Adhesion Behavior of Textured Electrosurgical Electrode in an Electric Cutting Process
Abstract
1. Introduction
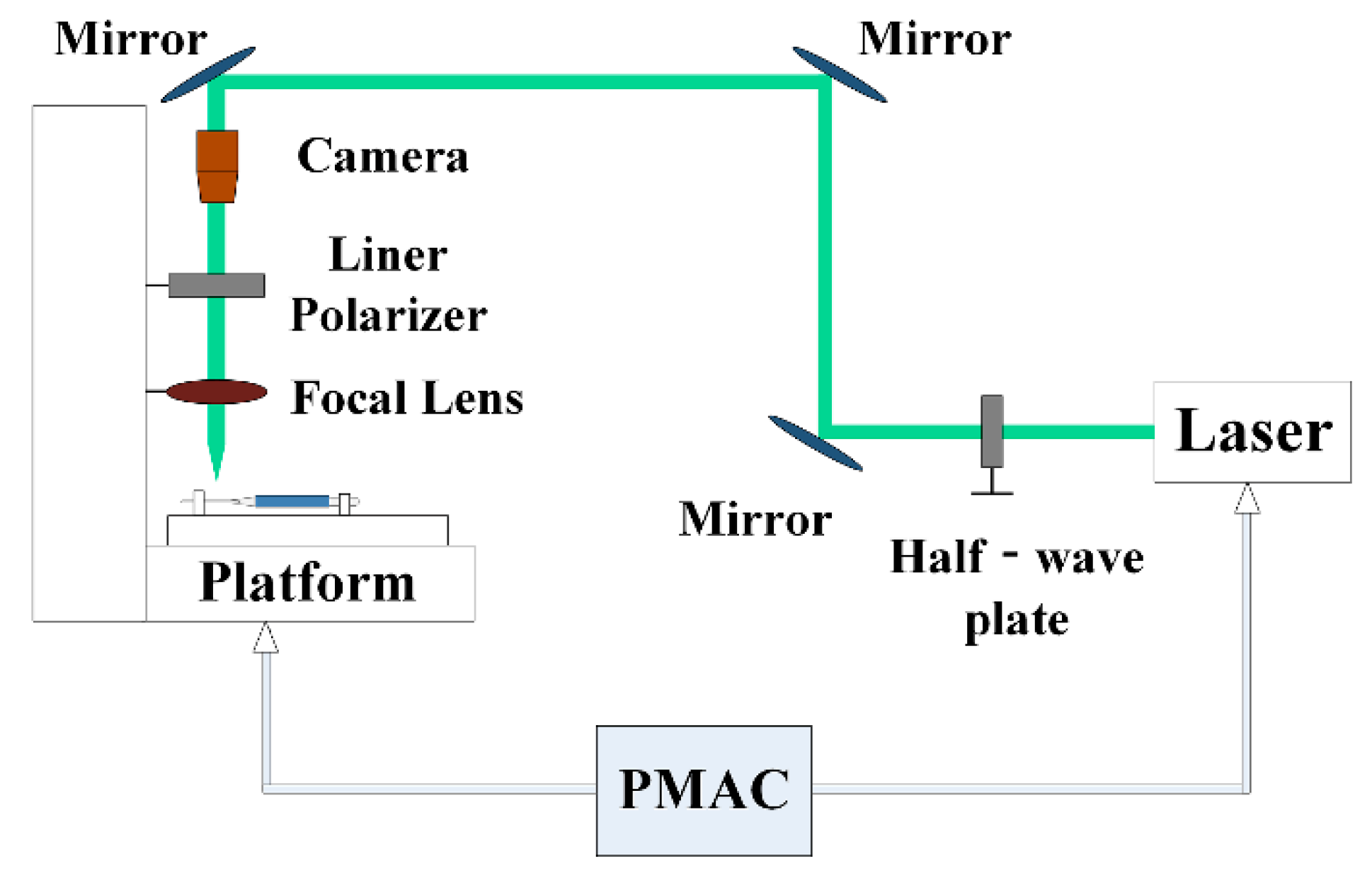
2. Experimental Section
2.1. Fabrication of Micro-Textures
