Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Material
2.2. PVD Coating Deposition
2.3. Microstructural Characterization
2.4. Electrochemical Technique
3. Results
3.1. Morphological Analysis
3.2. Corrosion Behaviour of PVD Coatings and Substrate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tiong, U.H.; Clark, G. The structural environment as a factor affecting coating failure in aircraft joints. Procedia Eng. 2010, 2, 1393–1401. [Google Scholar] [CrossRef]
- Clark, G. Corrosion and the management of structural integrity. In ICAF’99:Structural Integrity for the Next Millennium; Rudd, J.L., Ed.; EMAS Ltd.: Warley, UK, 1999. [Google Scholar]
- Trinstancho-Reyes, J.L.; Sanchez-Carrillo, M.; Sandoval-Jabalera, R.; Orozco-Carmona, V.M.; Almeraya-Calderon, F.; Chacon-Nava, J.G.; Gonzalez-Rodriguez, J.G.; Martinez-Villafane, A. Electrochemical impedance spectroscopy investigation of alloy Inconel 718 in molten salts at high temperature. J. Electrochem. Sci. 2011, 6, 419–431. [Google Scholar]
- Bianco, R.; Rapp, R.A. Pack cementation diffusion coatings. In Metallurgical and Ceramic Protective Coatings; Springer: Dordrecht, The Netherlands, 1996; pp. 236–260. [Google Scholar]
- Zhang, S.; Zhao, D. (Eds.) Aerospace Materials Handbook; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Mubarak, A.M.A.; Hamzah, E.H.E.; Toff, M.T.M. Review of Physical vapour depositetion (PVD) techinques for hard coating. J. Mek. 2005, 20, 42–51. [Google Scholar]
- Savisalo, T.; Lewis, D.; Luo, Q.; Bolton, M.; Hovsepian, P. Structure of duplex CrN/NbN coatings and their performance against corrosion and wear. Surf. Coat. Technol. 2008, 202, 1661–1667. [Google Scholar] [CrossRef]
- Bunshah, R.F.; Deshpandey, C.V. Hard coatings. Vacuum 1998, 30, 955–965. [Google Scholar]
- Kalinichenko, A.I.; Reshetnyak, E.; Strel’nitskij, V.; Abadias, G. Role of nonlocal thermoelastic peaks in the stress and texture evolution of TiN coatings formed by plasma based ion implantation and deposition. Surf. Coat. Technol. 2020, 391, 12565. [Google Scholar] [CrossRef]
- Cavaleiro, A.; de Hosson, J.T. (Eds.) Nanostructured Coatings; Springer: New York, NY, USA, 2006. [Google Scholar]
- D’Avico, L.; Beltrami, R.; Pargoletti, E.; Trasatti, S.P.; Cappelletti, G. Insight into the release agents/PVD coatings interaction for plastic mold technology. Coatings 2020, 10, 281. [Google Scholar] [CrossRef]
- D’Avico, L.; Beltrami, R.; Lecis, N.; Trasatti, S.P. Corrosion behavior and surface properties of PVD coatings for mold technology applications. Coatings 2018, 9, 7. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Kim, J.-K.; Kim, K.H. A comparative study on tribological behavior of TiN and TiAlN coatings prepared by arc ion plating technique. Surf. Coat. Technol. 2002, 161, 237–242. [Google Scholar] [CrossRef]
- Okumiya, M.; Griepentrog, M. Mechanical properties and tribological behavior of TiN–CrAlN and CrN–CrAlN multilayer coatings. Surf. Coat. Technol. 1999, 112, 123–128. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S. Single layer and multilayer wear resistant coatings of (Ti,Al)N: A review. Mater. Sci. Eng. A 2003, 342, 58–79. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion protection with hard coatings on steel: Past approaches and current research efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Caicedo, J.; Amaya, C.; Yate, L.; Aperador, W.; Zambrano, G.; Gomez, M.-E.; Alvarado-Rivera, J.; Muñoz-Saldaña, J.; Prieto, P. Effect of applied bias voltage on corrosion-resistance for TiC1−xNx and Ti1−xNbxC1−yNy coatings. Appl. Surf. Sci. 2010, 256, 2876–2883. [Google Scholar] [CrossRef]
- Yate, L.; Caicedo, J.C.; Macias, A.H.; Espinoza-Beltrán, F.J.; Zambrano, G.; Muñoz-Saldaña, J.; Prieto, P. Composition and mechanical properties of AlC, AlN and AlCN thin films obtained by rf magnetron sputtering. Surf. Coat. Technol. 2009, 203, 1904–1907. [Google Scholar] [CrossRef]
