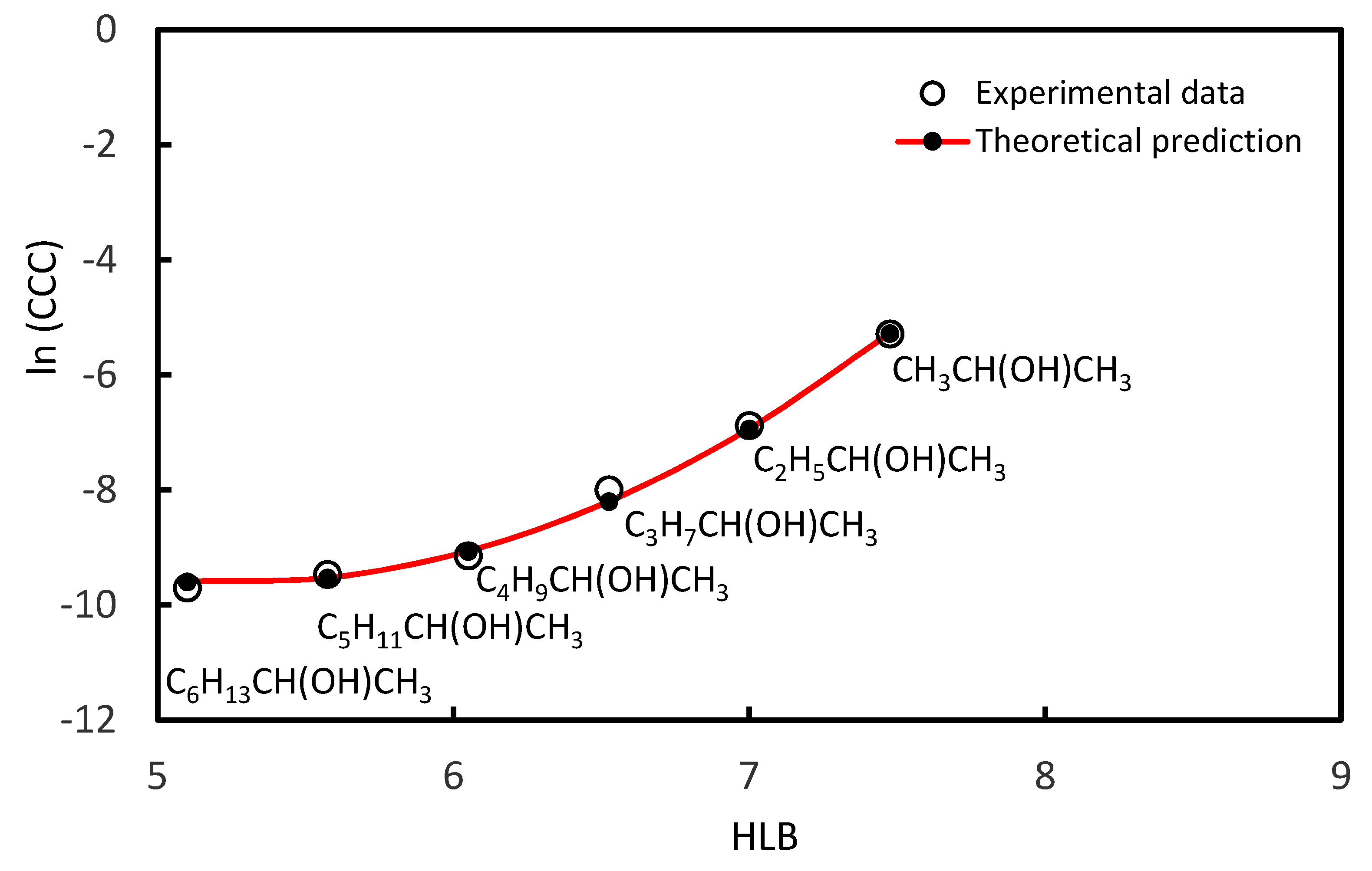
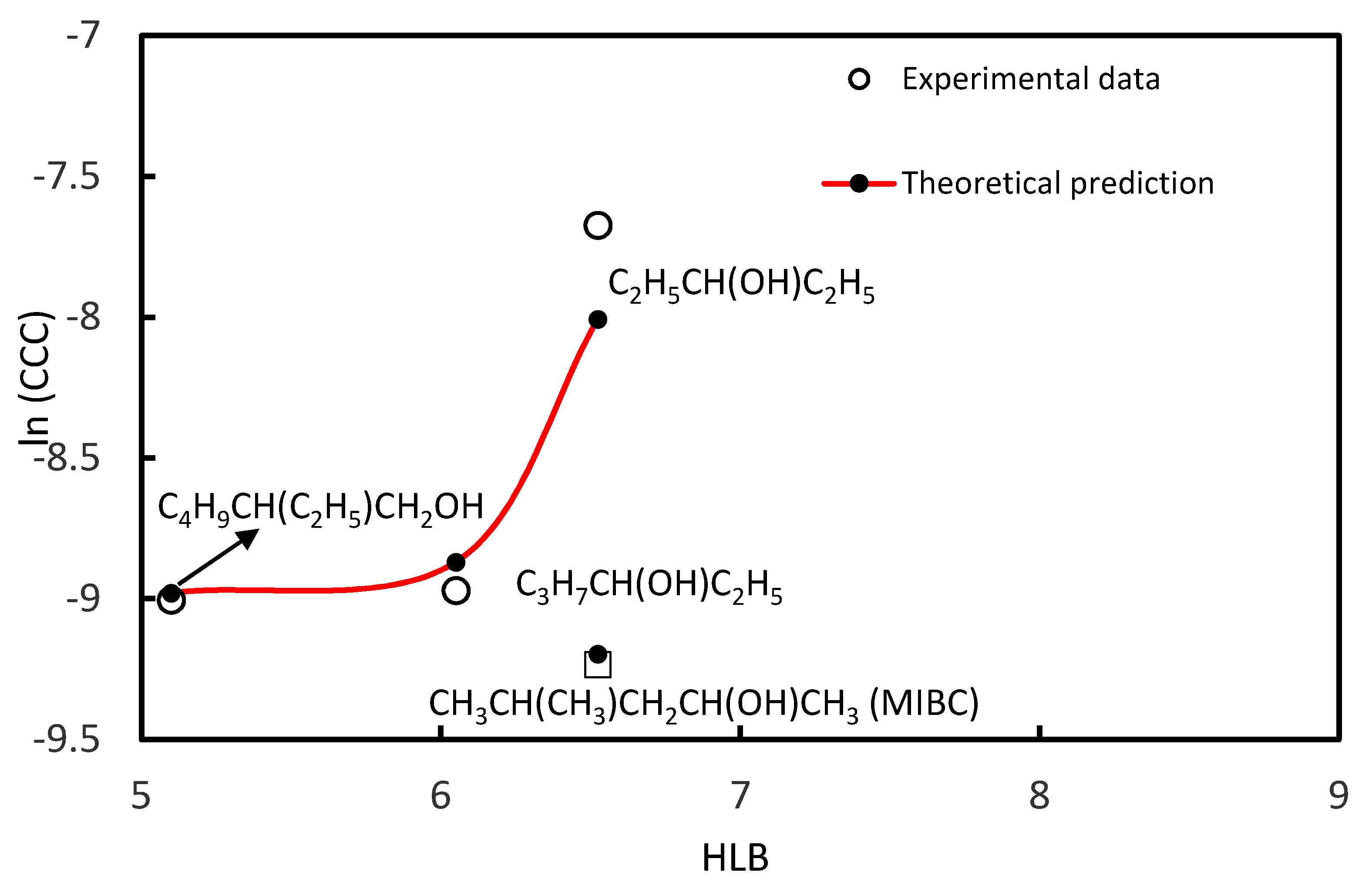
3.1. CCC Values of Homologue Series of Primary, Secondary Alcohols, and Tertiary Alcohols
3.2. CCC Values of Propoxy Frothers
3.3. CCC Values of Ethoxy Frothers
4. Conclusions
- For the homologue series of alcohol frothers, the position of the hydroxyl group (OH) on the main chain of the frother’s molecule and the branched alkyl groups attached to the main chain of the was accounted for;
- For the homologue series of Propoxy frothers, the number of the Propoxy (C3H6O) groups in the main chain of the molecule of the frother was taken into account;
- For the homologue series of Ethoxy frothers, the number of the Ethoxy (C2H4O) groups in the main chain of the molecule of the frother was considered.
Author Contributions
Funding
Conflicts of Interest
References
- Schick, M. Nonionic Surfactants; Marcel Dekker Inc.: New York, NY, USA, 1988. [Google Scholar]
- Lucassen-Reynders, E.H. Anionic Surfactants; Marcel Dekker: New York, NY, USA, 1981. [Google Scholar]
- Benrraou, M.; Bales, B.L.; Zana, R. Effect of the nature of the counterion on the properties of anionic surfactants. 1. Cmc, ionization degree at the cmc and aggregation number of micelles of sodium, cesium, tetramethylammonium, tetraethylammonium, tetrapropylammonium, and tetrabutylammonium dodecyl sulfates. J. Phys. Chem. B 2003, 107, 13432–13440. [Google Scholar]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley: New York, NY, USA, 2004; 464p. [Google Scholar]
- Bikerman, J.J. The unit of foaminess. Trans. Faraday Soc. 1938, 34, 0634–0638. [Google Scholar] [CrossRef]
- Rosen, M.J.; Solash, J. Factors Affecting Initial Foam Height in the Ross−Miles Foam Test. J. Am. Oil Chem. Soc. 1969, 46, 399–402. [Google Scholar] [CrossRef]
- Rosen, M.J.; Zhu, Z.H. Synergism in Binary-Mixtures of Surfactants. 7. Synergism in Foaming and Its Relation to Other Types of Synergism. J. Am. Oil Chem. Soc. 1988, 65, 663–668. [Google Scholar] [CrossRef]