2.2. Cutting Experiments
2.3. Numerical Implementation
3. Results and Discussion
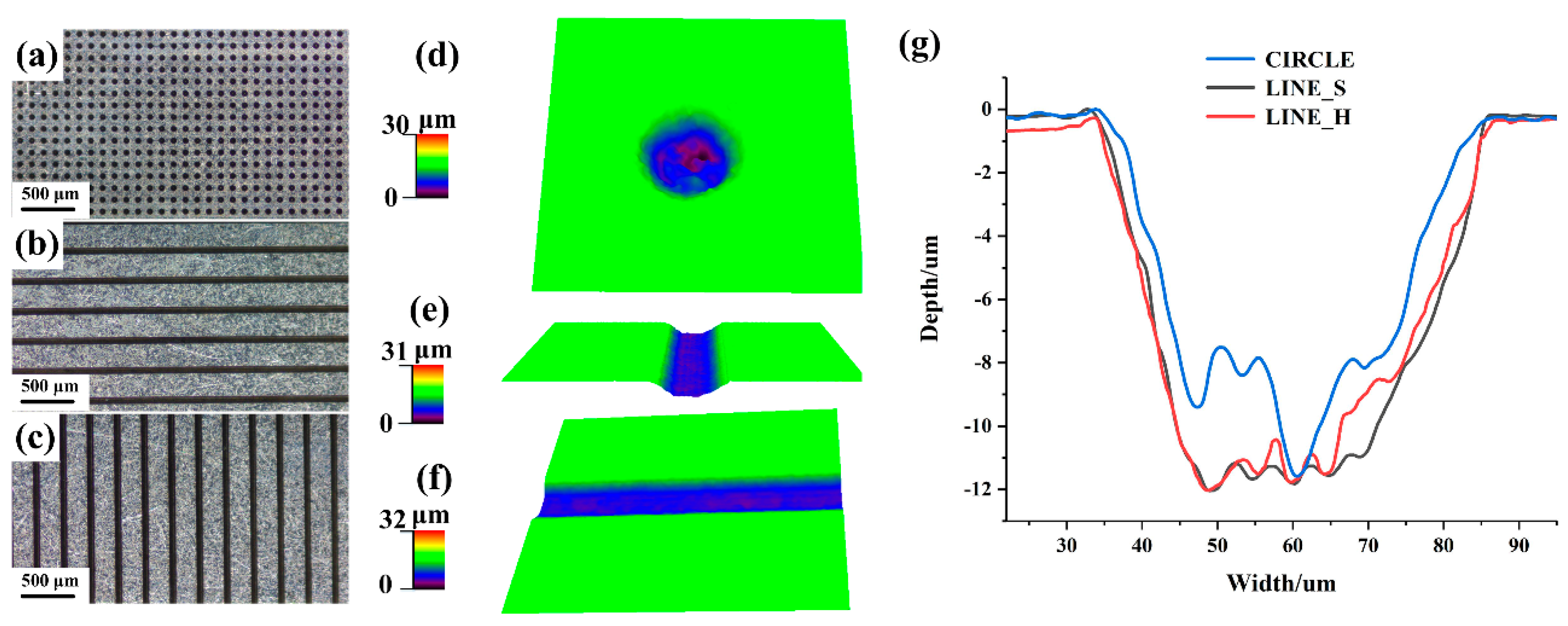
3.1. Morphologies of the Textured Electrode Surface
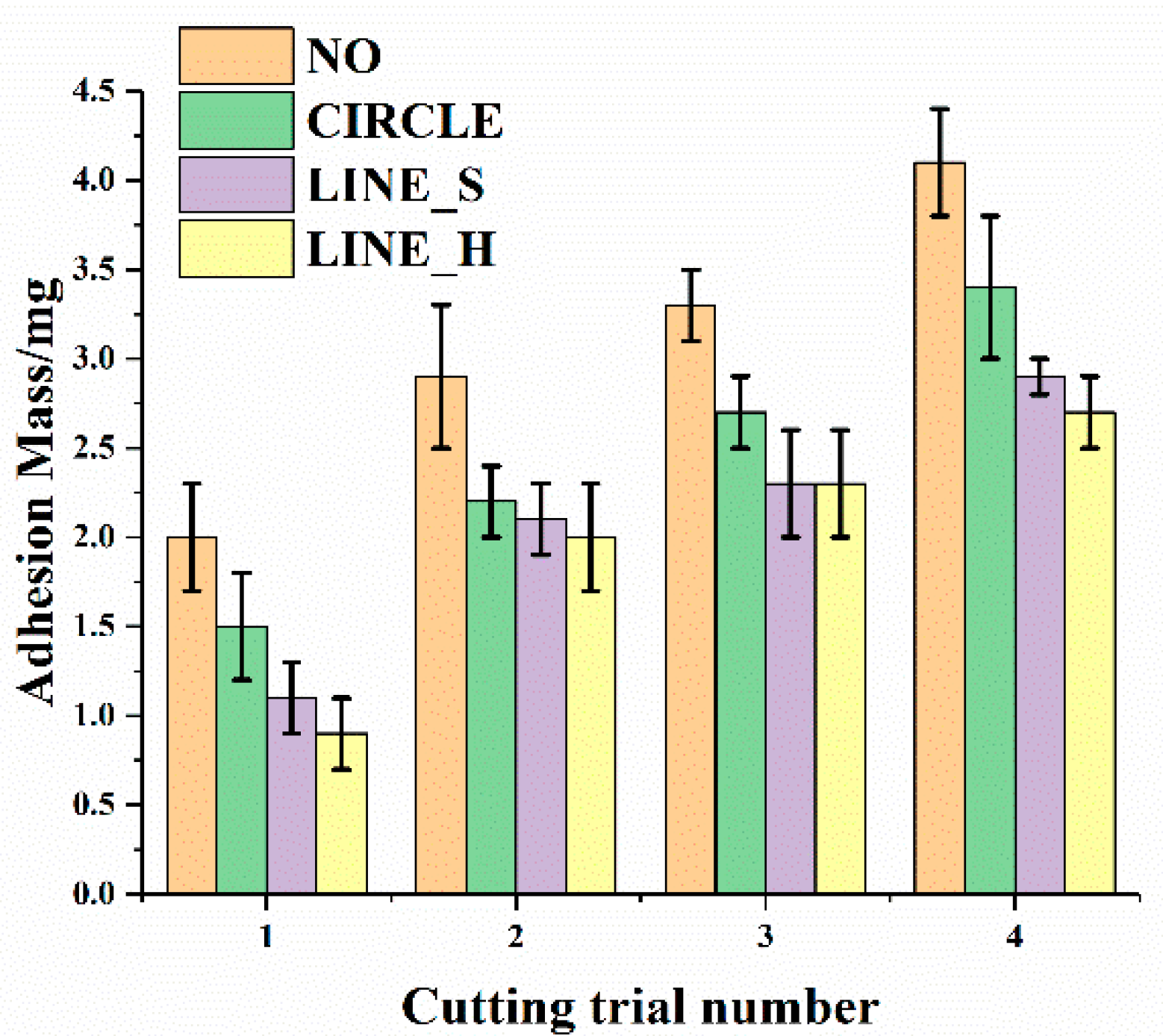
