Customized Therapeutic Surface Coatings for Dental Implants
Abstract
1. Introduction
2. Mode of Action of Drug-Releasing Coatings
3. Controlling the Drug Release from Coatings
4. Desired Properties for Drug-Releasing Dental Implants
5. Methods of Drugs Coating on Dental Implants
| Description | Problems/Benefits | Consequences | Examples |
|---|---|---|---|
| Physisorption or Adsorption [130,133,136,150,151] | |||
| Depending on the implant surface features (roughness, chemistry, surface energy, and wettability), which results in spontaneous adsorption of drugs and therapeutic agents on Ti implant surface via weaker van der Waals forces. | Lack of control over the delivery of molecules. Several parameters such as micro movement of the implant, pH, temperature, and solvent conditions | It is achieved by dipping method Uncontrolled adsorption from the surface | A burst release system, (80%–90%) in 1 h of adsorbed molecule Superficially adsorbed BMP-2 released rapidly in higher concentration |
| Covalent Binding [134,152,153,154,155] | |||
| Cellular adhesion of proteins via covalent bond to prevent systemic effects of drugs | Immobilization of molecules promote mineralization | Proteins bond to the implant surface directly or through a spacer such as hydroxyl (−OH) or amine (−NH) groups | Cell-adhesive proteins (collagen, osteopontin, fibronectin, or vitronectin) |
| Carrier Systems or Self-Organized Nanoporous Surfaces on Silicon, Aluminum, and Titanium [132,136,156,157] | |||
| Direct integration of drug into the coating material via carrier molecules (polylactide, polyglycolic acid, hydrogels, polypyrrole, and CaP/HA coating) | Growth factors or antibiotics are incorporated into a HA coating; can be delivered in a physiologic-like manner | Antibiotics, proteins, and growth factors are entrapped in crystals formed by precipitation of CaP/HA solution | A slow-release system, protein loaded into the carrier can be 10 times higher than adsorption |
6. Understanding Coating–Implant Adhesion Interface
7. Therapeutic Dental Implant Coatings
7.1. Biomimetic and Bioactive Coatings
7.2. Antibacterial Coatings
- (a)
- As acute infections occur immediately after implant surgery, short-term antimicrobial drug delivery coatings to the host tissues and device interface would help to prevent bacterial colonization, thus preventing the infection.
- (b)
- Similarly, long-term bacteria can colonize the implant surface; therefore, consistent antimicrobial drug delivery coatings are required to inhibit microbial colonization on the surface over time.
- (c)
- It is important that, while maintaining both long and short-term drug elution from implant coatings, there should be no alteration in the surface materials’ properties; otherwise, it may deteriorate the implant’s osseointegration.
7.3. Antimicrobial Metallic/Metalloid Coatings
7.4. Antimicrobial Peptides (AMPs) Coatings
7.5. Bisphosphonates Coatings
7.6. Zirconia Coatings
8. Limitations and Future Challenges for Coated Dental Implants
9. Conclusions and Future Trends
Funding
Conflicts of Interest
References
- Mijiritsky, E.; Mazor, Z.; Lorean, A.; Levin, L. Implant diameter and length influence on survival: Interim results during the first 2 years of function of implants by a single manufacturer. Implant Dent. 2013, 22, 394–398. [Google Scholar] [CrossRef]
- Sullivan, R.M. Implant dentistry and the concept of osseointegration: A historical perspective. J. Calif. Dent. Assoc. 2001, 29, 737–745. [Google Scholar]
- Oshida, Y.; Tuna, E.B.; Aktören, O.; Gençay, K. Dental implant systems. Int. J. Mol. Sci. 2010, 11, 1580–1678. [Google Scholar] [CrossRef]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Ng, D.Y.; Wong, A.Y.C.; Liston, P.N. Multidisciplinary approach to implants: A review. N. Z. Dent. J. 2012, 108, 123–128. [Google Scholar]
- Misch, C.E. Dental Implant Prosthetics; Elsevier Health Sciences: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Babbush, C.A. Dental Implants: Principles and Practice; WB Saunders Company: Philadelphia, PA, USA, 1991. [Google Scholar]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2014, CD003815, 1–93. [Google Scholar] [CrossRef]
- AlHelal, A.; AlBader, B.; Kattadiyil, M.T.; Garbacea, A.; Proussaefs, P. CAD-CAM implant-supported fixed complete dental prosthesis with titanium milled molars: A clinical report. J. Prosthet. Dent. 2017, 117, 463–469. [Google Scholar] [CrossRef]
- Konstantinidis, I.K.; Jacoby, S.; Rädel, M.; Böning, K. Prospective evaluation of zirconia based tooth-and implant-supported fixed dental prostheses: 3-year results. J. Dent. 2015, 43, 87–93. [Google Scholar] [CrossRef]
- Ekfeldt, A.; Zellmer, M.; Carlsson, G.E. Treatment with implant-supported fixed dental prostheses in patients with congenital and acquired neurologic disabilities: A prospective study. Int. J. Prosthodont. 2013, 26, 517–524. [Google Scholar] [CrossRef]
- Ibrahim, W.I.; ElKhadem, A.H.; Alqutaibi, A.Y.; Kaddah, A.F. Prosthodontic Maintenance of Fixed Implant Restorations vs. Implant Overdentures: A Systematic Review. Indian J. Sci. Technol. 2016, 9, 1–16. [Google Scholar] [CrossRef][Green Version]
- Albrektsson, T.; Brånemark, P.; Hansson, H.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [PubMed]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Tocco, I.; Roman, M.; Vindigni, V.; Stellini, E.; Gardin, C.; Ferroni, L.; Sivolella, S. Nanostructured surfaces of dental implants. Int. J. Mol. Sci. 2013, 14, 1918–1931. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Bioactivity and osseointegration of PEEK are inferior to those of titanium—A systematic review. J. Oral Implantol. 2016, 42, 512–516. [Google Scholar] [CrossRef]
- Geurs, N.C.; Jeffcoat, R.L.; McGlumphy, E.A.; Reddy, M.S.; Jeffcoat, M.K. Influence of implant geometry and surface characteristics on progressive osseointegration. Int. J. Oral Maxillofac. Implant. 2002, 17, 811–815. [Google Scholar]
- Osman, R.B.; Swain, M.V. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef]
- Nepal, M.; Li, L.; Bae, T.S.; Kim, B.I.; Soh, Y. Evaluation of Osseointegration around Tibial Implants in Rats by Ibandronate-Treated Nanotubular Ti-32Nb-5Zr Alloy. Biomol. Ther. 2014, 22, 563–569. [Google Scholar] [CrossRef]
- Vidigal, G.M.; Groisman, M.; Gregório, L.H.; Soares, G.d.A. Osseointegration of titanium alloy and HA-coated implants in healthy and ovariectomized animals: A histomorphometric study. Clin. Oral Implant. Res. 2009, 20, 1272–1277. [Google Scholar] [CrossRef]
- Chaturvedi, T.P. An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J. Dent. Res. 2009, 20, 91–98. [Google Scholar] [CrossRef]
- McCracken, M. Dental implant materials: Commercially pure titanium and titanium alloys. J. Prosthodont. 1999, 8, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, A.; Sandukas, S. Processing and evaluation of bioactive coatings on polymeric implants. J. Biomed. Mater. Res. Part A 2013, 101, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Richardson, J.; Lundgren, T. Comparative soft and hard tissue responses to titanium and polymer healing abutments. J. Oral Implantol. 2011, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Poulsson, A.H.; Eglin, D.; Zeiter, S.; Camenisch, K.; Sprecher, C.; Agarwal, Y.; Nehrbass, D.; Wilson, J.; Richards, R.G. Osseointegration of machined, injection moulded and oxygen plasma modified PEEK implants in a sheep model. Biomaterials 2014, 35, 3717–3728. [Google Scholar] [CrossRef]
- Schwitalla, A.; Müller, W. PEEK dental implants: A review of the literature. J. Oral Implantol. 2013, 39, 743–749. [Google Scholar] [CrossRef]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef]
- Özkurt, Z.; Kazazoğlu, E. Zirconia dental implants: A literature review. J. Oral Implantol. 2011, 37, 367–376. [Google Scholar] [CrossRef]
- Stübinger, S.; Homann, F.; Etter, C.; Miskiewicz, M.; Wieland, M.; Sader, R. Effect of Er: YAG, CO2 and diode laser irradiation on surface properties of zirconia endosseous dental implants. Lasers Surg. Med. 2008, 40, 223–228. [Google Scholar] [CrossRef]
- Badran, Z.; Struillou, X.; Hughes, F.J.; Soueidan, A.; Hoornaert, A.; Ide, M. Silicon Nitride (Si3N4) Implants: The Future of Dental Implantology? J. Oral Implantol. 2017, 43, 240–244. [Google Scholar] [CrossRef]
- Webster, T.J.; Patel, A.A.; Rahaman, M.; Bal, B.S. Anti-infective and osteointegration properties of silicon nitride, poly (ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef]