- Pugh, R.J. Foams and foaming. Handbook of Applied Surface and Colloid Chemistry; Wiley: Hoboken, NJ, USA, 2012; pp. 23–43. [Google Scholar]
- Petkova, B.; Tcholakova, S.; Chenkova, M.; Golemanov, K.; Denkov, N.; Thorley, D.; Stoyanov, S. Foamability of aqueous solutions: Role of surfactant type and concentration. Adv. Colloid Interface Sci. 2020, 276. [Google Scholar] [CrossRef]
- Umstatter, H. Foam stability and surface viscosity. Technik Die 1947, 2, 505–507. [Google Scholar]
- Barber, A.D.; Hartland, S. Collapse of cellular foams. Trans. Inst. Chem. Eng. 1975, 53, 106–111. [Google Scholar]
- Andersson, K.; Paul, S. Foams. Part I. Review on foam stabilization factor. Faerg Lack Scand. 1982, 28, 122–130. [Google Scholar]
- Aveyard, R.; Clint, J.H. Foam and thin film breakdown processes. Curr. Opin. Colloid Interface Sci. 1996, 1, 764–770. [Google Scholar] [CrossRef]
- Weaire, D.; Phelan, R. The physics of foam. J. Phys. Condens. Matter 1996, 8, 9519–9524. [Google Scholar] [CrossRef]
- Exerowa, D.; Kruglyakov, P.M. Foam and Foam Films: Theory, Experiment, Application; Marcel Dekker: New York, NY, USA, 1997; 796p. [Google Scholar]
- Varade, D.; Carriere, D.; Arriaga, L.R.; Fameau, A.L.; Rio, E.; Langevin, D.; Drenckhan, W. On the origin of the stability of foams made from catanionic surfactant mixtures. Soft Matter 2011, 7, 6557–6570. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Georgiev, P.; Balashev, K. Foam production—Ratio between foaminess and rate of foam decay. J. Colloid Interface Sci. 2012, 379, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Laskowski, J.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Miner. Process 2002, 64, 69–80. [Google Scholar] [CrossRef]
- Malysa, K.; Czubak-Pawlikowska, J.; Pomianowski, A. Frothing Properties of Solutions and Their Influence on the Floatabaility. In Proceedings of the 7th International Congress Surface Active Substances, Moscow, Russia, 12–18 September 1978; pp. 513–520. [Google Scholar]