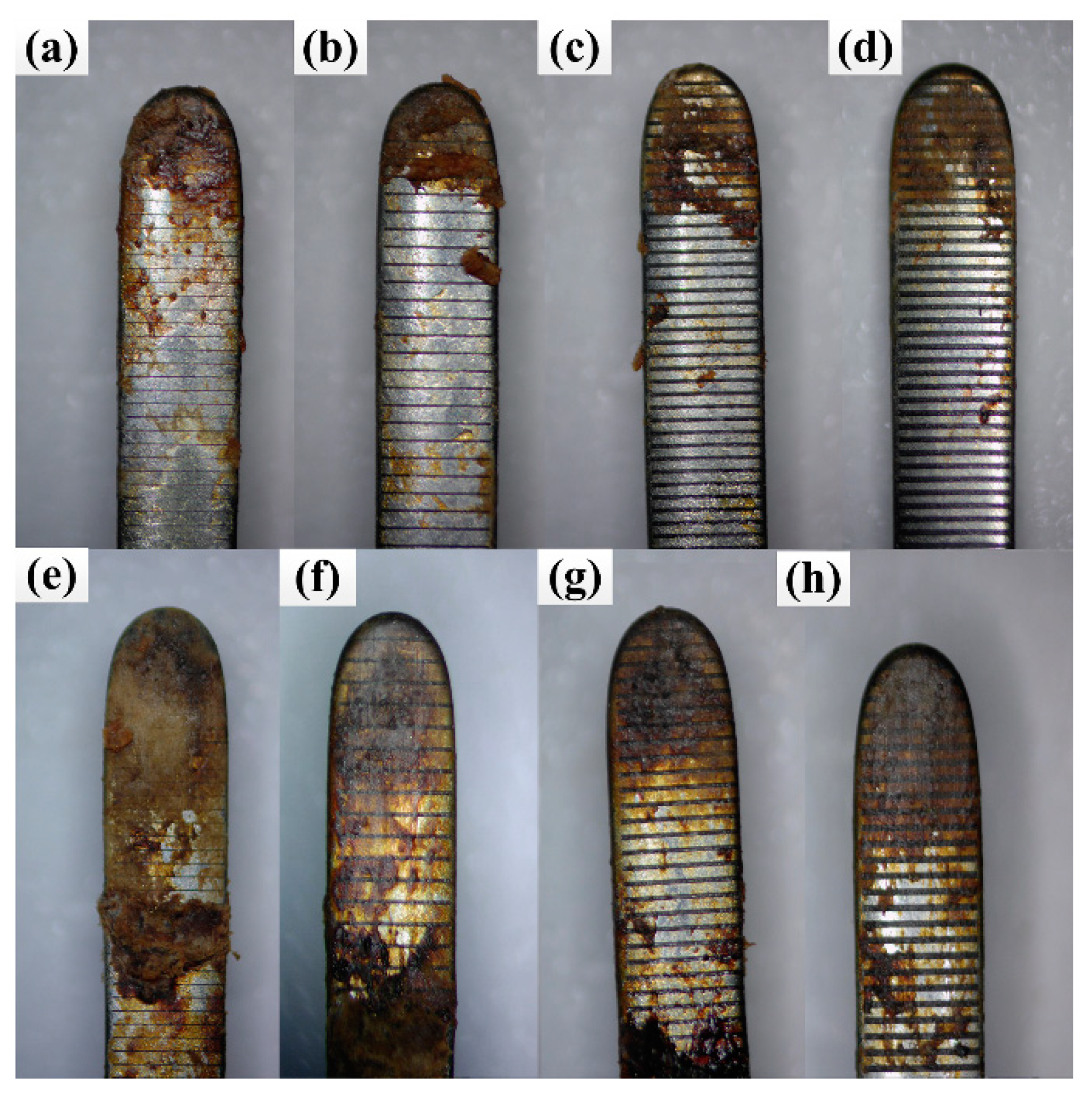
3.2. Effect of the Morphology of Micro-Texture on Adhesion Properties
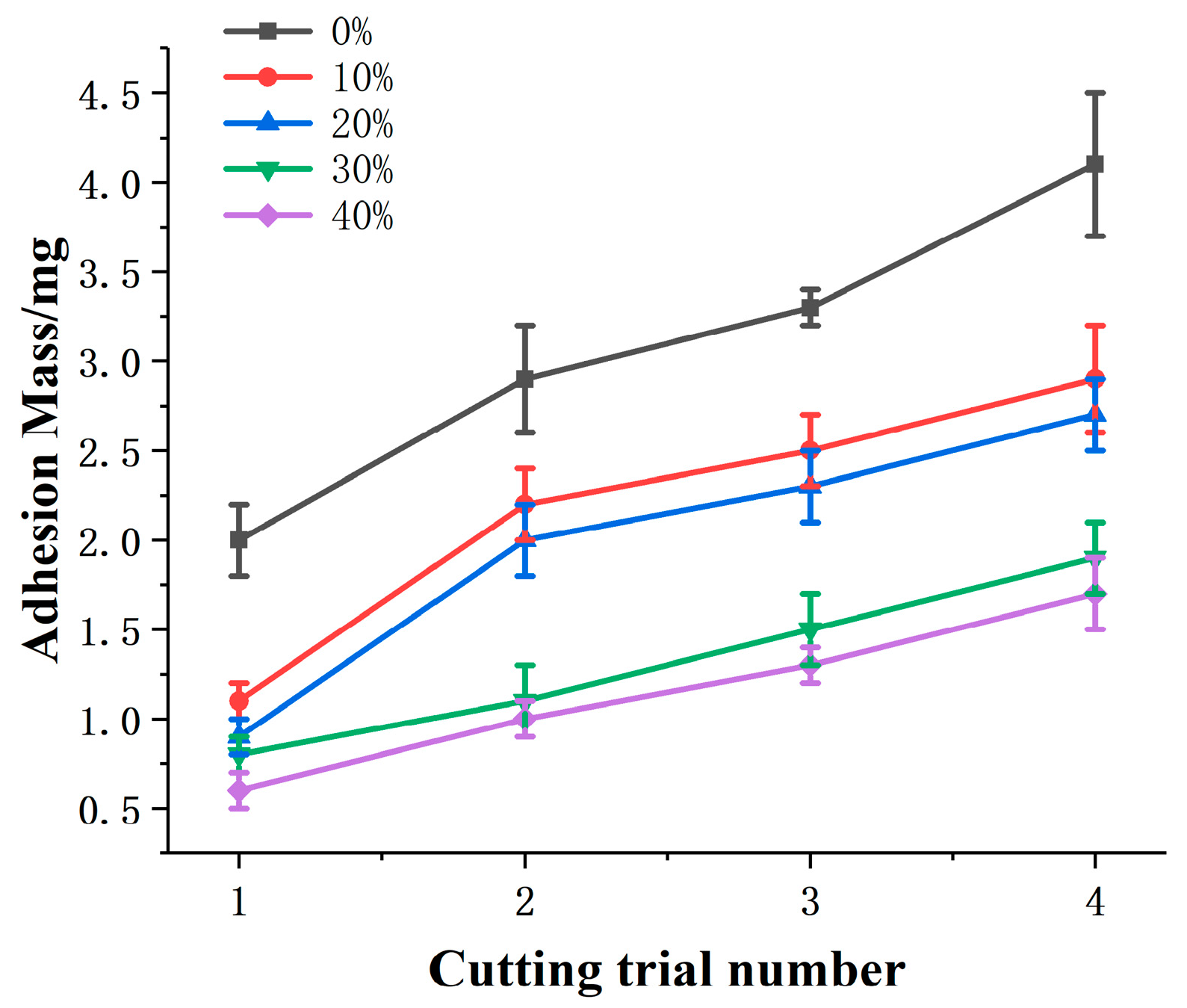
3.3. Effect of Area Density on Adhesion Properties