- Elias, C.N.; Fernandes, D.J.; Resende, C.R.; Roestel, J. Mechanical properties, surface morphology and stability of a modified commercially pure high strength titanium alloy for dental implants. Dent. Mater. 2015, 31, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Fernandes, D.J.; de Souza, F.M.; dos Santos Monteiro, E.; de Biasi, R.S. Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J. Mater. Res. Technol. 2019, 8, 1060–1069. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Triplett, R.G.; Frohberg, U.; Sykaras, N.; Woody, R.D. Implant materials, design, and surface topographies: Their influence on osseointegration of dental implants. J. Long. Term. Eff. Med. 2003, 13, 485–502. [Google Scholar] [CrossRef]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Gepreel, M.A.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Sumitomo, N.; Noritake, K.; Hattori, T.; Morikawa, K.; Niwa, S.; Sato, K.; Niinomi, M. Experiment study on fracture fixation with low rigidity titanium alloy. J. Mater. Sci. Mater. Med. 2008, 19, 1581–1586. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface engineering for bone implants: A trend from passive to active surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef]
- Suzuki, M.; Guimaraes, M.V.M.; Marin, C.; Granato, R.; Gil, J.N.; Coelho, P.G. Histomorphometric Evaluation of Alumina-Blasted/Acid-Etched and Thin Ion Beam-Deposited Bioceramic Surfaces: An Experimental Study in Dogs. J. Oral Maxillofac. Surg. 2009, 67, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A. The importance of surface roughness for implant incorporation. Int. J. Mach. Tools Manuf. 1998, 38, 657–662. [Google Scholar] [CrossRef]
- Elias, C.N.; Meirelles, L. Improving osseointegration of dental implants. Expert Rev. Med Devices 2010, 7, 241–256. [Google Scholar] [CrossRef]
- Zafar, M.S.; Farooq, I.; Awais, M.; Najeeb, S.; Khurshid, Z.; Zohaib, S. Chapter 11-Bioactive Surface Coatings for Enhancing Osseointegration of Dental Implants. Biomed. Ther. Clin. Appl. Bioact. Glasses 2019, 313–329. [Google Scholar] [CrossRef]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. Part A 2017. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Hasan, S.M.; Khan, R.S. Bisphosphonate releasing dental implant surface coatings and osseointegration: A systematic review. J. Taibah Univ. Med. Sci. 2017, 12, 369–375. [Google Scholar] [CrossRef]
- Ballo, A.M.; Akca, E.; Ozen, T.; Moritz, N.; Lassila, L.; Vallittu, P.; Närhi, T. Effect of implant design and bioactive glass coating on biomechanical properties of fiber-reinforced composite implants. Eur. J. Oral Sci. 2014, 122, 303–309. [Google Scholar] [CrossRef]
- Barkarmo, S.; Wennerberg, A.; Hoffman, M.; Kjellin, P.; Breding, K.; Handa, P.; Stenport, V. Nano-hydroxyapatite-coated PEEK implants: A pilot study in rabbit bone. J. Biomed. Mater. Res. Part A 2013, 101, 465–471. [Google Scholar] [CrossRef]
- Mehdikhani-Nahrkhalaji, M.; Fathi, M.; Mortazavi, V.; Mousavi, S.; Hashemi-Beni, B.; Razavi, S. Novel nanocomposite coating for dental implant applications in vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2012, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7, S515–S527. [Google Scholar] [CrossRef] [PubMed]
- Kettenberger, U.; Luginbuehl, V.; Procter, P.; Pioletti, D.P. In vitro and in vivo investigation of bisphosphonate-loaded hydroxyapatite particles for peri-implant bone augmentation. J. Tissue Eng. Regen. Med. 2017, 11, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.S.; Bosco, R.; Both, S.K.; Iafisco, M.; Leeuwenburgh, S.C.; Jansen, J.A.; van den Beucken, J.J. Synergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic rats. Biomaterials 2014, 35, 5482–5490. [Google Scholar] [CrossRef]
- Abtahi, J.; Tengvall, P.; Aspenberg, P. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 2012, 50, 1148–1151. [Google Scholar] [CrossRef]
- Norowski, P.A.; Courtney, H.S.; Babu, J.; Haggard, W.O.; Bumgardner, J.D. Chitosan coatings deliver antimicrobials from titanium implants: A preliminary study. Implant Dent. 2011, 20, 56–67. [Google Scholar] [CrossRef]
- Rodriguez, G.M.; Bowen, J.; Grossin, D.; Ben-Nissan, B.; Stamboulis, A. Functionalisation of Ti6Al4V and hydroxyapatite surfaces with combined peptides based on KKLPDA and EEEEEEEE peptides. Colloids Surf. B Biointerfaces 2017, 160, 154–160. [Google Scholar] [CrossRef]
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial dental implant functionalization strategies—A systematic review. Dent. Mater. J. 2016, 35, 545–558. [Google Scholar] [CrossRef]
- Alves, D.; Olívia Pereira, M. Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Stevenson, C.L.; Santini, J.T.; Langer, R. Reservoir-based drug delivery systems utilizing microtechnology. Adv. Drug Deliv. Rev. 2012, 64, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Peng, X.; Zhou, X.; Zou, J.; Cheng, L. Emerging Applications of Drug Delivery Systems in Oral Infectious Diseases Prevention and Treatment. Molecules 2020, 25, 516. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, V.C.; Fadda, H.M.; Parsons, G.E.; Basit, A.W. A comparative in vitro assessment of the drug release performance of pH-responsive polymers for ileo-colonic delivery. Int. J. Pharm. 2006, 308, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Gultepe, E.; Nagesha, D.; Sridhar, S.; Amiji, M. Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv. Drug Deliv. Rev. 2010, 62, 305–315. [Google Scholar] [CrossRef]
- Son, G.; Lee, B.; Cho, C. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siegel, R.A.; Rathbone, M.J. Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Boston, MA, USA, 2012; Volume 3. [Google Scholar]
- Exner, A.A.; Saidel, G.M. Drug-eluting polymer implants in cancer therapy. Expert Opin. Drug Deliv. 2008, 5, 775–788. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Mauri, E.; Rossi, F.; Sacchetti, A. Tunable drug delivery using chemoselective functionalization of hydrogels. Mater. Sci. Eng. C 2016, 61, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Castiglione, F.; Ferro, M.; Moioli, M.; Mele, A.; Masi, M. The role of drug–drug interactions in hydrogel delivery systems: Experimental and model study. ChemPhysChem 2016, 17, 1615–1622. [Google Scholar] [CrossRef]
- Reza Saboktakin, M.; Tabatabaei, R.M. Supramolecular hydrogels as drug delivery systems. Int. J. Biol. Macromol. 2015, 75, 426–436. [Google Scholar] [CrossRef]
- Aw, M.S.; Gulati, K.; Losic, D. Controlling drug release from titania nanotube arrays using polymer nanocarriers and biopolymer coating. J. Biomater. Nanobiotechnology 2011, 2, 477–484. [Google Scholar] [CrossRef]
- Han, C.; Jang, T.; Kim, H.; Koh, Y. Creation of nanoporous TiO2 surface onto polyetheretherketone for effective immobilization and delivery of bone morphogenetic protein. J. Biomed. Mater. Res. Part A 2014, 102, 793–800. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. USA 2015, 112, 14444–14451. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.; Goyal, A.K. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 2016, 23, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Zelikin, A.N. Drug releasing polymer thin films: New era of surface-mediated drug delivery. ACS Nano 2010, 4, 2494–2509. [Google Scholar] [CrossRef] [PubMed]
- Creel, C.J.; Lovich, M.A.; Edelman, E.R. Arterial paclitaxel distribution and deposition. Circ. Res. 2000, 86, 879–884. [Google Scholar] [CrossRef]
- Pakulska, M.M.; Donaghue, I.E.; Obermeyer, J.M.; Tuladhar, A.; McLaughlin, C.K.; Shendruk, T.N.; Shoichet, M.S. Encapsulation-free controlled release: Electrostatic adsorption eliminates the need for protein encapsulation in PLGA nanoparticles. Sci. Adv. 2016, 2, e1600519. [Google Scholar] [CrossRef]
- Chang, M.; Zhang, F.; Wei, T.; Zuo, T.; Guan, Y.; Lin, G.; Shao, W. Smart linkers in polymer–drug conjugates for tumor-targeted delivery. J. Drug Target. 2016, 24, 475–491. [Google Scholar] [CrossRef]
- Kamath, K.R.; Barry, J.J.; Miller, K.M. The Taxus™ drug-eluting stent: A new paradigm in controlled drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Vulic, K.; Shoichet, M.S. Tunable growth factor delivery from injectable hydrogels for tissue engineering. J. Am. Chem. Soc. 2012, 134, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, D.; Kootala, S.; Yi, Z.; Yang, X.; Hilborn, J. Orthogonal chemoselective assembly of hyaluronic acid networks and nanogels for drug delivery. Macromolecules 2013, 46, 4105–4113. [Google Scholar] [CrossRef]
- Rossi, F.; van Griensven, M. Polymer functionalization as a powerful tool to improve scaffold performances. Tissue Eng. Part A 2014, 20, 2043–2051. [Google Scholar] [CrossRef]
- Venkatraman, S.; Boey, F. Release profiles in drug-eluting stents: Issues and uncertainties. J. Control. Release 2007, 120, 149–160. [Google Scholar] [CrossRef]
- Wang, X.; Venkatraman, S.S.; Boey, F.Y.C.; Loo, J.S.C.; Tan, L.P. Controlled release of sirolimus from a multilayered PLGA stent matrix. Biomaterials 2006, 27, 5588–5595. [Google Scholar] [CrossRef]