- Czarnecki, J.; Małysa, K.; Pomianowski, A. Dynamic frothability index. J. Colloid Interface Sci. 1982, 86, 570–572. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Bubble coalescence and its effect on dynamic foam stability. Can. J. Chem. Eng. 2002, 80, 299–305. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Tlhone, T.; Williams, P.; Ding, K. Fundamental properties of the polyoxypropylene alkyl ether flotation frothers. Int. J. Miner. Process. 2003, 72, 289–299. [Google Scholar] [CrossRef]
- Laskowski, J.S. Fundamental properties of flotation frothers. In Proceedings of the 22nd International Mineral Processing Congress, Cape Town, South Africa, 29 Steptember–3 October 2003; pp. 788–797. [Google Scholar]
- Gungoren, C.; Islek, E.; Baktarhan, Y.; Unver, I.K.; Ozdemir, O. A novel technique to investigate the bubble coalescence in the presence of surfactant (MIBC) and electrolytes (NaCl and CaCl2). Physicochem. Probl. Miner. Process. 2018, 54, 1215–1222. [Google Scholar] [CrossRef]
- Tan, Y.H.; Zhang, W.; Finch, J.A. Frother structure-property relationship: Effect of polyethylene glycols on bubble rise velocity. Miner. Eng. 2018, 116, 56–61. [Google Scholar] [CrossRef]
- Jávor, Z.; Schreithofer, N.; Heiskanen, K. Validity of critical coalescence concentration in dynamic conditions. Int. J. Miner. Process. 2014, 127, 16–22. [Google Scholar] [CrossRef]
- Laskowski, J.S. Testing flotation frothers. In Proceedings of the 2004 SME Annual Meeting Preprints, Denver, CO, USA, 23–25 February2004; pp. 419–422. [Google Scholar]
- Nassif, M.; Finch, J.A.; Waters, K.E. Developing critical coalescence concentration curves for industrial process waters using dilution. Miner. Eng. 2013, 50-51, 64–68. [Google Scholar] [CrossRef]
- Veras, M.M.; Baltar, C.A.M.; Paulo, J.B.A.; Leite, J.Y.P. Comparative study of the main flotation frothers using a new HYDROMESS adapted technique. Rev. Escola Minas 2014, 67, 87–92. [Google Scholar] [CrossRef][Green Version]