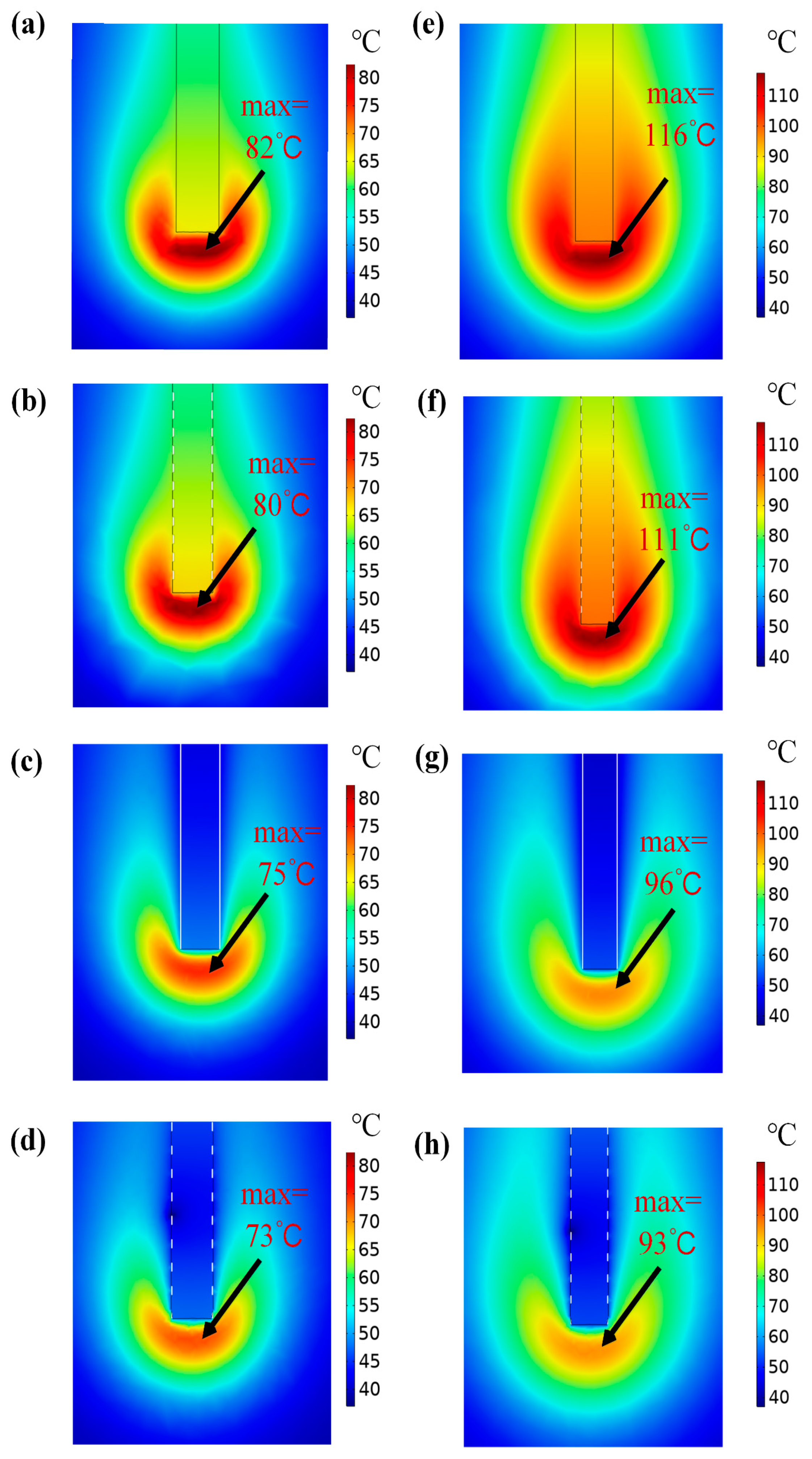
3.4. Temperature Distribution
3.5. Discussion
4. Conclusions
- Micro-textures with different morphologies and area densities were fabricated on the electrode surfaces. The micro-textures improved the adhesion properties of the electrode due to the reduction of contact area between the electrode and soft tissue, and the effect of air in the micro-textures.