- Ranade, S.V.; Richard, R.E.; Helmus, M.N. Styrenic block copolymers for biomaterial and drug delivery applications. Acta Biomater. 2005, 1, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Shameem, M.; Lee, H.; DeLuca, P.P. A short-term (accelerated release) approach to evaluate peptide release from PLGA depot formulations. AAPS PharmSci 1999, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, M.; Koshika, A.; Okada, J.; Ikeda, M. Mechanism of drug release from poly(l-lactic acid) matrix containing acidic or neutral drugs. J. Control. Release 1999, 60, 199–209. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Robinson, D.H. A bioresorbable, polylactide reservoir for diffusional and osmotically controlled drug delivery. AAPS PharmSciTech 2000, 1, 26–34. [Google Scholar] [CrossRef]
- Frank, A.; Kumar Rath, S.; Boey, F.; Venkatraman, S. Study of the initial stages of drug release from a degradable matrix of poly(d,l-lactide-co-glycolide). Biomaterials 2004, 25, 813–821. [Google Scholar] [CrossRef]
- Hurrell, S.; Cameron, R.E. The effect of initial polymer morphology on the degradation and drug release from polyglycolide. Biomaterials 2002, 23, 2401–2409. [Google Scholar] [CrossRef]
- Grassi, M.; Grassi, G. Mathematical modelling and controlled drug delivery: Matrix systems. Curr. Drug Deliv. 2005, 2, 97–116. [Google Scholar] [CrossRef]
- Chen, M.; Liang, H.; Chiu, Y.; Chang, Y.; Wei, H.; Sung, H. A novel drug-eluting stent spray-coated with multi-layers of collagen and sirolimus. J. Control. Release 2005, 108, 178–189. [Google Scholar] [CrossRef]
- Acharya, G.; Park, K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006, 58, 387–401. [Google Scholar] [CrossRef]
- Pan, C.J.; Tang, J.J.; Weng, Y.J.; Wang, J.; Huang, N. Preparation, characterization and anticoagulation of curcumin-eluting controlled biodegradable coating stents. J. Control. Release 2006, 116, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, K.; Kramer, S.; Nischan, C.; Grabow, N.; Langer, T.; Hennighausen, G.; Schmitz, K. In vitro study of drug-eluting stent coatings based on poly (L-lactide) incorporating cyclosporine A–drug release, polymer degradation and mechanical integrity. J. Mater. Sci. Mater. Med. 2007, 18, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Saylor, D.M.; Kim, C.; Patwardhan, D.V.; Warren, J.A. Modeling microstructure development and release kinetics in controlled drug release coatings. J. Pharm. Sci. 2009, 98, 169–186. [Google Scholar] [CrossRef]
- Alexis, F.; Venkatraman, S.S.; Rath, S.K.; Boey, F. In vitro study of release mechanisms of paclitaxel and rapamycin from drug-incorporated biodegradable stent matrices. J. Control. Release 2004, 98, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.; McClean, D.; Kar, S.; Takizawa, K.; Varghese, K.; Baek, N.; Park, K.; Fishbein, M.C.; Makkar, R.; Litvack, F. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation 2003, 107, 777–784. [Google Scholar] [CrossRef]
- Raval, A.; Choubey, A.; Engineer, C.; Kotadia, H.; Kothwala, D. Novel biodegradable polymeric matrix coated cardiovascular stent for controlled drug delivery. Trends Biomater. Artif. Organs 2007, 20, 101–110. [Google Scholar]
- Balakrishnan, B.; Dooley, J.F.; Kopia, G.; Edelman, E.R. Intravascular drug release kinetics dictate arterial drug deposition, retention, and distribution. J. Control. Release 2007, 123, 100–108. [Google Scholar] [CrossRef]
- Leon, M.; Abizaid, A.; Moses, J. The Cypher Stent: A New Gold Standard in the Treatment of Coronary Artery Disease; The Cardiovascular Research Foundation: New York, NY, USA, 2003. [Google Scholar]
- Otsuka, Y.; Chronos, N.A.; Apkarian, R.P.; Robinson, K.A. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: Comparison of BiodivYsio, Taxus and Cypher stents. J. Invasive Cardiol. 2007, 19, 71–76. [Google Scholar]
- Rajurkar, R.M.; Rathod, C.P.; Thonte, S.S.; Sugave, R.V.; Sugave, B.K.; Phadtare, A.A.; Bhosale, P.H. Gastroretentive Mucoadhesive Microsphere As Carriers In Drug Delivery: A Review. Indo Am. J. Pharm. Res. 2013, 3, 2751–2777. [Google Scholar]
- Albrektsson, T.; Jemt, T.; Mölne, J.; Tengvall, P.; Wennerberg, A. On inflammation-immunological balance theory—A critical apprehension of disease concepts around implants: Mucositis and marginal bone loss may represent normal conditions and not necessarily a state of disease. Clin. Implant Dent. Relat. Res. 2019, 21, 183–189. [Google Scholar] [CrossRef]
- Yeo, I.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2020, 13, 89. [Google Scholar] [CrossRef]
- Lavenus, S.; Rozé, J.; Hoornaert, A.; Louarn, G.; Layrolle, P. Chapter 5-Impact of Nanotechnology on Dental Implants. Emerg. Nanotechnol. Dent. 2012, 71–84. [Google Scholar] [CrossRef]
- Subramani, K.; Ahmed, W.; Pachauri, P. Chapter 7-Titanium nanotubes as carriers of osteogenic growth factors and antibacterial drugs for applications in dental implantology. Emerg. Nanotechnol. Dent. 2018, 125–136. [Google Scholar] [CrossRef]
- Sakka, S.; Coulthard, P. Implant failure: Etiology and complications. Med. Oral Patol. Oral Cir. Bucal 2011, 16, 42–44. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Liu, N.; Xu, X.; Qu, X.; Lu, E. Smoking, radiotherapy, diabetes and osteoporosis as risk factors for dental implant failure: A meta-analysis. PLoS ONE 2013, 8, e71955. [Google Scholar] [CrossRef] [PubMed]
- Baqain, Z.H.; Moqbel, W.Y.; Sawair, F.A. Early dental implant failure: Risk factors. Br. J. Oral Maxillofac. Surg. 2012, 50, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Vohra, F.; Zafar, S.; Almas, K. Significance of Osteogenic Surface Coatings on Implants to Enhance Osseointegration Under Osteoporotic-like Conditions. Implant Dent. 2014, 23, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Welter, J.F.; Goldberg, V.M. Effect of hydroxyapatite/tricalcium-phosphate coating on osseointegration of plasma-sprayed titanium alloy implants. J. Biomed. Mater. Res. Part A 2004, 69, 1–10. [Google Scholar] [CrossRef]
- Alzubaydi, T.L.; AlAmeer, S.S.; Ismaeel, T.; AlHijazi, A.Y.; Geetha, M. In vivo studies of the ceramic coated titanium alloy for enhanced osseointegration in dental applications. J. Mater. Sci. Mater. Med. 2009, 20, 35. [Google Scholar] [CrossRef]
- Zafar, M.S.; Al-Samadani, K.H. Potential use of natural silk for bio-dental applications. J. Taibah Univ. Med. Sci. 2014, 9, 171–177. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Tsoi, J.K.; de Vries, J.; Busscher, H.J. Characterization of novel silane coatings on titanium implant surfaces. Clin. Oral Implant. Res. 2013, 24, 688–697. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Parr, G.R.; Gardner, L.K.; Toth, R.W. Titanium: The mystery metal of implant dentistry. Dental materials aspects. J. Prosthet. Dent. 1985, 54, 410–414. [Google Scholar] [CrossRef]
- Smith, D.C. Dental implants: Materials and design considerations. Int. J. Prosthodont. 1993, 6, 106–117. [Google Scholar] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial–cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef]
- Bruschi, M.; Steinmüller-Nethl, D.; Goriwoda, W.; Rasse, M. Composition and modifications of dental implant surfaces. J. Oral Implant. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Steinmüller-Nethl, D.; Kloss, F.R.; Najam-Ul-Haq, M.; Rainer, M.; Larsson, K.; Linsmeier, C.; Köhler, G.; Fehrer, C.; Lepperdinger, G.; Liu, X.; et al. Strong binding of bioactive BMP-2 to nanocrystalline diamond by physisorption. Biomaterials 2006, 27, 4547–4556. [Google Scholar] [CrossRef]
- Mistry, S.; Kundu, D.; Datta, S.; Basu, D. Comparison of bioactive glass coated and hydroxyapatite coated titanium dental implants in the human jaw bone. Aust. Dent. J. 2011, 56, 68–75. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Abduljabbar, T.; Vohra, F.; Malignaggi, V.R.; Malmstrom, H.; Romanos, G.E.; Javed, F. Role of local alendronate delivery on the osseointegration of implants: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, B.; Barca, A.; Bogani, F.; Boniardi, M.; Carlino, P.; Mele, C.; Verri, T.; Romano, A. Electrodeposition of nanostructured bioactive hydroxyapatite-heparin composite coatings on titanium for dental implant applications. J. Mater. Sci. Mater. Med. 2014, 25, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zeng, Q.; Lingling, E.; Wang, D.; He, H.; Liu, H. Sustained Topical Delivery of Insulin From Fibrin Gel Loaded With Poly(Lactic-Co-Glycolic Acid) Microspheres Improves the Biomechanical Retention of Titanium Implants in Type 1 Diabetic Rats. J. Oral Maxillofac. Surg. 2012, 70, 2299–2308. [Google Scholar] [CrossRef]
- Wang, F.; Song, Y.; Li, C.; Li, D.; Zhang, H.; Ma, A.; Xi, X.; Zhang, N.; Wang, B.; Wang, Y.; et al. Sustained release of insulin-like growth factor-1 from poly(lactide-co-glycolide) microspheres improves osseointegration of dental implants in type 2 diabetic rats. Eur. J. Pharmacol. 2010, 640, 226–232. [Google Scholar] [CrossRef]