- Grandón, F.; Álvarez, J.; Gómez, C. Frother Dosage in Laboratory Flotation Testing. In Proceedings of the XXVII International Mineral Processing Congress, Santiago, Chile, 20–24 October 2014; pp. 1–11. [Google Scholar]
- Szyszka, D. Critical Coalescence Concentration (CCC) for surfactants in aqueous solutions. Minerals 2018, 8, 431. [Google Scholar] [CrossRef]
- Corona-Arroyo, M.A.; López-Valdivieso, A.; Laskowski, J.S.; Encinas-Oropesa, A. Effect of frothers and dodecylamine on bubble size and gas holdup in a downflow column. Miner. Eng. 2015, 81, 109–115. [Google Scholar] [CrossRef]
- Grau, R.A.; Laskowski, J.S. Role of frothers in bubble generation and coalescence in a mechanical flotation cell. Can. J. Chem. Eng. 2006, 84, 170–182. [Google Scholar] [CrossRef]
- Zhang, W. Water Overflow Rate and Bubble Surface Area Flux in Flotation; McGill University: Montreal, QC, Canada, 2009. [Google Scholar]
- Gupta, A.K.; Banerjee, P.K.; Mishra, A. Effect of frothers on foamability, foam stability, and bubble size. Coal Prep. 2007, 27, 107–125. [Google Scholar] [CrossRef]
- Drzymala, J.; Kowalczuk, P.B. Classification of flotation frothers. Minerals 2018, 8, 53. [Google Scholar] [CrossRef]
- Zhang, W.; Nesset, J.E.; Rao, R.; Finch, J.A. Characterizing frothers through critical coalescence concentration (CCC)95-hydrophile-lipophile balance (HLB) relationship. Minerals 2012, 2, 208–227. [Google Scholar] [CrossRef]
- Castillo, P.; Alvarez, J.; Gomez, C. Analytical method to calculate the critical coalescence concentration (CCC). In Proceedings of the XXVII International Mineral Processing Congress –IMPC, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Kowalczuk, P.B. Determination of critical coalescence concentration and bubble size for surfactants used as flotation frothers. Ind. Eng. Chem. Res. 2013, 52, 11752–11757. [Google Scholar] [CrossRef]