- Different morphologies of micro-textures lead to different adhesion behavior of electrodes. The order of adhesion mass from more to less is the electrode without micro-texture, the electrode with micro-dimples, longitudinal micro-channels, and lateral micro-channels. Furthermore, the influence of micro-texture with different morphologies on temperature and adhesion behavior in the cutting process was verified by COMSOL multiphysics simulation software. The simulation results showed that different micro-textures can segment the temperature field in different ways, and reduce the transfer of heat to different degrees, thereby reducing soft tissue adhesion.
- Different area densities of micro-textures can lead to different effects on adhesion behavior of the electrode. With the increase of area density, adhesion mass decreased clearly. When the area density increased to a larger value, adhesion mass no longer decreased significantly. The better anti-adhesion effect can be obtained by combining lateral micro-channels with a large area density.
Author Contributions
Funding
Conflicts of Interest
References
- Massarweh, N.N.; Cosgriff, N.; Slakey, D.P. Electrosurgery: History, principles, and current and future uses. J. Am. Coll. Surg. 2006, 202, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Meakin, L.B.; Murrell, J.C.; Doran, I.C.P.; Knowles, T.G.; Tivers, M.S.; Chanoit, G.P.A. Electrosurgery reduces blood loss and immediate postoperative inflammation compared to cold instruments for midline celiotomy in dogs: A randomized controlled trial. Vet. Surg. 2017, 46, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.A.; Awad, S.; Perkins, A.C.; Lobo, D.N. Comparison of lateral thermal spread using monopolar and bipolar diathermy, the Harmonic Scalpel and the Ligasure. Br. J. Surg. 2010, 97, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Friedrich, T.; Metternich, F.; Tannapfel, A.; Reimann, H.; Eichfeld, U. Determination of temperature elevation in tissue during the application of the harmonic scalpel. Ultrasound Med. Biol. 2003, 29, 301–309. [Google Scholar] [CrossRef]
- Zheng, L.; Wan, J.; Long, Y.; Fu, H.; Zheng, J.; Zhou, Z. Effect of high-frequency electric distributions on the tissue sticking of minimally invasive electrosurgical devices. R. Soc. Open Sci. 2018, 5, 180125. [Google Scholar] [CrossRef]
- Sibbons, P.D.; Southgate, A. Comparison of wound-healing and tissue effects using the Gyrus PlasmaKnife with monopolar Coblation, and Harmonic Scalpel methodologies. Comp. Clin. Pathol. 2006, 15, 17–26. [Google Scholar] [CrossRef]
- Dodde, R.E.; Gee, J.S.; Geiger, J.D.; Shih, A.J. Monopolar electrosurgical thermal management for minimizing tissue damage. IEEE. Trans. Biomed. Eng. 2012, 59, 167–173. [Google Scholar] [CrossRef]
- Donzelli, J.; Leonetti, J.P.; Wurster, R.D.; Lee, J.M.; Young, M.R.I. Neuroprotection due to irrigation during bipolar cauter. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 149. [Google Scholar] [CrossRef]
- Morales, J.P.; Sabharwal, T.; Georganas, M.; Dourado, R.; Cahill, D.; Adam, A. Cold saline irrigation of the renal pelvis during Radiofrequency Ablation of a central renal neoplasm: A case report. J. Med. Case Rep. 2008, 2, 40. [Google Scholar] [CrossRef]
- Kang, S.K.; Kim, P.Y.; Koo, I.G.; Kim, H.Y.; Jung, J.-C.; Choi, M.Y.; Lee, J.K.; Collins, G.J. Non-stick polymer coatings for energy-based surgical devices employed in vessel sealing. Plasma Process. Polym. 2012, 9, 446–452. [Google Scholar] [CrossRef]