- Amaya, C.; Aperador, W.; Caicedo, J.C.; Espinoza-Beltrán, F.J.; Muñoz-Saldaña, J.; Zambrano, G.; Prieto, P. Corrosion study of alumina/yttria-stabilized zirconia (Al2O3/YSZ) nanostructured thermal barrier coatings (TBC) exposed to high temperature treatment. Corros. Sci. 2009, 51, 2994–2999. [Google Scholar] [CrossRef]
- Nordin, M.; Larsson, M.; Hogmark, S. Mechanical and tribological properties of multilayered PVD TiN/CrN, TiN/MoN, TiN/NbN and TiN/TaN coatings on cemented carbide. Surf. Coat. Technol. 1998, 106, 234–241. [Google Scholar] [CrossRef]
- Nordin, M.; Larsson, M.; Hogmark, S. Mechanical and tribological properties of multilayered PVD TiN/CrN. Wear 1999, 232, 221–225. [Google Scholar] [CrossRef]
- Ries, L.; Azambuja, D.; Baumvol, I. Corrosion resistance of steel coated with Ti/TiN multilayers. Surf. Coat. Technol. 1997, 89, 114–120. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Lukaszkowicz, K.; Kriz, A. Properties of the multi-layer Ti/CrN and Ti/TiAlN coatings deposited with the PVD technique onto the brass substrate. J. Mater. Process. Technol. 2003, 143, 832–837. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Rajam, K. Structure and properties of reactive DC magnetron sputtered TiN/NbN hard superlattices. Surf. Coat. Technol. 2004, 183, 174–183. [Google Scholar] [CrossRef]
- Oliveira, V.; Aguiar, C.; Vázquez, A.; Robin, A.; Barboza, M. Improving corrosion resistance of Ti–6Al–4V alloy through plasma-assisted PVD deposited nitride coatings. Corros. Sci. 2014, 88, 317–327. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. EIS comparison on corrosion performance of PVD TiN and CrN coated mild steel in 0.5 N NaCl aqueous solution. Corros. Sci. 2001, 43, 1953–1961. [Google Scholar] [CrossRef]
- Chipatecua, Y.; Olaya, J.; Arias, D.F. Corrosion behaviour of CrN/Cr multilayers on stainless steel deposited by unbalanced magnetron sputtering. Vacuum 2012, 86, 1393–1401. [Google Scholar] [CrossRef]
- Marulanda, D.; Olaya, J.; Piratoba, U.; Marino, A.; Camps, E. The effect of bilayer period and degree of unbalancing on magnetron sputtered Cr/CrN nano-multilayer wear and corrosion. Thin Solid Films 2011, 519, 1886–1893. [Google Scholar] [CrossRef]
- Olia, H.; Ebrahimi-Kahrizsangi, R.; Ashrafizadeh, F.; Ebrahimzadeh, I. Corrosion study of TiN, TiAlN and CrN multilayer coatings deposit on martensitic stainless steel by arc cathodic physical vapour deposition. Mater. Res. Express 2019, 6, 046425. [Google Scholar] [CrossRef]
- Grabke, H.J. High Nitrogen Steels. The Role of nitrogen in the corrosion of iron and steels. ISIJ Int. 1996, 36, 777–786. [Google Scholar] [CrossRef]
- Li, Z.; Di, S. The Microstructure and wear resistance of microarc oxidation composite coatings containing nano-hexagonal boron nitride (HBN) particles. J. Mater. Eng. Perform. 2017, 26, 1551–1561. [Google Scholar] [CrossRef]
- Wang, L.; Nie, X. Effect of annealing temperature on tribological properties and material transfer phenomena of CrN and CrAlN coatings. J. Mater. Eng. Perform. 2013, 23, 560–571. [Google Scholar] [CrossRef]
- Hong, L.; Bian, G.; Hu, S.; Wang, L.; Dacosta, H. Tribological properties of CrAlN and TiN coatings tested in nano- and micro-scale laboratory wear tests. J. Mater. Eng. Perform. 2015, 24, 2670–2677. [Google Scholar] [CrossRef]
- Srinath, M.K.; Prasad, M.S.G. Wear and corrosion resistance of titanium carbo-nitride coated Al-7075 produced through PVD. Bull. Mater. Sci. 2020, 43, 1–11. [Google Scholar] [CrossRef]
- ASTM G106-15. Standard Practice for Verification of Algorithm and Equip for Electrochemical Impedance Measurements; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Taberna, P.L.; Simon, P.; Fauvarque, J.F. Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J. Electrochem. Soc. 2003, 150, A292–A300. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, D. An investigation of the electrochemical properties of PVD TiN-coated SS410 in simulated PEM fuel cell environments. Int. J. Hydrogen Energy 2007, 32, 895–902. [Google Scholar] [CrossRef]