- Srinivas, A.; Ghosh, P. Coalescence of bubbles in aqueous alcohol solutions. Ind. Eng. Chem. Res. 2012, 51, 795–806. [Google Scholar] [CrossRef]
- Kracht, W.; Rebolledo, H. Study of the local critical coalescence concentration (l-CCC) of alcohols and salts at bubble formation in two-phase systems. Miner. Eng. 2013, 50-51, 77–82. [Google Scholar] [CrossRef]
- Khoshdast, H.; Sam, A. Flotation Frothers: Review of Their Classifications, Properties and Preparation. Open Miner. Process. J. 2011, 4, 25–44. [Google Scholar] [CrossRef]
- Zhu, H.; Valdivieso, A.L.; Zhu, J.; Song, S.; Min, F.; Corona Arroyo, M.A. A study of bubble size evolution in Jameson flotation cell. Chem. Eng. Res. Des. 2018, 137, 461–466. [Google Scholar] [CrossRef]
- Szyszka, D.; Glapiak, E.; Drzymała, J. Entrainment-flotation activity of quartz in the presence of selected frothers. Physicochem. Probl. Miner. Process. 2008, 42, 85–90. [Google Scholar]
- Szyzska, D. Krytyczne stężenie koalescencji potencjalnych spieniaczy do flotacji łupka miedzionośnego. In Lupek Miedzionosny II; Kowalczuk, P.B., Drzymala, J., Eds.; WGGG PWr: Wroclaw, Poland, 2016; pp. 222–227. [Google Scholar]
- Davies, J.T. A quantitative kinetic theory of emulsion type, I. Physical chemistry of the emulsifying agent, Gas/Liquid and Liquid/Liquid Interface. In Proceedings of the 2nd International Congress of Surface Actvity, London, UK, 1957. [Google Scholar]
- Wang, Z.; Li, G.; Zhang, X.; Wang, R.; Lou, A. A quantitative structure-property relationship study for the prediction of critical micelle concentration of nonionic surfactants. Coll. Surf. A 2002, 197, 37–45. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Konouz, E. Multiple linear regression modeling of the critical micelle concentration of alkyltrimethylammonium and alkylpyridinium salts. J. Surfactants Deterg. 2003, 6, 25–30. [Google Scholar] [CrossRef]
- Chen, M.L.; Wang, Z.W.; Zhang, G.X.; Wang, W.D.; Wang, Z.N. Prediction on hydrophile-lipophile balance values of anionic surfactants with QSPR method. Acta Chim. Sin. 2007, 65, 1265–1272. [Google Scholar]