- Hanisch, O.; Sorensen, R.G.; Kinoshita, A.; Spiekermann, H.; Wozney, J.M.; Wikesjö, U.M. Effect of recombinant human bone morphogenetic protein-2 in dehiscence defects with non-submerged immediate implants: An experimental study in Cynomolgus monkeys. J. Periodontol. 2003, 74, 648–657. [Google Scholar] [CrossRef]
- Wen, B.; Karl, M.; Pendrys, D.; Shafer, D.; Freilich, M.; Kuhn, L. An evaluation of BMP-2 delivery from scaffolds with miniaturized dental implants in a novel rat mandible model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 315–326. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, L.; Peel, S.; Yang, G.; Zhao, S.; He, F. Bone response to the multilayer BMP-2 gene coated porous titanium implant surface. Clin. Oral Implant. Res. 2013, 24, 853–861. [Google Scholar] [CrossRef]
- Sykaras, N.; Iacopino, A.M.; Triplett, R.G.; Marker, V.A. Effect of recombinant human bone morphogenetic protein-2 on the osseointegration of dental implants: A biomechanics study. Clin. Oral Investig. 2004, 8, 196–205. [Google Scholar] [CrossRef]
- Schuckert, K.; Jopp, S.; Muller, U. De novo grown bone on exposed implant surfaces using photodynamic therapy and recombinant human bone morphogenetic protein-2: Case report. Implant Dent. 2006, 15, 361–365. [Google Scholar] [CrossRef]
- Pokrowiecki, R. The paradigm shift for drug delivery systems for oral and maxillofacial implants. Drug Deliv. 2018, 25, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, C.; Hung, C.; Kao, C. Comparison of antibacterial activities of root-end filling materials by an agar diffusion assay and Alamar blue assay. J. Dent. Sci. 2012, 7, 336–341. [Google Scholar] [CrossRef]
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cell. Mater. 2006, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Kloss, F.R.; Singh, S.; Hächl, O.; Rentenberger, J.; Auberger, T.; Kraft, A.; Klima, G.; Mitterlechner, T.; Steinmüller-Nethl, D.; Lethaus, B. BMP-2 immobilized on nanocrystalline diamond–coated titanium screws; demonstration of osteoinductive properties in irradiated bone. Head Neck 2013, 35, 235–241. [Google Scholar] [CrossRef]
- Liu, Y.; Huse, R.O.; de Groot, K.; Buser, D.; Hunziker, E.B. Delivery mode and efficacy of BMP-2 in association with implants. J. Dent. Res. 2007, 86, 84–89. [Google Scholar] [CrossRef]
- Pye, A.; Lockhart, D.; Dawson, M.; Murray, C.; Smith, A. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.; Kang, E.; Poh, C.K.; Wang, W. Surface functionalization of titanium with carboxymethyl chitosan and immobilized bone morphogenetic protein-2 for enhanced osseointegration. Biomacromolecules 2009, 10, 1603–1611. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. 2009, 91B, 470–480. [Google Scholar] [CrossRef]
- McLean, K.M.; McArthur, S.L.; Chatelier, R.C.; Kingshott, P.; Griesser, H.J. Hybrid biomaterials: Surface-MALDI mass spectrometry analysis of covalent binding versus physisorption of proteins. Colloids Surf. B Biointerfaces 2000, 17, 23–35. [Google Scholar] [CrossRef]
- De Giglio, E.; De Gennaro, L.; Sabbatini, L.; Zambonin, G. Analytical characterization of collagen- and/or hydroxyapatite-modified polypyrrole films electrosynthesized on Ti-substrates for the development of new bioactive surfaces. J. Biomater. Sci. Polym. Ed. 2001, 12, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tanihara, M.; Suzuki, K.; Saitou, A.; Sufan, W.; Nishimura, Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J. Biomed. Mater. Res. 2000, 50, 405–409. [Google Scholar] [CrossRef]
- Wysocki, B.; Idaszek, J.; Zdunek, J.; Rożniatowski, K.; Pisarek, M.; Yamamoto, A.; Święszkowski, W. The influence of selective laser melting (SLM) process parameters on in-vitro cell response. Int. J. Mol. Sci. 2018, 19, 1619. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Li, J.; Zhou, X.; Wang, A. Structure and properties of a personalized bio-fixed implant prepared with selective laser melting. Comput. Methods Biomech. Biomed. Engin. 2019, 22, 1034–1042. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chen, F.; Ge, Y.J.; Wei, L.; Pan, S.X.; Feng, H.L. Influence of implants prepared by selective laser melting on early bone healing. Beijing Da Xue Xue Bao Yi Xue Ban 2018, 50, 117–122. [Google Scholar]
- Mroz, W.; Budner, B.; Syroka, R.; Niedzielski, K.; Golański, G.; Slosarczyk, A.; Schwarze, D.; Douglas, T.E. In vivo implantation of porous titanium alloy implants coated with magnesium-doped octacalcium phosphate and hydroxyapatite thin films using pulsed laser depostion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 151–158. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; JÄ™drzejewski, T.; Sadowska, B.; WiÄ™ckowska-Szakiel, M.; Holopainen, J.; Ritala, M.; Leskelä, M.; Bartmański, M.; Szkodo, M. Titania nanotubes/hydroxyapatite nanocomposites produced with the use of the atomic layer deposition technique: Estimation of bioactivity and nanomechanical properties. Nanomaterials 2019, 9, 123. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Ripamonti, U.; Roden, L.C.; Renton, L.F. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 2012, 33, 3813–3823. [Google Scholar] [CrossRef]
- Gotfredsen, K. Implant coatings and its application in clinical reality. In Implant Surfaces and Their Biological and Clinical Impact; Springer: Berlin/Heidelberg, Germany, 2015; pp. 147–155. [Google Scholar]
- Yeo, I. Surface modification of dental biomaterials for controlling bone response. Bone Response Dent. Implant Mater. 2017, 43–64. [Google Scholar] [CrossRef]
- Kunrath, M.; Hübler, R. A bone preservation protocol that enables evaluation of osseointegration of implants with micro-and nanotextured surfaces. Biotech. Histochem. 2019, 94, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagam, D.; Oyewumi, M.O. Critical assessment of implantable drug delivery devices in glaucoma management. J. Drug Deliv. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, L.; Yu, Y.; Li, W.; Zhi, J. Synthesis and characterization of diamond–silver composite with anti-bacterial property. Mater. Lett. 2014, 114, 92–95. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Agwan, A.S.; Zafar, M.S.; Alrahabi, M.; Qasim, S.B.; Sefat, F. Dental Applications of Nanodiamonds. Sci. Adv. Mater. 2016, 8, 2064–2070. [Google Scholar] [CrossRef]
- Gonçalves, J.P.L.; Shaikh, A.Q.; Reitzig, M.; Kovalenko, D.A.; Michael, J.; Beutner, R.; Cuniberti, G.; Scharnweber, D.; Opitz, J. Detonation nanodiamonds biofunctionalization and immobilization to titanium alloy surfaces as first steps towards medical application. Beilstein J. Org. Chem. 2014, 10, 2765–2773. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Lee, D.; Choi, K.S.; Lim, H.; Kim, J. Graphene as an enabling strategy for dental implant and tissue regeneration. Tissue Eng. Regen. Med. 2017, 14, 481–493. [Google Scholar] [CrossRef]
- Kaviya, M.; Ramakrishnan, P.; Mohamed, S.; Ramakrishnan, R.; Gimbun, J.; Veerabadran, K.; Kuppusamy, M.; Kaviyarasu, K.; Sridhar, T. Synthesis and characterization of nano-hydroxyapatite/graphene oxide composite materials for medical implant coating applications. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Kaya, C.; Singh, I.; Boccaccini, A.R. Multi-walled carbon nanotube-reinforced hydroxyapatite layers on Ti6Al4V medical implants by Electrophoretic Deposition (EPD). Adv. Eng. Mater. 2008, 10, 131–138. [Google Scholar] [CrossRef]
- Hirata, E.; Uo, M.; Takita, H.; Akasaka, T.; Watari, F.; Yokoyama, A. Multiwalled carbon nanotube-coating of 3D collagen scaffolds for bone tissue engineering. Carbon 2011, 49, 3284–3291. [Google Scholar] [CrossRef]
- Hahn, B.; Lee, J.; Park, D.; Choi, J.; Ryu, J.; Yoon, W.; Lee, B.; Shin, D.; Kim, H. Mechanical and in vitro biological performances of hydroxyapatite–carbon nanotube composite coatings deposited on Ti by aerosol deposition. Acta Biomater. 2009, 5, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Abe, S.; Akasaka, T.; Uo, M.; Kitagawa, Y.; Watari, F. Multiwalled carbon nanotube coating on titanium. Biomed. Mater. Eng. 2009, 19, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Arciola, C.; Bertoglio, F.; Curtosi, S.; Dacarro, G.; D’Agostino, A.; Ferrari, F.; Merli, D.; Milanese, C.; Rossi, S. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J. Colloid Interface Sci. 2017, 498, 271–281. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K.; Wolke, J.G.C.; Jansen, J.A. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 137–147. [Google Scholar] [CrossRef]
- Kay, J.F. Calcium phosphate coatings for dental implants. Current status and future potential. Dent. Clin. N. Am. 1992, 36, 1–18. [Google Scholar]