- Shen, Y.D.; Lin, L.H.; Chiang, H.J.; Ou, K.L.; Cheng, H.Y. Research of electrosurgical unit with novel antiadhesion composite thin film for tumor ablation: Microstructural characteristics, thermal conduction properties, and biological behaviors. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Subbiahdoss, G.; Swartjes, J.; van der Mei, H.C.; Busscher, H.J.; Libera, M. Length-scale mediated differential adhesion of mammalian cells and microbes. Adv. Funct. Mater. 2011, 21, 3916–3923. [Google Scholar] [CrossRef]
- Zhang, B.; Guan, F.; Zhao, X.; Zhang, Y.; Li, Y.; Duan, J.; Hou, B. Micro-nano textured superhydrophobic 5083 aluminum alloy as a barrier against marine corrosion and sulfate-reducing bacteria adhesion. J. Taiwan Inst. Chem. Eng. 2019, 97, 433–440. [Google Scholar] [CrossRef]
- Hejazi, I.; Seyfi, J.; Hejazi, E.; Sadeghi, G.M.; Jafari, S.H.; Khonakdar, H.A. Investigating the role of surface micro/nano structure in cell adhesion behavior of superhydrophobic polypropylene/nanosilica surfaces. Colloids Surf. B Biointerfaces 2015, 127, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Peillon, S.; Autricque, A.; Redolfi, M.; Stancu, C.; Gensdarmes, F.; Grisolia, C.; Pluchery, O. Adhesion of tungsten particles on rough tungsten surfaces using Atomic Force Microscopy. J. Aerosol Sci. 2019, 137, 105431. [Google Scholar] [CrossRef]
- Kim, T.; Min, C.; Jung, M.; Lee, J.; Park, C.; Kang, S. Design methodology for nano-engineered surfaces to control adhesion: Application to the anti-adhesion of particles. Appl. Surf. Sci. 2016, 389, 889–893. [Google Scholar] [CrossRef]
- Xun, X.; Wan, Y.; Zhang, Q.; Gan, D.; Hu, J.; Luo, H. Low adhesion superhydrophobic AZ31B magnesium alloy surface with corrosion resistant and anti-bioadhesion properties. Appl. Surf. Sci. 2020, 505, 144566. [Google Scholar] [CrossRef]
- Ling, T.D.; Liu, P.; Xiong, S.; Grzina, D.; Cao, J.; Wang, Q.J.; Xia, Z.C.; Talwar, R. Surface Texturing of Drill Bits for Adhesion Reduction and Tool Life Enhancement. Tribol. Lett. 2013, 52, 113–122. [Google Scholar] [CrossRef]
- Sugihara, T.; Enomoto, T. Development of a cutting tool with a nano/micro-textured surface—Improvement of anti-adhesive effect by considering the texture patterns. Precis. Eng. 2009, 33, 425–429. [Google Scholar] [CrossRef]
- Liang, J.; Song, R.; Huang, Q.; Yang, Y.; Lin, L.; Zhang, Y.; Jiang, P.; Duan, H.; Dong, X.; Lin, C. Electrochemical construction of a bio-inspired micro/nano-textured structure with cell-sized microhole arrays on biomedical titanium to enhance bioactivity. Electrochim. Acta 2015, 174, 1149–1159. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics inspired surfaces for drag reduction and oleophobicity/philicity. Beilstein J. Nanotechnol. 2011, 2, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shan, H.; Tong, X.; Zhang, Z.; Ren, L. The adhesion of bionic non-smooth characteristics on sample surfaces against parts. Mater. Sci. Eng A 2006, 417, 190–196. [Google Scholar] [CrossRef]
- Orazi, L.; Gnilitskyi, I.; Serro, A. Laser nanopatterning for wettability applications. J. Micro Nano Manuf. 2017, 5, 021008. [Google Scholar] [CrossRef]
- Wang, X.; Xu, B.; Chen, Y.; Ma, C.; Huang, Y. Fabrication of micro/nano-hierarchical structures for droplet manipulation via velocity-controlled picosecond laser surface texturing. Opt. Lasers Eng. 2019, 122, 319–327. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Hong, W.; Ma, C.; Xing, Y.; Feng, J. Fabrication of ordered hierarchical structures on stainless steel by picosecond laser for modified wettability applications. Opt. Express 2018, 26, 18998–19008. [Google Scholar] [CrossRef]