- Chen, M.-L.; Wang, Z.-W.; Zhang, G.-X.; Gu, J.; Cun, Z.; Tao, F.-M. Studies on the Cloud Points of Nonionic Surfactants with QSPR. Chem. Res. Chin. Univ. 2007, 23, 715–719. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Pacureanu, L.M.; Slavov, S.H.; Dobchev, D.A.; Karelson, M. QSPR Study of Critical Micelle Concentrations of Nonionic Surfactants. Ind. Eng. Chem. Res. 2008, 47, 9687–9695. [Google Scholar] [CrossRef]
- Ghasemi, J.; Abdolmaleki, A.; Asadpour, S.; Shiri, F. Prediction of solubility of nonionic solutes in anionic micelle (SDS) using a QSPR model. Quant. Sruct.-Activ. Relatsh. Comb. Sci. 2008, 27, 338–346. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Pacureanu, L.M.; Slavov, S.H.; Dobchev, D.A.; Shah, D.O.; Karelson, M. QSPR study of the first and second critical micelle concentrations of cationic surfactants. Comput. Chem. Eng. 2009, 33, 321–332. [Google Scholar] [CrossRef]
- Lindgren, Å.; Sjöström, M.; Wold, S. QSAR modelling of the toxicity of some technical non-ionic surfactants towards fairy shrimps. Quant. Sruct.-Activ. Relatsh. 1996, 15, 208–218. [Google Scholar] [CrossRef]
- Lindgren, Å.; Sjöström, M.; Wold, S. Quantitative-structure-effect relationship for some technical nonionic surfactants. J. Am. Oil Chem. Sci. 1996, 73, 863–875. [Google Scholar] [CrossRef]
- Guven, O.; Batjargal, K.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Experimental procedure for the determination of the critical coalescence concentration (CCC) of simple frothers. Minerals 2020. submitted. [Google Scholar]
- Davies, J.T. Study of foam stabilizers using a new “viscous-traction” surface viscometer. In Proceedings of the 2nd International Congress Surface Activity, London, UK, 1957; pp. 220–224. [Google Scholar]
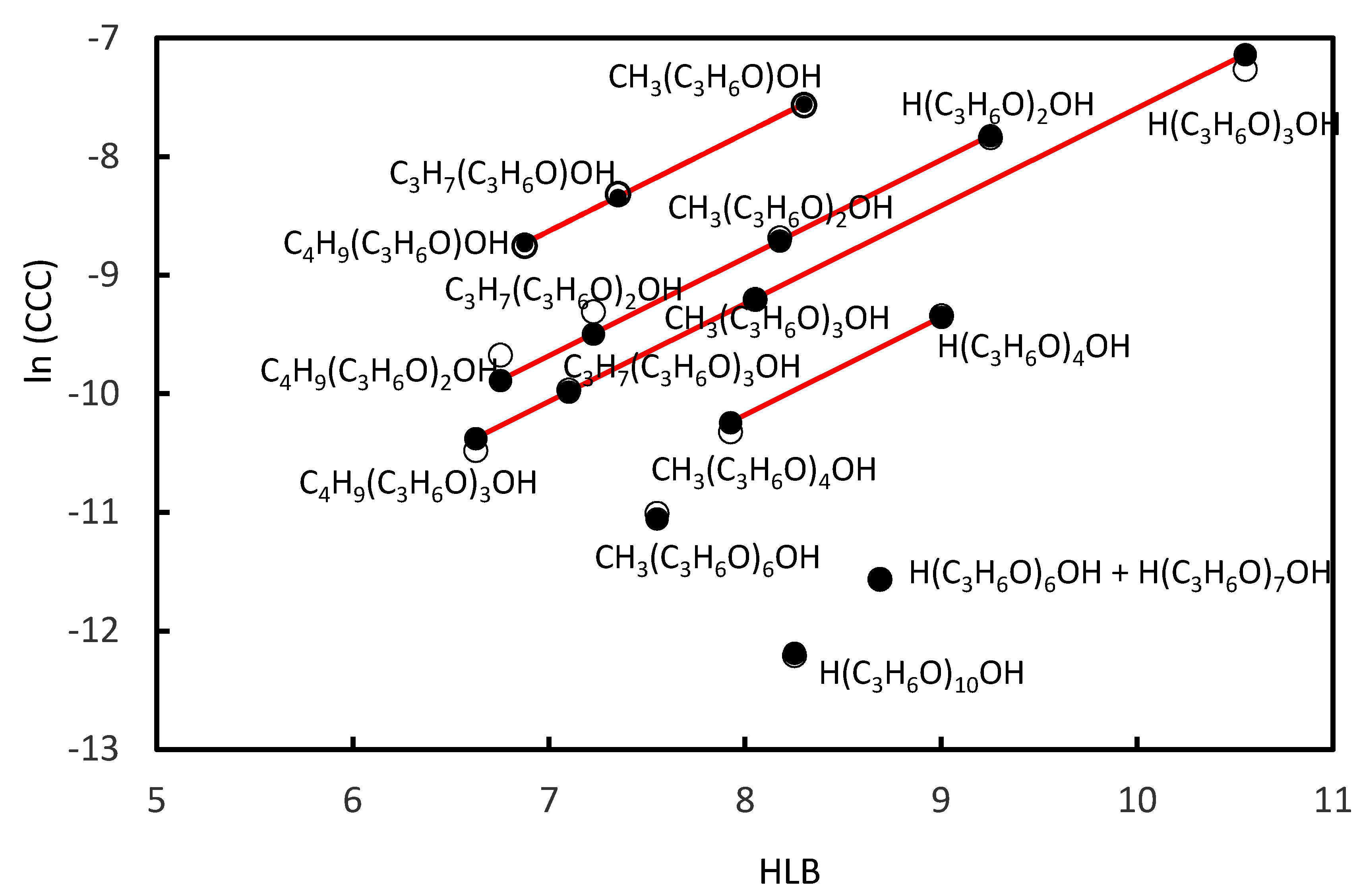
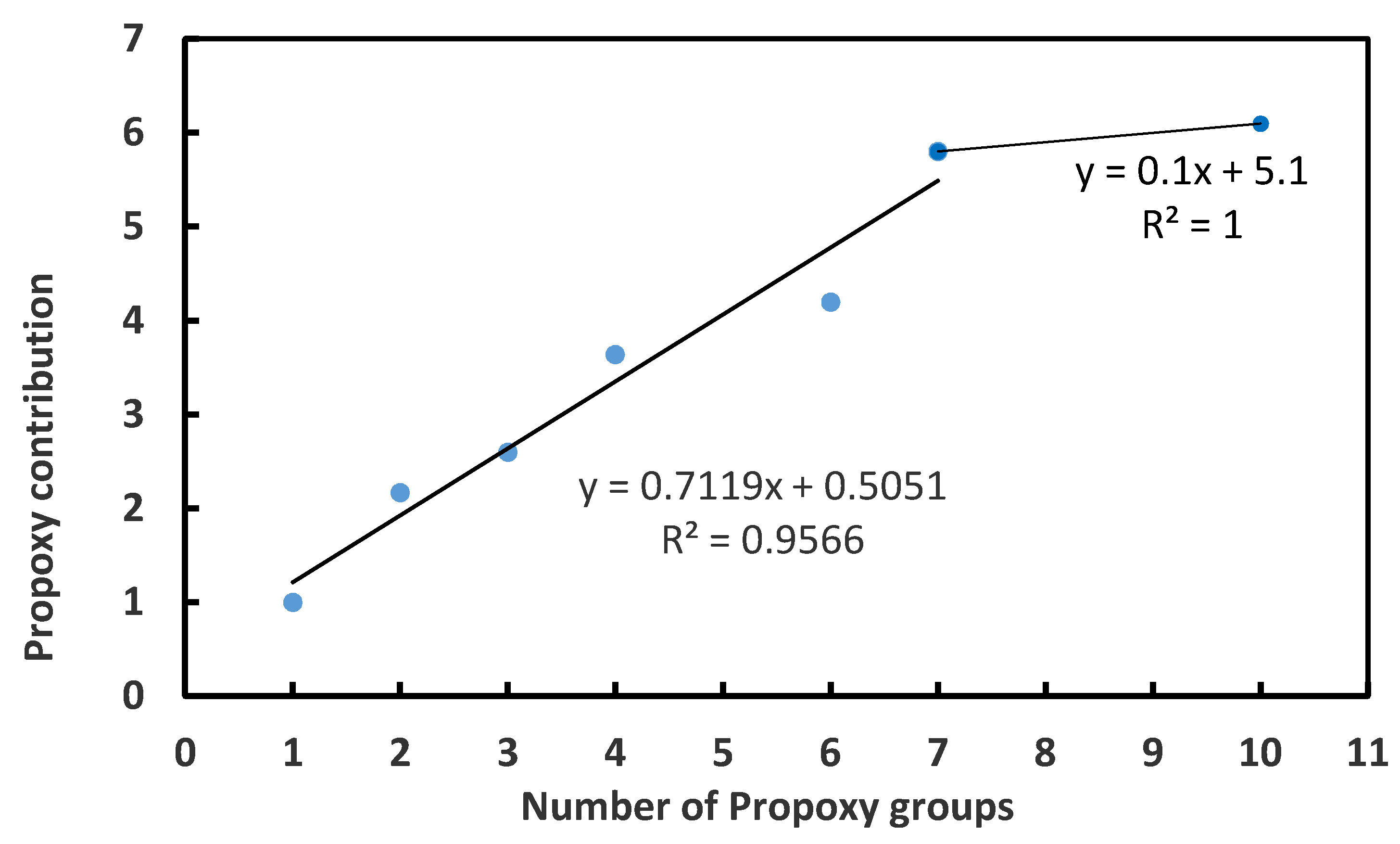
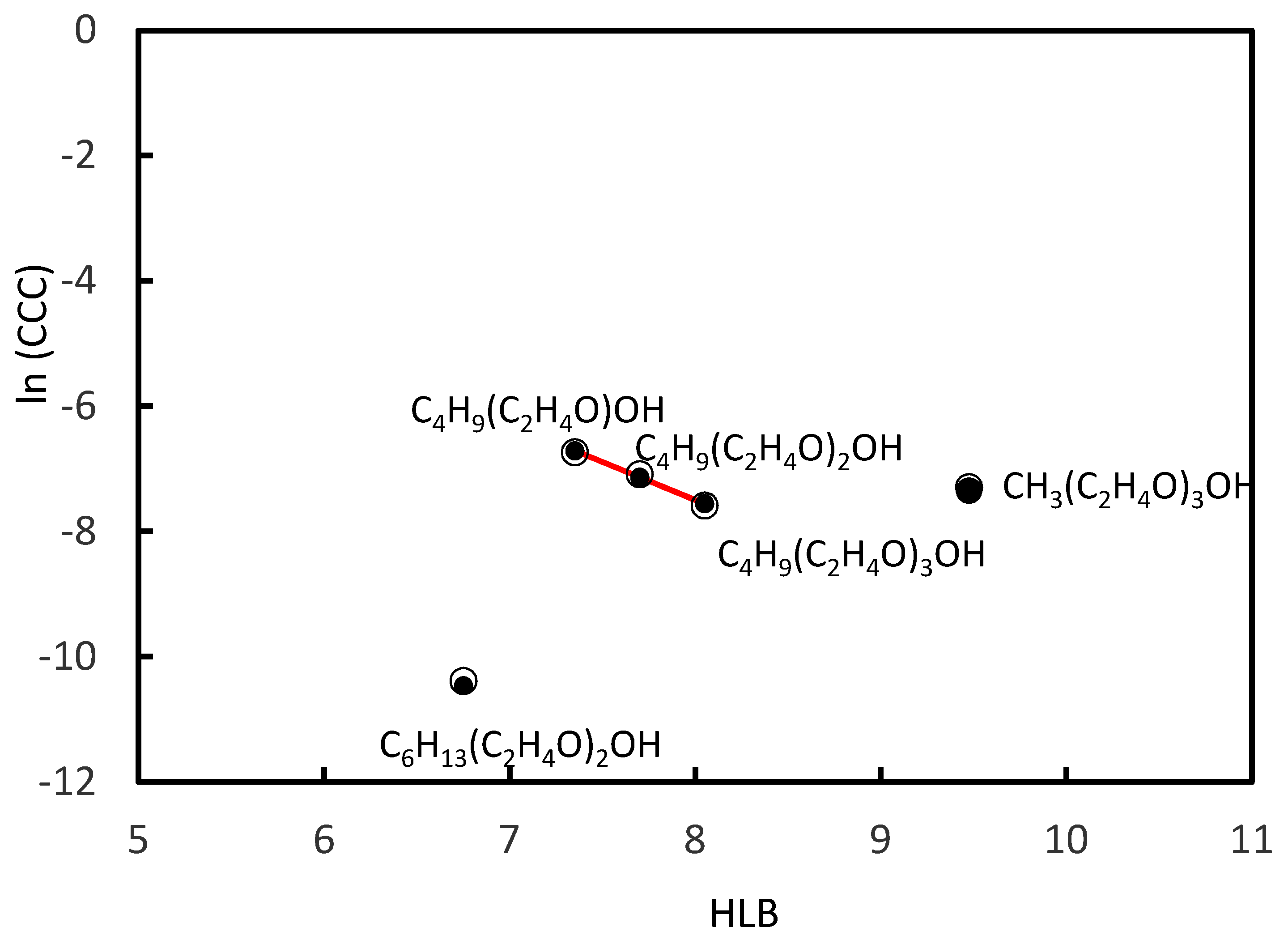