- Alghamdi, H.S.; Cuijpers, V.M.J.I.; Wolke, J.G.C.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium-phosphate-coated Oral Implants Promote Osseointegration in Osteoporosis. J. Dent. Res. 2013, 92, 982–988. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.; Wolke, J.G.; Siebers, M.C.; Schoonman, J.; Jansen, J.A. In vitro and in vivo reactivity of porous, electrosprayed calcium phosphate coatings. Biomaterials 2006, 27, 3368–3378. [Google Scholar] [CrossRef]
- Bjursten, L.M.; Rasmusson, L.; Oh, S.; Smith, G.C.; Brammer, K.S.; Jin, S. Titanium dioxide nanotubes enhance bone bonding in vivo. J. Biomed. Mater. Res. Part A 2010, 92, 1218–1224. [Google Scholar]
- Hilbig, H.; Kirsten, M.; Rupietta, R.; Graf, H.; Tbalbammer, S.; Strasser, S.; Armbruster, F. Implant surface coatings with bone sialoprotein, collagen, and fibronectin and their effects on cells derived from human maxillar bone. Eur. J. Med. Res. 2007, 12, 6. [Google Scholar]
- Raphel, J.; Karlsson, J.; Galli, S.; Wennerberg, A.; Lindsay, C.; Haugh, M.G.; Pajarinen, J.; Goodman, S.B.; Jimbo, R.; Andersson, M.; et al. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials 2016, 83, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Huck, O.; Frisch, B.; Vautier, D.; Elkaim, R.; Voegel, J.; Brunel, G.; Tenenbaum, H. The effect of microstructured surfaces and laminin-derived peptide coatings on soft tissue interactions with titanium dental implants. Biomaterials 2009, 30, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- El-Ghannam, A.; Starr, L.; Jones, J. Laminin-5 coating enhances epithelial cell attachment, spreading, and hemidesmosome assembly on Ti-6Al-4V implant material in vitro. J. Biomed. Mater. Res. 1998, 41, 30–40. [Google Scholar] [CrossRef]
- Graf, H.L.; Stoeva, S.; Armbruster, F.P.; Neuhaus, J.; Hilbig, H. Effect of bone sialoprotein and collagen coating on cell attachment to TICER® and pure titanium implant surfaces. Int. J. Oral Maxillofac. Surg. 2008, 37, 634–640. [Google Scholar] [CrossRef]
- Fiorellini, J.P.; Glindmann, S.; Salcedo, J.; Weber, H.; Park, C.; Sarmiento, H.L. The effect of osteopontin and an osteopontin-derived synthetic peptide coating on osseointegration of implants in a canine model. Int. J. Periodontics Restor. Dent. 2016, 36, e88–e94. [Google Scholar] [CrossRef]
- Lee, D.; Yun, Y.; Park, K.; Kim, S.E. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone 2012, 50, 974–982. [Google Scholar] [CrossRef]
- Liu, Y.; de Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [CrossRef]
- Aebli, N.; Stich, H.; Schawalder, P.; Theis, J.; Krebs, J. Effects of bone morphogenetic protein-2 and hyaluronic acid on the osseointegration of hydroxyapatite-coated implants: An experimental study in sheep. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 73, 295–302. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, W.; Tu, K.; Huang, Z.; Zhou, Q.; Sun, T.; Lv, Y.; Cui, W.; Yang, L. The effects of combined human parathyroid hormone (1–34) and simvastatin treatment on osseous integration of hydroxyapatite-coated titanium implants in the femur of ovariectomized rats. Injury 2015, 46, 2164–2169. [Google Scholar] [CrossRef]
- Ardura, J.A.; Portal-Núñez, S.; Lozano, D.; Gutiérrez-Rojas, I.; Sánchez-Salcedo, S.; López-Herradón, A.; Mulero, F.; Villanueva-Peñacarrillo, M.L.; Vallet-Regí, M.; Esbrit, P. Local delivery of parathyroid hormone-related protein-derived peptides coated onto a hydroxyapatite-based implant enhances bone regeneration in old and diabetic rats. J. Biomed. Mater. Res. Part A 2016, 104, 2060–2070. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Jiang, X.; Rowe, D.; Wei, M. Biomimetic CaP coating incorporated with parathyroid hormone improves the osseointegration of titanium implant. J. Mater. Sci. Mater. Med. 2012, 23, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, N.; Chen, S.; Lu, R.; Li, H.; Zhang, Z. Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide. Int. J. Nanomed. 2017, 12, 2995–3007. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hirt, H.; Li, Y.; Gorr, S.U.; Aparicio, C. Antimicrobial GL13K peptide coatings killed and ruptured the wall of Streptococcus gordonii and prevented formation and growth of biofilms. PLoS ONE 2014, 9, e111579. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Waite, J.H.; Tirrell, M. Promotion of osteoblast proliferation on complex coacervation-based hyaluronic acid – recombinant mussel adhesive protein coatings on titanium. Biomaterials 2010, 31, 1080–1084. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Gerard, P.D.; Bergin, P.; Chestnutt, B.; Marini, M.; Ramsey, V.; Elder, S.H.; Gilbert, J.A. Chitosan: Potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J. Biomater. Sci. Polym. Ed. 2003, 14, 423–438. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Kokkonen, H.; Cassinelli, C.; Verhoef, R.; Morra, M.; Schols, H.; Tuukkanen, J. Differentiation of osteoblasts on pectin-coated titanium. Biomacromolecules 2008, 9, 2369–2376. [Google Scholar] [CrossRef]
- Kokkonen, H.; Niiranen, H.; Schols, H.A.; Morra, M.; Stenbäck, F.; Tuukkanen, J. Pectin-coated titanium implants are well-tolerated in vivo. J. Biomed. Mater. Res. Part A 2010, 93, 1404–1409. [Google Scholar] [CrossRef]
- Abtahi, J.; Agholme, F.; Sandberg, O.; Aspenberg, P. Effect of Local vs. Systemic Bisphosphonate Delivery on Dental Implant Fixation in a Model of Osteonecrosis of the Jaw. J. Dent. Res. 2013, 92, 279–283. [Google Scholar] [CrossRef]
- Zuffetti, F.; Bianchi, F.; Volpi, R.; Trisi, P.; Del Fabbro, M.; Capelli, M.; Galli, F.; Capsoni, F.; Testori, T. Clinical application of bisphosphonates in implant dentistry: Histomorphometric evaluation. Int. J. Periodontics Restor. Dent. 2009, 29, 31–39. [Google Scholar]
- Yang, G.; Song, L.; Guo, C.; Zhao, S.; Liu, L.; He, F. Bone responses to simvastatin-loaded porous implant surfaces in an ovariectomized model. Int. J. Oral Maxillofac. Implant. 2012, 27, 369–374. [Google Scholar]
- Du, Z.; Chen, J.; Yan, F.; Xiao, Y. Effects of Simvastatin on bone healing around titanium implants in osteoporotic rats. Clin. Oral Implant. Res. 2009, 20, 145–150. [Google Scholar] [CrossRef]
- Maïmoun, L.; Brennan, T.C.; Badoud, I.; Dubois-Ferriere, V.; Rizzoli, R.; Ammann, P. Strontium ranelate improves implant osseointegration. Bone 2010, 46, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.P.; Raschke, M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef]
- Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol–gel films on titanium alloy fracture plate material. Biomaterials 2007, 28, 1721–1729. [Google Scholar] [CrossRef]
- Adams, C.S.; Antoci, V., Jr.; Harrison, G.; Patal, P.; Freeman, T.A.; Shapiro, I.M.; Parvizi, J.; Hickok, N.J.; Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J. Orthop. Res. 2009, 27, 701–709. [Google Scholar] [CrossRef]
- Antoci, V., Jr.; King, S.B.; Jose, B.; Parvizi, J.; Zeiger, A.R.; Wickstrom, E.; Freeman, T.A.; Composto, R.J.; Ducheyne, P.; Shapiro, I.M. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J. Orthop. Res. 2007, 25, 858–866. [Google Scholar] [CrossRef]
- Xing, R.; Witsø, I.L.; Jugowiec, D.; Tiainen, H.; Shabestari, M.; Lyngstadaas, S.P.; Lönn-Stensrud, J.; Haugen, H.J. Antibacterial effect of doxycycline-coated dental abutment surfaces. Biomed. Mater. 2015, 10, 055003. [Google Scholar] [CrossRef]
- Ferreira, C.F.; Babu, J.; Hamlekhan, A.; Patel, S.; Shokuhfar, T. Efficiency of Nanotube Surface-Treated Dental Implants Loaded with Doxycycline on Growth Reduction of Porphyromonas gingivalis. Int. J. Oral Maxillofac. Implant. 2017, 32, 322–328. [Google Scholar] [CrossRef]
- Baghdan, E.; Pinnapireddy, S.R.; Vögeling, H.; Schäfer, J.; Eckert, A.W.; Bakowsky, U. Nano spray drying: A novel technique to prepare well-defined surface coatings for medical implants. J. Drug Deliv. Sci. Technol. 2018, 48, 145–151. [Google Scholar] [CrossRef]
- Vitti, R.P.; Pacheco, R.R.; Silva, E.J.N.L.; Prati, C.; Gandolfi, M.G.; Piva, E.; Ogliari, F.A.; Zanchi, C.H.; Sinhoreti, M.A.C. Addition of phosphates and chlorhexidine to resin-modified MTA materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2195–2201. [Google Scholar] [CrossRef]
- Nasar, A. Hydroxyapatite and its coatings in dental implants. In Applications of Nanocomposite Materials in Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–160. [Google Scholar]
- Xiong, J.Y.; Li, Y.C.; Hodgson, P.D.; Wen, C.E. Bioactive hydroxyapatite coating on titanium-niobium alloy through a sol-gel process. In Materials Science Forum; Trans Tech Publ.: Zurich, Switzerland, 2009; Volume 618, pp. 325–328. [Google Scholar]