- Sun, N.; Shan, H.; Zhou, H.; Chen, D.; Ren, L. Adhesion resistance surfaces against clay resulting from biomimetic adaptation. Surf. Coat. Technol. 2012, 206, 3559–3565. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, G.; Zhang, D.; Chen, H. Liquid-infused surfaces on electrosurgical instruments with exceptional antiadhesion and low-damage performances. ACS Appl. Mater. Interfaces 2018, 10, 33713–33720. [Google Scholar] [CrossRef]
- Han, Z.; Fu, J.; Feng, X.; Niu, S.; Zhang, J.; Ren, L. Bionic anti-adhesive electrode coupled with maize leaf microstructures and TiO2 coating. RSC Adv. 2017, 7, 45287–45293. [Google Scholar] [CrossRef]
- Han, Z.; Fu, J.; Fang, Y.; Zhang, J.; Niu, S.; Ren, L. Anti-adhesive property of maize leaf surface related with temperature and humidity. J. Bionic Eng. 2017, 14, 540–548. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Ma, C.; Li, C.; Tian, S. Influence of blended textures generated by laser surface texturing on friction behavior in soft tissue cutting. Tribol. Int. 2019, 134, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Han, P.; Giovannini, M.; Ehmann, K. Modeling of machined depth in laser surface texturing of medical needles. Precis. Eng. 2017, 47, 10–18. [Google Scholar] [CrossRef]
- Tungjitkusolmun, S.; Staelin, S.T.; Haemmerich, D.; Tsai, J.-Z.; Cao, H.; Webster, J.G.; Lee, F.T., Jr.; Mahvi, D.M.; Vorperian, V.R. Three-dimensional finite-element analyses for radio-frequency hepatic tumor ablation. IEEE Trans. Biomed. Eng. 2002, 49, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1998, 85, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Lewis, E.W.; Mason, A.M.; Barrow, D.L. Evaluation of a new bipolar coagulation forceps in a thermal damage assessment. Neurosurgery 2009, 65, 1182–1187, discussion 1187. [Google Scholar] [CrossRef] [PubMed]


| Electrode No. | Width (μm) | Depth (μm) | Micro-Texture Characteristics | Area Density (%) |
|---|---|---|---|---|
| 1 | 0 | 0 | Without micro-texture | 0 |
| 2 | 50 | 12 | Micro-dimples | 20 |
| 3 | 50 | 12 | Longitudinal micro-channels | 20 |
| 4 | 50 | 12 | Lateral micro-channels | 20 |
| 5 | 25 | 12 | Lateral micro-channels | 10 |
| 6 | 75 | 12 | Lateral micro-channels | 30 |
| 7 | 100 | 12 | Lateral micro-channels | 40 |
| Finite Element Analysis (FEA) Region | Material | c (J/kg·K) | ρ (kg/m³) | k (W/m·K) | σ (s/m) |
|---|---|---|---|---|---|
| Biological Tissue | Porcine Liver | 3600 | 1060 | 0.512 | 0.333 |
| Electrode | 304 stainless steel | 132 | 21,500 | 71 | 4 × 106 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Lu, J.; Wang, X. Adhesion Behavior of Textured Electrosurgical Electrode in an Electric Cutting Process. Coatings 2020, 10, 596. https://doi.org/10.3390/coatings10060596
Zhou C, Lu J, Wang X. Adhesion Behavior of Textured Electrosurgical Electrode in an Electric Cutting Process. Coatings. 2020; 10(6):596. https://doi.org/10.3390/coatings10060596
Chicago/Turabian StyleZhou, Caiying, Juncheng Lu, and Xingsheng Wang. 2020. "Adhesion Behavior of Textured Electrosurgical Electrode in an Electric Cutting Process" Coatings 10, no. 6: 596. https://doi.org/10.3390/coatings10060596
APA StyleZhou, C., Lu, J., & Wang, X. (2020). Adhesion Behavior of Textured Electrosurgical Electrode in an Electric Cutting Process. Coatings, 10(6), 596. https://doi.org/10.3390/coatings10060596