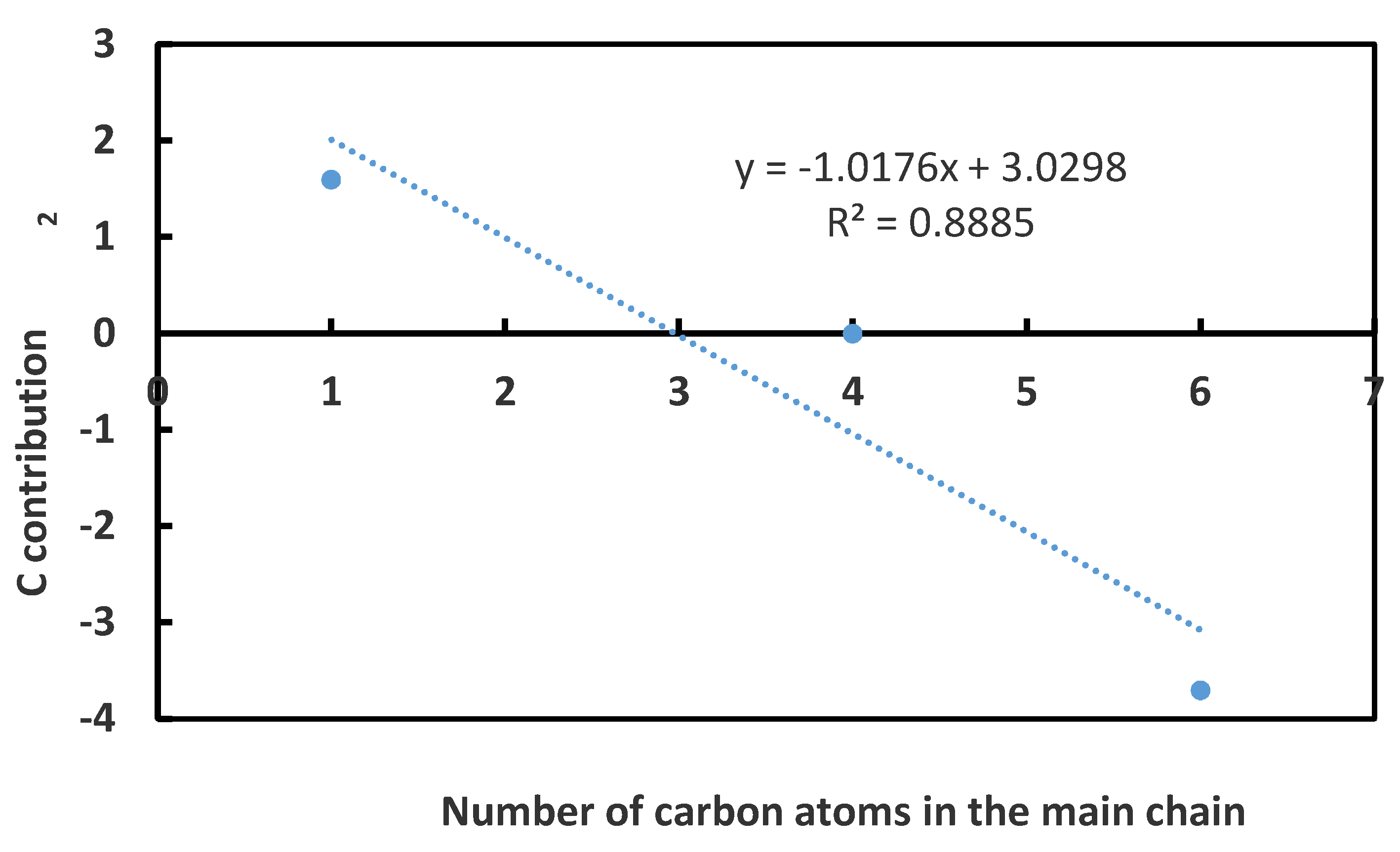
| Frother | Molecular Formula | HLB | CCC, mol/L | Reference |
|---|---|---|---|---|
| 1-Propanol | C3H7OH | 7.48 | 3.93 × 10−3 | [37] |
| 1-Butanol | C4H9OH | 7.00 | 8.50 × 10−4 | [37] |
| 1-Pentanol | C5H11OH | 6.53 | 2.84 × 10−4 | [37] |
| 1-Hexanol | C6H13OH | 6.05 | 7.90 × 10−5 | [37] |
| 1-Heptanol | C7H15OH | 5.58 | 5.52 × 10−5 | [37] |
| 1-Octanol | C8H17OH | 5.10 | 6.20 × 10−5 | [37] |
| 2-Propanol | CH3CH(OH)CH3 | 7.48 | 5.11 × 10−3 | [37] |
| 2-Butanol | C2H5CH(OH)CH3 | 7.00 | 1.04 × 10−3 | [37] |
| 2-Pentanol | C3H7CH(OH)CH3 | 6.53 | 3.40 × 10−4 | [37] |
| 2-Hexanol | C4H9CH(OH)CH3 | 6.05 | 1.08 × 10−4 | [37] |
| 2-Heptanol | C5H11CH(OH)CH3 | 5.58 | 7.76 × 10−5 | [37] |
| 2-Octanol | C6H13CH(OH)CH3 | 5.10 | 6.14 × 10−5 | [37] |
| MIBC | CH3CH(CH3)CH2CH(OH)CH3 | 6.53 | 9.78 × 10−5 | Present study |
| 3-Pentanol | C2H5CH(OH)C2H5 | 6.53 | 4.65 × 10−4 | [37] |
| 3-Hexanol | C3H7CH(OH)C2H5 | 6.05 | 1.27 × 10−4 | [37] |
| 2-Ethyl 1-Hexanol | C4H9CH(C2H5)CH2OH | 5.10 | 1.23 × 10−4 | [56] |
| Propylene Glycol Methyl Ether | CH3O(C3H6O)H | 8.30 | 5.19 × 10−4 | [37] |
| Propylene Glycol Propyl Ether | C3H7O(C3H6O)H | 7.35 | 2.45 × 10−4 | [37] |
| Propylene Glycol Butyl Ether | C4H9O(C3H6O)H | 6.88 | 1.59 × 10−4 | [37] |
| Dipropylene Glycol | HO(C3H6O)2H | 9.25 | 3.95 × 10−4 | [37] |
| Dipropylene Glycol Methyl Ether | CH3O(C3H6O)2H | 8.18 | 1.69 × 10−4 | [37] |
| Dipropylene Glycol Propyl Ether | C3H7O(C3H6O)2H | 7.23 | 9.08 × 10−5 | [37] |
| BDPG | C4H9O(C3H6O)2H | 6.75 | 6.31 × 10−5 | Present study |
| PPG200 | HO(C3H6O)3H | 10.55 | 7.03 × 10−4 | Present study |
| Dowfroth 200 | CH3O(C3H6O)3H | 8.05 | 1.01 × 10−4 | [26] |
| Tri(Propylene Glycol) Propyl Ether | C3H7O(C3H6O)3H | 7.10 | 4.69 × 10−5 | [37] |
| BTPG | C4H9O(C3H6O)3H | 6.63 | 2.82 × 10−5 | Present study |
| Tetrapropylene Glycol | HO(C3H6O)4H | 9.00 | 8.79 × 10−5 | [37] |
| Dowfroth 250 | CH3O(C3H6O)4H | 7.93 | 3.30 × 10−5 | [27] |