- Visentin, F.; El Habra, N.; Fabrizio, M.; Brianese, N.; Gerbasi, R.; Nodari, L.; Zin, V.; Galenda, A. TiO2-HA bi-layer coatings for improving the bioactivity and service-life of Ti dental implants. Surf. Coat. Technol. 2019, 378, 125049. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. Osteoblast proliferation on neat and apatite-like calcium phosphate-coated titanium foam scaffolds. Mater. Sci. Eng. C 2007, 27, 432–440. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B.; Matinlinna, J.P.; Conway, R.C. Current perspectives: Calcium phosphate nanocoatings and nanocomposite coatings in dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Choi, A.H. Sol-gel production of bioactive nanocoatings for medical applications. Part 1: An introduction. Nanomedicine 2006, 1, 311–319. [Google Scholar] [CrossRef]
- Lin, O.C.; Chao, E. Perspectives on Biomaterials: Proceedings of the 1985 International Symposium on Biomaterials, Taipei, Taiwan, 25–27 February 1985; Elsevier Publishing Company: Amsterdam, The Netherlands, 1986; Volume 33. [Google Scholar]
- Morris, H.F.; Ochi, S.; Spray, J.R.; Olson, J.W. Periodontal-Type Measurements Associated With Hydroxyapatite-Coated and Non—HA-Coated Implants: Uncovering to 36 Months. Ann. Periodontol. 2000, 5, 56–67. [Google Scholar] [CrossRef]
- Habibovic, P.; Li, J.; Van Der Valk, C.M.; Meijer, G.; Layrolle, P.; Van Blitterswijk, C.A.; De Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36. [Google Scholar] [CrossRef]
- Barrere, F.; Van Der Valk, C.; Meijer, G.; Dalmeijer, R.; De Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Yarramaneni, V.; Aparna, I.; Sachdeva, A.; Balakrishnan, D.; Prabhu, N. Emerging antibacterial coated dental implants: A preventive measure for peri-implantitis. World J. Dent. 2016, 7, 195–198. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Karacan, I.; Ben-Nissan, B.; Wang, H.A.; Juritza, A.; Swain, M.V.; Müller, W.H.; Chou, J.; Stamboulis, A.; Macha, I.J.; Taraschi, V. Mechanical testing of antimicrobial biocomposite coating on metallic medical implants as drug delivery system. Mater. Sci. Eng. C 2019, 104, 109757. [Google Scholar] [CrossRef]
- De Avila, E.D.; Castro, A.G.; Tagit, O.; Krom, B.P.; Löwik, D.; van Well, A.A.; Bannenberg, L.J.; Vergani, C.E.; van den Beucken, J.J. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl. Surf. Sci. 2019, 488, 194–204. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An antimicrobial dental light curable bioadhesive hydrogel for treatment of peri-implant diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef]
- Water, J.J.; Bohr, A.; Boetker, J.; Aho, J.; Sandler, N.; Nielsen, H.M.; Rantanen, J. Three-dimensional printing of drug-eluting implants: Preparation of an antimicrobial polylactide feedstock material. J. Pharm. Sci. 2015, 104, 1099–1107. [Google Scholar] [CrossRef]
- De Cremer, K.; Braem, A.; Gerits, E.; De Brucker, K.; Vandamme, K.; Martens, J.A.; Michiels, J.; Vleugels, J.; Cammue, B.P.; Thevissen, K. Controlled release of chlorhexidine from a mesoporous silica-containing macroporous titanium dental implant prevents microbial biofilm formation. Eur. Cell. Mater. 2017, 33, 13–27. [Google Scholar] [CrossRef]
- Baghdan, E.; Raschpichler, M.; Lutfi, W.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Nano spray dried antibacterial coatings for dental implants. Eur. J. Pharm. Biopharm. 2019, 139, 59–67. [Google Scholar] [CrossRef]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials 2007, 28, 4880–4888. [Google Scholar] [CrossRef]
- Cox, S.C.; Jamshidi, P.; Eisenstein, N.M.; Webber, M.A.; Hassanin, H.; Attallah, M.M.; Shepherd, D.E.; Addison, O.; Grover, L.M. Adding functionality with additive manufacturing: Fabrication of titanium-based antibiotic eluting implants. Mater. Sci. Eng. C 2016, 64, 407–415. [Google Scholar] [CrossRef]
- Swanson, T.; Cheng, X.; Friedrich, C. Development of chitosan–vancomycin antimicrobial coatings on titanium implants. J. Biomed. Mater. Res. Part A 2011, 97, 167–176. [Google Scholar] [CrossRef]
- Rojas-Montoya, I.D.; Fosado-Esquivel, P.; Henao-Holguín, L.V.; Esperanza-Villegas, A.E.; Bernad-Bernad, M.; Gracia-Mora, J. Adsorption/desorption studies of norfloxacin on brushite nanoparticles from reverse microemulsions. Adsorption 2019, 1–10. [Google Scholar] [CrossRef]
- Russell, A.D.; Day, M.J. Antibacterial activity of chlorhexidine. J. Hosp. Infect. 1993, 25, 229–238. [Google Scholar] [CrossRef]
- Wood, N.J.; Jenkinson, H.F.; Davis, S.A.; Mann, S.; O’Sullivan, D.J.; Barbour, M.E. Chlorhexidine hexametaphosphate nanoparticles as a novel antimicrobial coating for dental implants. J. Mater. Sci. Mater. Med. 2015, 26, 201. [Google Scholar] [CrossRef]
- Cortizo, M.C.; Oberti, T.G.; Cortizo, M.S.; Cortizo, A.M.; de Mele, M.A.F.L. Chlorhexidine delivery system from titanium/polybenzyl acrylate coating: Evaluation of cytotoxicity and early bacterial adhesion. J. Dent. 2012, 40, 329–337. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Bauab, T.M.; Hernandes, A.C.; de Souza Rastelli, A.N. Titanium dioxide and modified titanium dioxide by silver nanoparticles as an anti biofilm filler content for composite resins. Dent. Mater. 2019, 35, e36–e46. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. In vitro antimicrobial and biological properties of laser assisted tricalcium phosphate coating on titanium for load bearing implant. Mater. Sci. Eng. C 2009, 29, 1965–1968. [Google Scholar] [CrossRef] [PubMed]
- Rottmar, M.; Müller, E.; Guimond-Lischer, S.; Stephan, M.; Berner, S.; Maniura-Weber, K. Assessing the osteogenic potential of zirconia and titanium surfaces with an advanced in vitro model. Dent. Mater. 2019, 35, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Sanpo, N.; Ang, S.M.; Cheang, P.; Khor, K.A. Antibacterial Property of Cold Sprayed Chitosan-Cu/Al Coating. J. Therm. Spray Technol. 2009, 18, 600. [Google Scholar] [CrossRef]
- Xie, Y.; Li, H.; Ding, C.; Zheng, X.; Li, K. Effects of graphene plates’ adoption on the microstructure, mechanical properties, and in vivo biocompatibility of calcium silicate coating. Int. J. Nanomed. 2015, 10, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pei, X.; Yang, S.; Qin, H.; Cai, H.; Hu, S.; Sui, L.; Wan, Q.; Wang, J. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coat. Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Neitzel, I.; Etzold, B.J.M.; Peterson, A.; Palmese, G.; Gogotsi, Y. Covalent incorporation of aminated nanodiamond into an epoxy polymer network. ACS Nano 2011, 5, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Vaitkuviene, A.; McDonald, M.; Vahidpour, F.; Noben, J.; Sanen, K.; Ameloot, M.; Ratautaite, V.; Kaseta, V.; Biziuleviciene, G.; Ramanaviciene, A. Impact of differently modified nanocrystalline diamond on the growth of neuroblastoma cells. New Biotechnol. 2015, 32, 7–12. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Ratautaite, V.; Ramanaviciene, A.; Sanen, K.; Paesen, R.; Ameloot, M.; Petrakova, V.; McDonald, M.; Vahidpour, F.; Kaseta, V. Impact of diamond nanoparticles on neural cells. Mol. Cell. Probes 2015, 29, 25–30. [Google Scholar] [CrossRef]
- Alcaide, M.; Taylor, A.; Fjorback, M.; Zachar, V.; Pennisi, C.P. Boron-doped nanocrystalline diamond electrodes for neural interfaces: In vivo biocompatibility evaluation. Front. Neurosci. 2016, 10, 87. [Google Scholar] [CrossRef]
- Mohan, N.; Chen, C.; Hsieh, H.; Wu, Y.; Chang, H. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett. 2010, 10, 3692–3699. [Google Scholar] [CrossRef]
- Liu, K.; Cheng, C.; Chang, C.; Chao, J. Biocompatible and detectable carboxylated nanodiamond on human cell. Nanotechnology 2007, 18, 325102. [Google Scholar] [CrossRef]
- Townsend, L.; Williams, R.L.; Anuforom, O.; Berwick, M.R.; Halstead, F.; Hughes, E.; Stamboulis, A.; Oppenheim, B.; Gough, J.; Grover, L. Antimicrobial peptide coatings for hydroxyapatite: Electrostatic and covalent attachment of antimicrobial peptides to surfaces. J. R. Soc. Interface 2017, 14, 20160657. [Google Scholar] [CrossRef]
- Frieden, T. Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013; pp. 1–114.
- Bazli, L.; Chahardehi, A.M.; Arsad, H.; Malekpouri, B.; Jazi, M.A.; Azizabadi, N. Factors influencing the failure of dental implants: A Systematic Review. J. Compos. Compd. 2020, 2, 18–25. [Google Scholar]
- Kelly, M.; Williams, R.; Aojula, A.; O’Neill, J.; Trzińscka, Z.; Grover, L.; Scott, R.A.; Peacock, A.F.; Logan, A.; Stamboulis, A. Peptide aptamers: Novel coatings for orthopaedic implants. Mater. Sci. Eng. C 2015, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Mali, M.; Yaqin, S.A.U.; Zafar, M.S.; Khurshid, Z.; Alwadaani, A.; Matinlinna, J.P. Dental implants materials and surface treatments. In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 581–598. [Google Scholar]