| PPG400 | HO(C3H6O)6H + HO(C3H6O)7H | 8.69 | 9.52 × 10−6 | Present study |
| PPG600 | HO(C3H6O)10H | 8.25 | 5.00 × 10−6 | Present study |
| Dowfroth 1012 | CH3O(C3H6O)6H | 7.55 | 1.66 × 10−5 | [27] |
| Ethylene Glycol Butyl Ether | C4H9O(C2H4O)H | 7.35 | 1.19 × 10−3 | [31] |
| BDEG | C4H9O(C2H4O)2H | 7.70 | 8.4 × 10−4 | [31] |
| BTEG | C4H9O(C2H4O)3H | 8.05 | 5.10 × 10−4 | Present study |
| Triethylene Glycol Methyl Ether | CH3O(C2H4O)3H | 9.48 | 6.80 × 10−4 | [25] |
| Diethoxy Hexanol | HO(C2H4O)2C6H12OH | 6.75 | 3.10 × 10−5 | [41] |
| Hydrophilic Groups | Group Value |
|---|---|
| −SO4− Na+ | 38.7 |
| −COO− K+ | 21.1 |
| −COO− Na+ | 19.1 |
| N (tertiary amine) | 9.4 |
| Ester (sorbitan ring) | 6.8 |
| Ester (free) | 2.4 |
| −COOH | 2.1 |
| Hydroxyl (free) | 1.9 |
| −O− | 1.3 |
| Hydroxyl (sorbitan ring) | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakashev, S.I.; Grozev, N.A.; Batjargal, K.; Guven, O.; Ozdemir, O.; Boylu, F.; Çelik, M.S. Correlations for Easy Calculation of the Critical Coalescence Concentration (CCC) of Simple Frothers. Coatings 2020, 10, 612. https://doi.org/10.3390/coatings10070612
Karakashev SI, Grozev NA, Batjargal K, Guven O, Ozdemir O, Boylu F, Çelik MS. Correlations for Easy Calculation of the Critical Coalescence Concentration (CCC) of Simple Frothers. Coatings. 2020; 10(7):612. https://doi.org/10.3390/coatings10070612
Chicago/Turabian StyleKarakashev, Stoyan I., Nikolay A. Grozev, Khandjamts Batjargal, Onur Guven, Orhan Ozdemir, Feridun Boylu, and Mehmet Sabri Çelik. 2020. "Correlations for Easy Calculation of the Critical Coalescence Concentration (CCC) of Simple Frothers" Coatings 10, no. 7: 612. https://doi.org/10.3390/coatings10070612
APA StyleKarakashev, S. I., Grozev, N. A., Batjargal, K., Guven, O., Ozdemir, O., Boylu, F., & Çelik, M. S. (2020). Correlations for Easy Calculation of the Critical Coalescence Concentration (CCC) of Simple Frothers. Coatings, 10(7), 612. https://doi.org/10.3390/coatings10070612