- Zhang, L.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral Antimicrobial Peptides: Types and Role in the Oral Cavity. Saudi Pharm. J. 2015, 24, 515–524. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Yahya I Asiri, F.; Mali, M.; Sannam Khan, R.; Sahibzada, H.A.; Zafar, M.S.; Faraz Moin, S.; Khan, E. Significance and Diagnostic Role of Antimicrobial Cathelicidins (LL-37) Peptides in Oral Health. Biomolecules 2017, 7, 80. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Rehman, R.; Rehman, I.U. Advances of Proteomic Sciences in Dentistry. Int. J. Mol. Sci. 2016, 17, 728. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.S.; Naseem, M.; Khan, R.S.; Najeeb, S. Human Oral Defensins Antimicrobial Peptides: A Future Promising Antimicrobial Drug. Curr. Pharm. Des. 2018, 24, 1130–1137. [Google Scholar] [CrossRef]
- Lepage, P.; Heckel, C.; Humbert, S.; Stahl, S.; Rautmann, G. Recombinant technology as an alternative to chemical peptide synthesis: Expression and characterization of HIV-1 Rev recombinant peptides. Anal. Biochem. 1993, 213, 40–48. [Google Scholar] [CrossRef]
- Coin, I.; Beyermann, M.; Bienert, M. Solid-phase peptide synthesis: From standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2007, 2, 3247–3256. [Google Scholar] [CrossRef]
- Javaid, M.A.; Khurshid, Z.; Zafar, M.S.; Najeeb, S. Immediate Implants: Clinical Guidelines for Esthetic Outcomes. Dent. J. 2016, 4, 21. [Google Scholar] [CrossRef]
- Yoshinari, M.; Matsuzaka, K.; Inoue, T. Surface modification by cold-plasma technique for dental implants—Bio-functionalization with binding pharmaceuticals. Jpn. Dent. Sci. Rev. 2011, 47, 89–101. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E.; Chen, X.; Gutierrez-Gonzalez, C.F.; Fernandez, A.; Lopez-Esteban, S.; Aparicio, C. Peptide-functionalized zirconia and new zirconia/titanium biocermets for dental applications. J. Dent. 2015, 43, 1162–1174. [Google Scholar] [CrossRef]
- Zhou, L.; Lai, Y.; Huang, W.; Huang, S.; Xu, Z.; Chen, J.; Wu, D. Biofunctionalization of microgroove titanium surfaces with an antimicrobial peptide to enhance their bactericidal activity and cytocompatibility. Colloids Surf. B Biointerfaces 2015, 128, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1–11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Fernández-Calderón, M.C.; Pérez-Giraldo, C.; Manero, J.M.; Albericio, F.; Gil, F.J.; Rodríguez, D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014, 10, 3522–3534. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z. Synthesis and Characterization of Antimicrobial Peptides for Medical and Dental Applications; University of Birmingham: Birmingham, England, 2015. [Google Scholar]
- Righino, B.; Pirolli, D.; Radicioni, G.; Marzano, V.; Longhi, R.; Arcovito, A.; Sanna, M.T.; De Rosa, M.C.; Paoluzi, S.; Cesareni, G. Structural studies and SH3 domain binding properties of a human antiviral salivary proline-rich peptide. Pept. Sci. 2016, 106, 714–725. [Google Scholar] [CrossRef]
- Warnke, P.H.; Voss, E.; Russo, P.A.; Stephens, S.; Kleine, M.; Terheyden, H.; Liu, Q. Antimicrobial peptide coating of dental implants: Biocompatibility assessment of recombinant human beta defensin-2 for human cells. Int. J. Oral Maxillofac. Implant. 2013, 28, 982–988. [Google Scholar] [CrossRef][Green Version]
- Wisdom, C.; Chen, C.; Yuca, E.; Zhou, Y.; Tamerler, C.; Snead, M.L. Repeatedly applied peptide film kills bacteria on dental implants. JOM 2019, 71, 1271–1280. [Google Scholar] [CrossRef]
- Abtahi, J.; Tengvall, P.; Aspenberg, P. Bisphosphonate coating might improve fixation of dental implants in the maxilla: A pilot study. Int. J. Oral Maxillofac. Surg. 2010, 39, 673–677. [Google Scholar] [CrossRef]
- Im, G.; Qureshi, S.A.; Kenney, J.; Rubash, H.E.; Shanbhag, A.S. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 2004, 25, 4105–4115. [Google Scholar] [CrossRef]
- Jobke, B.; Milovanovic, P.; Amling, M.; Busse, B. Bisphosphonate-osteoclasts: Changes in osteoclast morphology and function induced by antiresorptive nitrogen-containing bisphosphonate treatment in osteoporosis patients. Bone 2014, 59, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.C.; Stewart, M.; Roehm, M.; Schneider, G.B. Osteoporosis-like bone conditions affect osseointegration of implants. Int. J. Oral Maxillofac. Implant. 2004, 19, 687–694. [Google Scholar]
- Giro, G.; Chambrone, L.; Goldstein, A.; Rodrigues, J.A.; Zenobio, E.; Feres, M.; Figueiredo, L.C.; Cassoni, A.; Shibli, J.A. Impact of osteoporosis in dental implants: A systematic review. World J. Orthop. 2015, 6, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Curi, M.M.; Cossolin, G.S.; Koga, D.H.; Zardetto, C.; Christianini, S.; Feher, O.; Cardoso, C.L.; dos Santos, M.O. Bisphosphonate-related osteonecrosis of the jaws—An initial case series report of treatment combining partial bone resection and autologous platelet-rich plasma. J. Oral Maxillofac. Surg. 2011, 69, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A. Clinical efficacy of 1% alendronate gel in adjunct to mechanotherapy in the treatment of aggressive periodontitis: A randomized controlled clinical trial. J. Periodontol. 2012, 83, 19–26. [Google Scholar] [CrossRef]
- De Sarkar, A.; Singhvi, N.; Shetty, J.N.; Ramakrishna, T.; Shetye, O.; Islam, M.; Keerthy, H. The local effect of alendronate with intra-alveolar collagen sponges on post extraction alveolar ridge resorption: A clinical trial. J. Maxillofac. Oral Surg. 2015, 14, 344–356. [Google Scholar] [CrossRef]
- Levin, L.; Bryson, E.C.; Caplan, D.; Trope, M. Effect of topical alendronate on root resorption of dried replanted dog teeth. Dent. Traumatol. 2001, 17, 120–126. [Google Scholar] [CrossRef]
- Najeeb, S.; Siddiqui, F.; Khurshid, Z.; Zohaib, S.; Zafar, M.S.; Ansari, S.A. Effect of bisphosphonates on root resorption after tooth replantation–a systematic review. Dent. Traumatol. 2017, 33, 77–83. [Google Scholar] [CrossRef]
- Pura, J.A.; Bobyn, J.D.; Tanzer, M. Implant-delivered Alendronate Causes a Dose-dependent Response on Net Bone Formation Around Porous Titanium Implants in Canines. Clin. Orthop. Relat. Res. 2016, 474, 1224–1233. [Google Scholar] [CrossRef]
- Denissen, H.; Montanari, C.; Martinetti, R.; van Lingen, A.; van den Hooff, A. Alveolar bone response to submerged bisphosphonate-complexed hydroxyapatite implants. J. Periodontol. 2000, 71, 279–286. [Google Scholar] [CrossRef]
- Pyo, S.W.; Kim, Y.M.; Kim, C.S.; Lee, I.S.; Park, J.U. Bone formation on biomimetic calcium phosphate-coated and zoledronate-immobilized titanium implants in osteoporotic rat tibiae. Int. J. Oral Maxillofac. Implant. 2014, 29, 478–484. [Google Scholar] [CrossRef]
- Lee, S.; Oh, T.; Bae, T.; Lee, M.; Soh, Y.; Kim, B.; Kim, H.S. Effect of bisphosphonates on anodized and heat-treated titanium surfaces: An animal experimental study. J. Periodontol. 2011, 82, 1035–1042. [Google Scholar] [CrossRef]
- Karlsson, J.; Harmankaya, N.; Allard, S.; Palmquist, A.; Halvarsson, M.; Tengvall, P.; Andersson, M. Ex vivo alendronate localization at the mesoporous titania implant/bone interface. J. Mater. Sci. Mater. Med. 2015, 26, 1–8. [Google Scholar] [CrossRef]
- Pilathadka, S.; Vahalová, D.; Vosáhlo, T. The Zirconia: A new dental ceramic material. An overview. Prague Med. Rep. 2007, 108, 5–12. [Google Scholar] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Luthardt, R.; Holzhüter, M.; Sandkuhl, O.; Herold, V.; Schnapp, J.; Kuhlisch, E.; Walter, M. Reliability and properties of ground Y-TZP-zirconia ceramics. J. Dent. Res. 2002, 81, 487–491. [Google Scholar] [CrossRef]
- Bona, A.D.; Pecho, O.E.; Alessandretti, R. Zirconia as a dental biomaterial. Materials 2015, 8, 4978–4991. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.F.; Swain, M.V. Grain-size-dependent transformation behavior in polycrystalline tetragonal zirconia. J. Am. Ceram. Soc. 1992, 75, 493–502. [Google Scholar] [CrossRef]
- Nistor, L.; Grădinaru, M.; Rîcă, R.; Mărășescu, P.; Stan, M.; Manolea, H.; Ionescu, A.; Moraru, I. Zirconia use in dentistry-manufacturing and properties. Curr. Health Sci. J. 2019, 45, 28. [Google Scholar] [PubMed]
- Malkondu, Ö.; Tinastepe, N.; Akan, E.; Kazazoğlu, E. An overview of monolithic zirconia in dentistry. Biotechnol. Biotechnol. Equip. 2016, 30, 644–652. [Google Scholar] [CrossRef]
- Apratim, A.; Eachempati, P.; Krishnappa Salian, K.K.; Singh, V.; Chhabra, S.; Shah, S. Zirconia in dental implantology: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Di Carlo, F.; Quaranta, M.; Piattelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef]
- Kohal, R.J.; Weng, D.; Bächle, M.; Strub, J.R. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J. Periodontol. 2004, 75, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, Y.; Ichikawa, Y.; Nikai, H.; Tsuru, H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J. Prosthet. Dent. 1993, 69, 599–604. [Google Scholar] [CrossRef]
- Pekkan, G.; Pekkan, K.; Hatipoglu, M.G.; Tuna, S.H. Comparative radiopacity of ceramics and metals with human and bovine dental tissues. J. Prosthet. Dent. 2011, 106, 109–117. [Google Scholar] [CrossRef]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torriceni, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. J. Oral Maxillofac. Implant. 2002, 17, 793–798. [Google Scholar]
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed titanium as a bioinert metal—A review. Quintessence Int. 2005, 36, 523–530. [Google Scholar] [CrossRef]
- Madfa, A.A.; Al-Sanabani, F.A.; Al-Qudami, N.H.; Al-Sanabani, J.S.; Amran, A.G. Use of zirconia in dentistry: An overview. Open Biomater. J. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.; Kiattavorncharoen, S.; Lauer, H.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef]
- Bächle, M.; Butz, F.; Hübner, U.; Bakalinis, E.; Kohal, R.J. Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin. Oral Implant. Res. 2007, 18, 53–59. [Google Scholar] [CrossRef]
- Degidi, M.; Artese, L.; Scarano, A.; Perrotti, V.; Gehrke, P.; Piattelli, A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J. Periodontol. 2006, 77, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Größner-Schreiber, B.; Herzog, M.; Hedderich, J.; Dück, A.; Hannig, M.; Griepentrog, M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: An in vitro study. Clin. Oral Implant. Res. 2006, 17, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.; Mine, A.; Egusa, H.; Shimazu, T.; Ko, R.; Nakano, T.; Yatani, H. Allergic Reaction to Titanium-Made Fixed Dental Restorations: A Clinical Report. J. Prosthodont. 2014, 23, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Sterner, T.; Schutze, N.; Saxler, G.; Jakob, F.; Rader, C.P. Effects of clinically relevant alumina ceramic, zirconia ceramic and titanium particles of different sizes and concentrations on TNF-alpha release in a human macrophage cell line. Biomed. Tech. 2004, 49, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.S. Methods to improve osseointegration of dental implants in low quality (type-IV) bone: An overview. J. Funct. Biomater. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Stanford, C. Surface modifications of dental implants. Aust. Dent. J. 2008, 53, S26–S33. [Google Scholar] [CrossRef]
- Ferguson, S.J.; Langhoff, J.D.; Voelter, K.; von Rechenberg, B.; Scharnweber, D.; Bierbaum, S.; Schnabelrauch, M.; Kautz, A.R.; Frauchiger, V.M.; Mueller, T.L.; et al. Biomechanical comparison of different surface modifications for dental implants. Int. J. Oral Maxillofac. Implant. 2008, 23, 1037–1046. [Google Scholar]
- Gahlert, M.; Gudehus, T.; Eichhorn, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral Implant. Res. 2007, 18, 662–668. [Google Scholar] [CrossRef]
- Yang, Y.; Ong, J.L.; Tian, J. Deposition of highly adhesive ZrO2 coating on Ti and CoCrMo implant materials using plasma spraying. Biomaterials 2003, 24, 619–627. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Li, C.; Yin, K.; Hao, D.; Lan, J. Application of Plasma-Sprayed Zirconia Coating in Dental Implants: Study in Implants. J. Oral Implantol. 2018, 44, 102–109. [Google Scholar] [CrossRef]
- Shon, W.; Chung, S.H.; Kim, H.; Han, G.; Cho, B.; Park, Y. Peri-implant bone formation of non-thermal atmospheric pressure plasma–treated zirconia implants with different surface roughness in rabbit tibiae. Clin. Oral Implant. Res. 2014, 25, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; van Rienen, U.; Iglic, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar]
- Salem, N.A.; Abo Taleb, A.L.; Aboushelib, M.N. Biomechanical and histomorphometric evaluation of osseointegration of fusion-sputtered zirconia implants. J. Prosthodont. 2013, 22, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Wenz, H.J.; Bartsch, J.; Wolfart, S.; Kern, M. Osseointegration and clinical success of zirconia dental implants: A systematic review. Int. J. Prosthodont. 2008, 21, 27–36. [Google Scholar]
- Alam, M.N.; Anand, N.; Chandrasekaran, S.; Kovendhan, Y. Is primary stability the gold standard factor in implant success. Dent. Hypotheses 2014, 5, 70. [Google Scholar]
- Cobo-Vazquez, C.; Reininger, D.; Molinero-Mourelle, P.; Gonzalez-Serrano, J.; Guisado-Moya, B.; Lopez-Quiles, J. Effect of the lack of primary stability in the survival of dental implants. J. Clin. Exp. Dent. 2018, 10, e14–e19. [Google Scholar] [CrossRef]
- Esposito, M. Biological factors contributing to failures of osseointegrated oral implants. (l). Success criteria and epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants, (II). Etiopathogenesis. Eur. J. Oral Sci. 1998, 106, 721–764. [Google Scholar] [CrossRef]
- Filiaggi, M.J.; Coombs, N.A.; Pilliar, R.M. Characterization of the interface in the plasma-sprayed HA coating/Ti-6Al-4V implant system. J. Biomed. Mater. Res. 1991, 25, 1211–1229. [Google Scholar] [CrossRef]
- Munting, E. The contributions and limitations of hydroxyapatite coatings to implant fixation. Int. Orthop. 1996, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aebli, N.; Krebs, J.; Stich, H.; Schawalder, P.; Walton, M.; Schwenke, D.; Gruner, H.; Gasser, B.; Theis, J. In vivo comparison of the osseointegration of vacuum plasma sprayed titanium- and hydroxyapatite-coated implants. J. Biomed. Mater. Res. Part A 2003, 66A, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coat. Technol. 2011, 205, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. Part A 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.L. Eight-year clinical retrospective study of titanium plasma-sprayed and hydroxyapatite-coated cylinder implants. Int. J. Oral Maxillofac. Implant. 1996, 11, 340–350. [Google Scholar] [CrossRef]
- Tinsley, D.; Watson, C.J.; Russell, J.L. A comparison of hydroxylapatite coated implant retained fixed and removable mandibular prostheses over 4 to 6 years. Clin. Oral Implant. Res. 2001, 12, 159–166. [Google Scholar] [CrossRef]
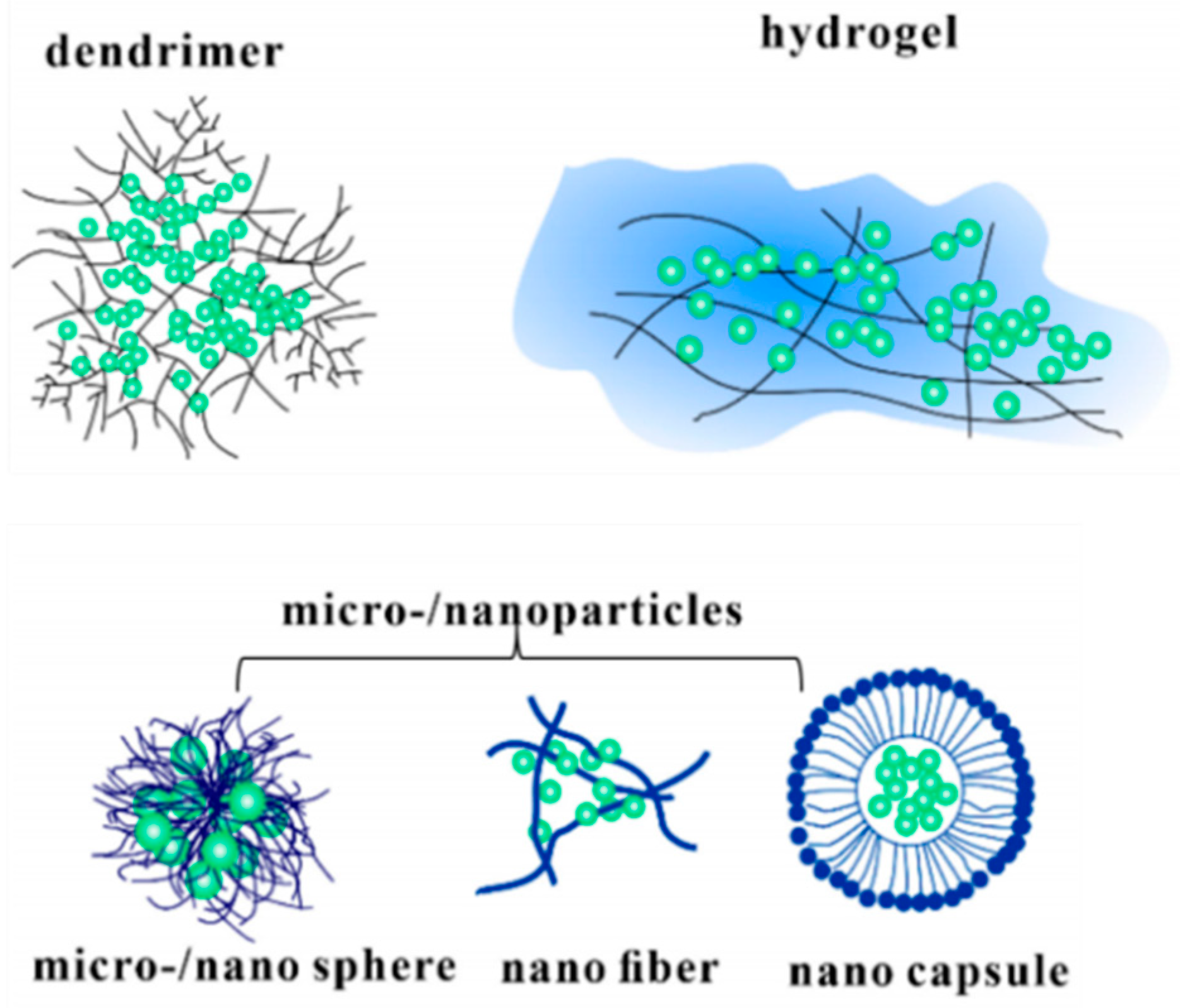
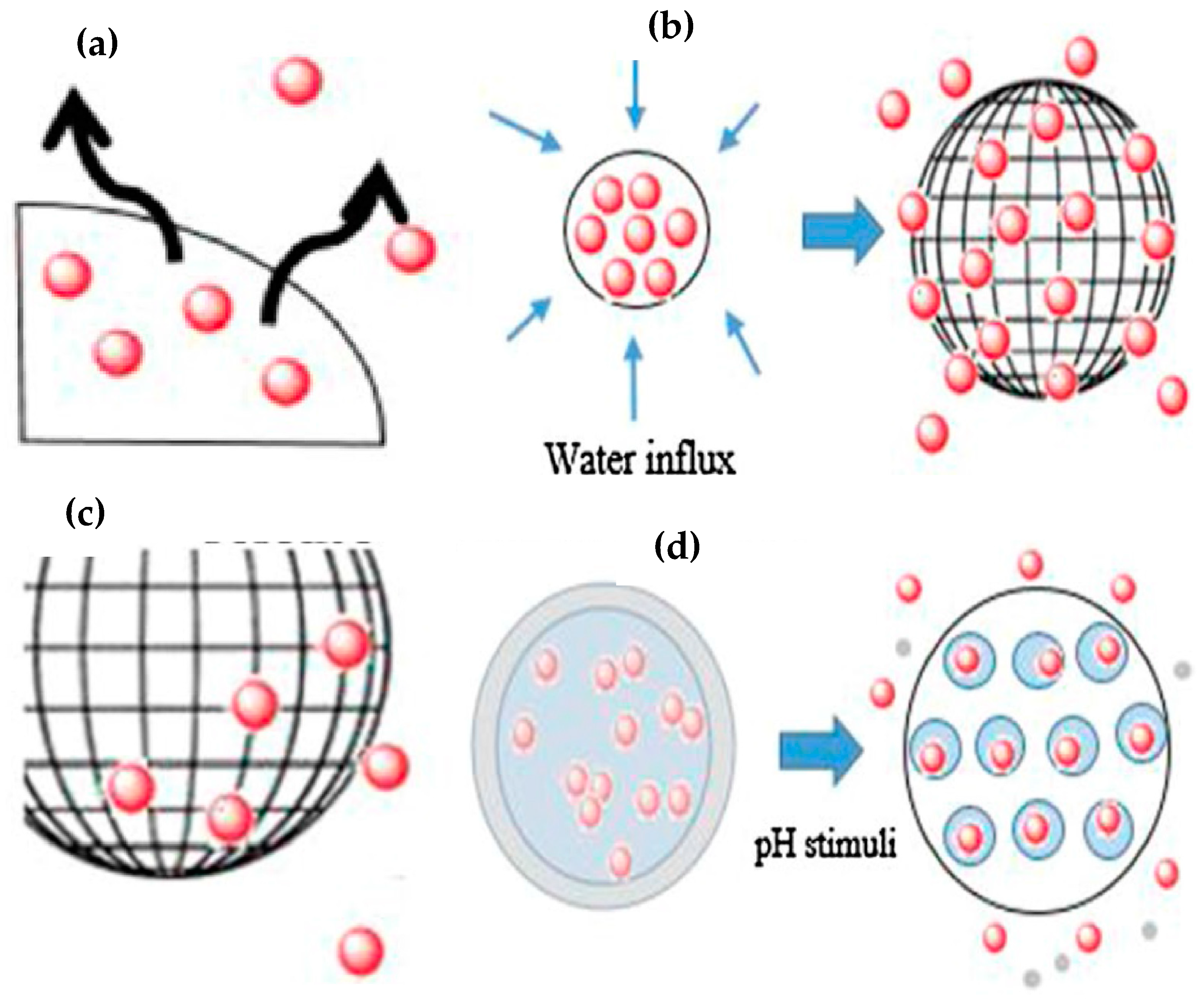
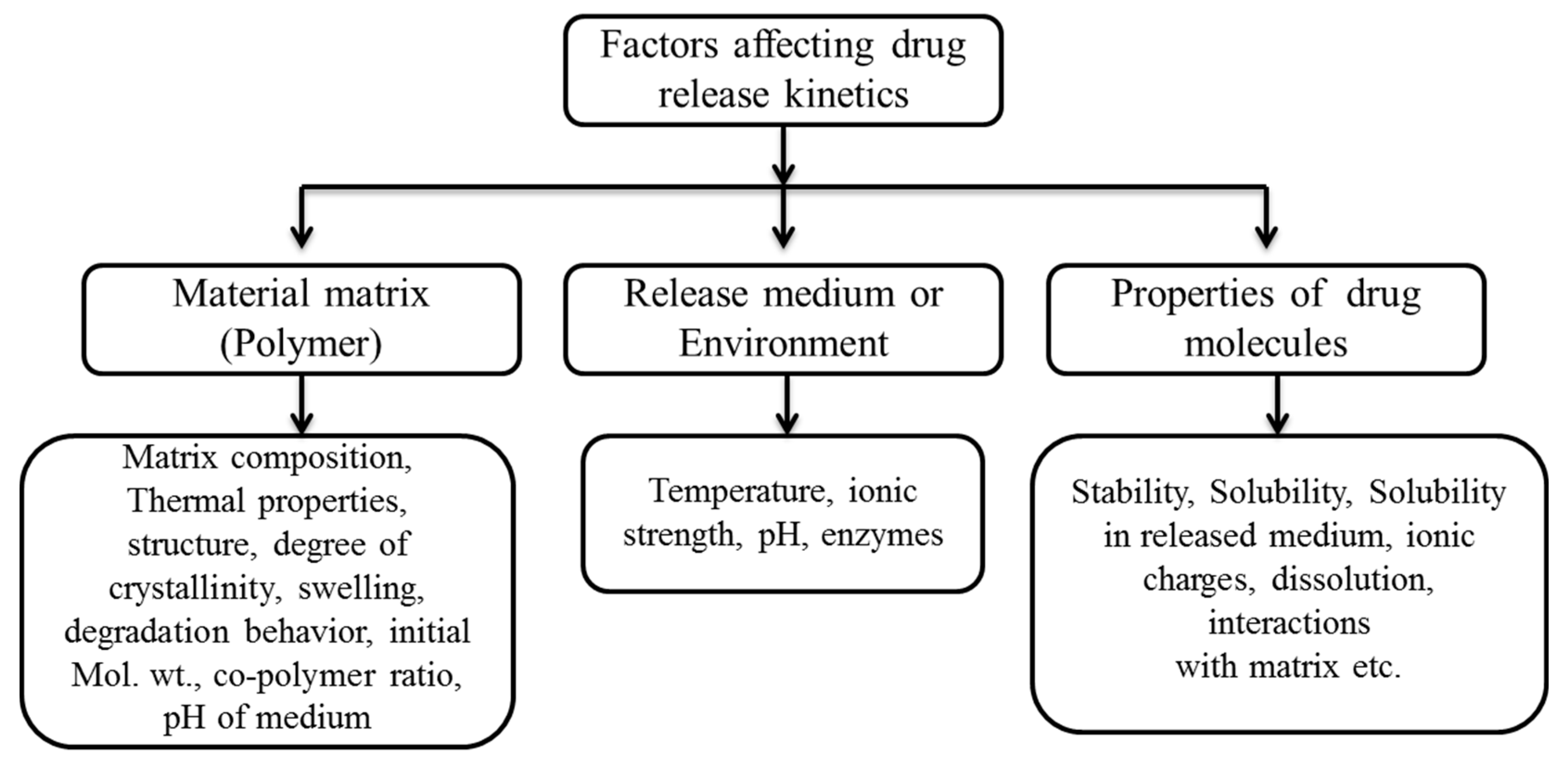
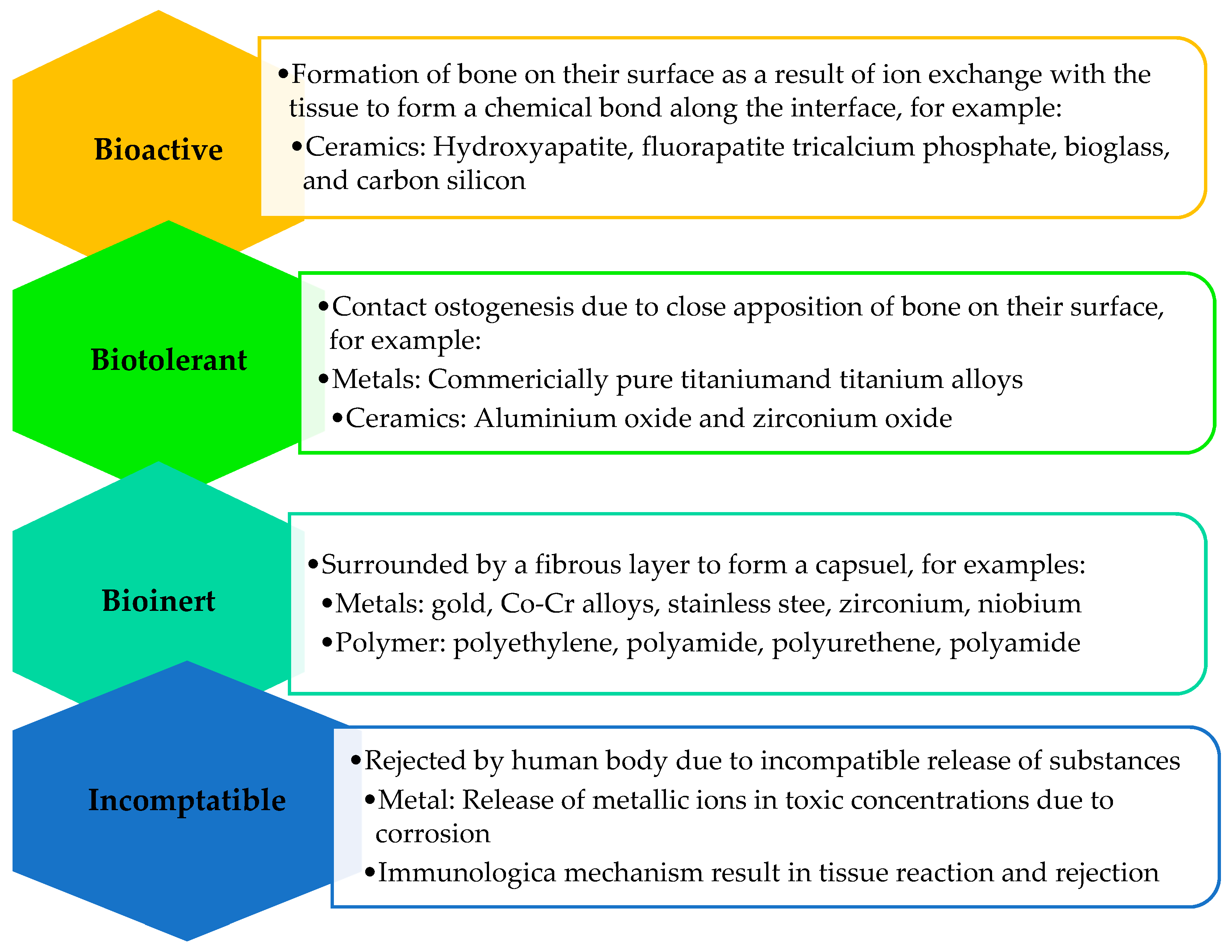

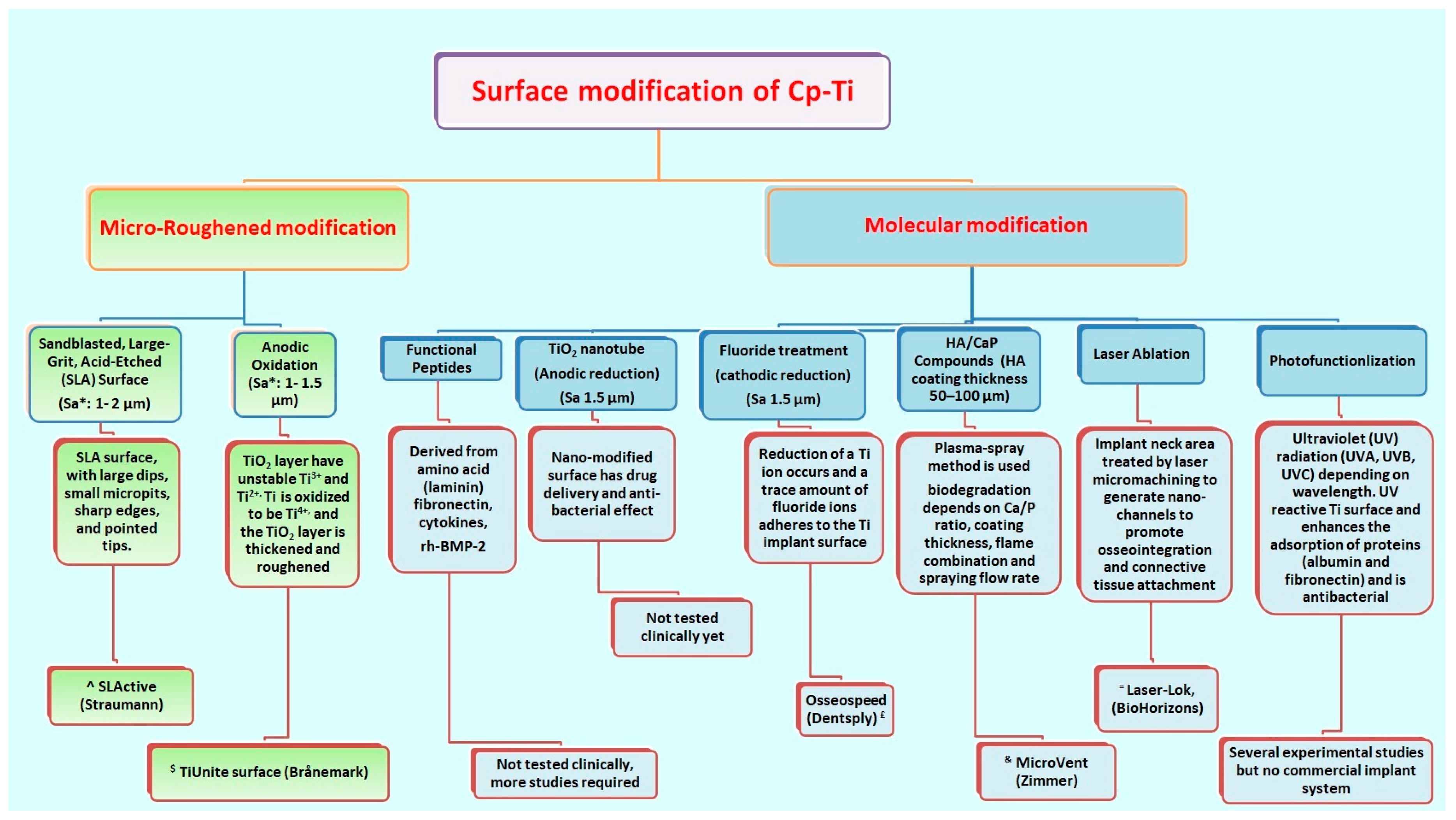
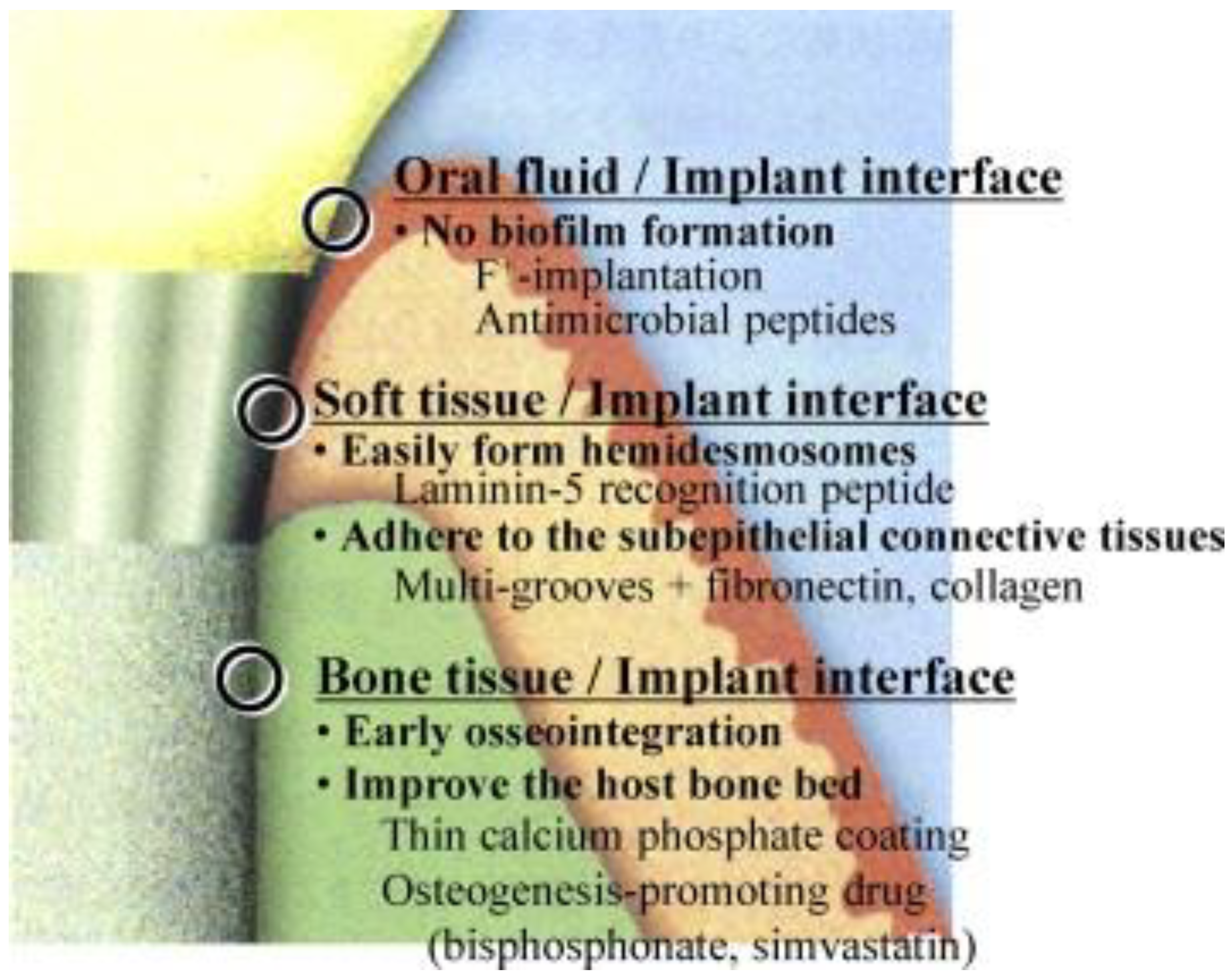
| Type | Subgroup | Substance/Biomolecule | References |
|---|---|---|---|
| Inorganic | Elemental | Nano-diamond | [169,170,171] |
| Graphene | [172,173] | ||
| Carbon nanotube | [174,175,176,177] | ||
| Silver | [178,179] | ||
| Ca-P | [180,181,182,183] | ||
| Titanium | [117,184] | ||
| Organic | Protein | Fibronectin | [185] |
| Elastin | [186] | ||
| Laminin | [187,188] | ||
| Collagen | [131,185,189] | ||
| Bone sialoprotein | [185,189] | ||
| Osteopontin | [190] | ||
| Growth factors | Bone morphogenetic proteins | [133,191,192,193] | |
| Peptides | Arginylglycylaspartic acid | [131] | |
| Parathyroid hormone | [194,195,196] | ||
| Antimicrobial GL13K | [197,198] | ||
| Polysaccharides | Hyaluronic acid | [193,199] | |
| Chondroitin 4-sulfate | [131] | ||
| Chitosan | [58,200,201] | ||
| Pectin | [179,202,203] | ||
| Drugs | Bisphosphonate | [50,57,204,205] | |
| Simvastatin | [194,206,207] | ||
| Strontium ranelate | [208] | ||
| Gentamycin | [191,209] | ||
| Tetracycline | [58] | ||
| Vancomycin | [210,211,212] | ||
| Doxycycline | [213,214] | ||
| Norfloxacin | [215]. | ||
| Chlorhexidine | [58,216] |
| Antimicrobial Peptides | Type | Site of Expression |
|---|---|---|
| α-Defensins | HNP-1 | Neutrophils (azurophilic granules), gingival crevicular fluid, and bone marrow |
| HNP-2 | ||
| HNP-3 | ||
| HNP-4 | Neutrophils | |
| β-Defensins | hBD-1 | Saliva and suprabasal layer of stratified epithelium |
| hBD-2 | Gingival epithelium and saliva | |
| hBD-3 | Skin and salivary gland | |
| Histatin | 1 | Saliva (parotid and submandibular) |
| 3 | ||
| 5 | ||
| Adrenomedullin | - | Epithelium |
| Cathelicidins | LL-37 | Neutrophils, inflamed epithelia, and saliva and submandibular glands |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings 2020, 10, 568. https://doi.org/10.3390/coatings10060568
Zafar MS, Fareed MA, Riaz S, Latif M, Habib SR, Khurshid Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings. 2020; 10(6):568. https://doi.org/10.3390/coatings10060568
Chicago/Turabian StyleZafar, Muhammad Sohail, Muhammad Amber Fareed, Samiya Riaz, Muhammad Latif, Syed Rashid Habib, and Zohaib Khurshid. 2020. "Customized Therapeutic Surface Coatings for Dental Implants" Coatings 10, no. 6: 568. https://doi.org/10.3390/coatings10060568
APA StyleZafar, M. S., Fareed, M. A., Riaz, S., Latif, M., Habib, S. R., & Khurshid, Z. (2020). Customized Therapeutic Surface Coatings for Dental Implants. Coatings, 10(6), 568. https://doi.org/10.3390/coatings10060568