- Moisel, M.; De Mele, M.A.F.L.; Müller, W.-D.; De Mele, M.F.L. Biomaterial interface investigated by electrochemical impedance spectroscopy. Adv. Eng. Mater. 2008, 10, B33–B46. [Google Scholar] [CrossRef]
- Chen, T.; Nutter, J.; Hawk, J.; Liu, X. Corrosion fatigue crack growth behavior of oil-grade nickel-base alloy 718. Part 1: Effect of corrosive environment. Corros. Sci. 2014, 89, 146–153. [Google Scholar] [CrossRef]
- Grips, V.W.; Barshilia, H.C.; Selvi, V.E.; Rajam, K. Electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive direct current magnetron sputtering. Thin Solid Films 2006, 514, 204–211. [Google Scholar] [CrossRef]
- Jehn, H. Improvement of the corrosion resistance of PVD hard coating–substrate systems. Surf. Coat. Technol. 2000, 125, 212–217. [Google Scholar] [CrossRef]
- Tsai, S.H.; Duh, J.-G. Microstructure and corrosion properties of multilayered CrAlN/SiNx coatings. J. Electrochem. Soc. 2010, 157, K89. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corros. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]
- Manohar, A.K.; Bretschger, O.; Nealson, K.H.; Mansfeld, F. The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry 2008, 72, 149–154. [Google Scholar] [CrossRef]
- Miranda, D.A.; Jaimes, S.A.; Bastidas, J.M. Assessment of carbon steel microbiologically induced corrosion by electrical impedance spectroscopy. J. Solid State Electrochem. 2014, 18, 389–398. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part II.: EIS interpretation of corrosion behaviour. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Lin, C.; Duh, J.-G. Electrochemical impedance spectroscopy (EIS) study on corrosion performance of CrAlSiN coated steels in 3.5 wt.% NaCl solution. Surf. Coat. Technol. 2009, 204, 784–787. [Google Scholar] [CrossRef]
- Wan, Q.; Ding, H.; Yousaf, M.; Chen, Y.; Liu, H.; Hu, L.; Yang, B. Corrosion behaviors of TiN and Ti–Si–N (with 2.9 at.% and 5.0 at.% Si) coatings by electrochemical impedance spectroscopy. Thin Solid Films 2016, 616, 601–607. [Google Scholar] [CrossRef]
- Er, D.; Azar, G.T.P.; Kazmanli, M.; Ürgen, M. The corrosion protection ability of TiAlN coatings produced with CA-PVD under superimposed pulse bias. Surf. Coat. Technol. 2018, 346, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Feng, Y.; Chen, S.; Wan, Q.-L.; Zhu, J.-F. Electrochemical characterization of AlTiN, AlCrN and AlCrSiWN coatings. Int. J. Refract. Met. Hard Mater. 2015, 53, 68–73. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Cai, F.; Yang, Y.; Wang, Q.; Zhang, S. Characterization and corrosion behaviors of TiN/TiAlN multilayer coatings by ion source enhanced hybrid arc ion plating. Surf. Coat. Technol. 2019, 366, 355–365. [Google Scholar] [CrossRef]
- Ruden, A.; Parra, E.R.; Paladines, A.; Sequeda, F. Corrosion resistance of CrN thin films produced by DC magnetron sputtering. Appl. Surf. Sci. 2013, 270, 150–156. [Google Scholar] [CrossRef]
- Vasilescu, E.; Drob, P.; Vasilescu, E.; Demetrescu, I.; Ionita, D.; Prodana, M.; Drob, S.I. Characterisation and corrosion resistance of the electrodeposited hydroxyapatite and bovine serum albumin/hydroxyapatite films on Ti–6Al–4V–1Zr alloy surface. Corros. Sci. 2011, 53, 992–999. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, S.H.; Kim, J.G. Effect of Al additions in WC-(Cr1−xAlx)N coatings on the corrosion resistance of coated AISI D2 steel in a deaerated 3.5 wt.% NaCl solution. Surf. Coat. Technol. 2005, 190, 417–427. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Montoya, R.M.; Garza Montes de Oca, N.; Castorena, G.J.H.; Estupiñan, L.F.; Cabral, M.J.; Maldonado, B.E.; Gaona-Tiburcio, C. Corrosion behavior of multilayer coatings deposited by PVD on Inconel 718 in Chloride and Sulphuric Acid solutions. J. Electrochem. Sci. 2019, 14, 9596–9609. [Google Scholar] [CrossRef]
- Amin, M.A.; El-Bagoury, N.; Saracoglu, M.; Ramadan, M. Electrochemical and corrosion behavior of cast Re-containing Inconel 718 alloys in Sulphuric Acid solutions and the effect of Cl. Int. J. Electrochem. Sci. 2014, 9, 5352–5374. [Google Scholar]

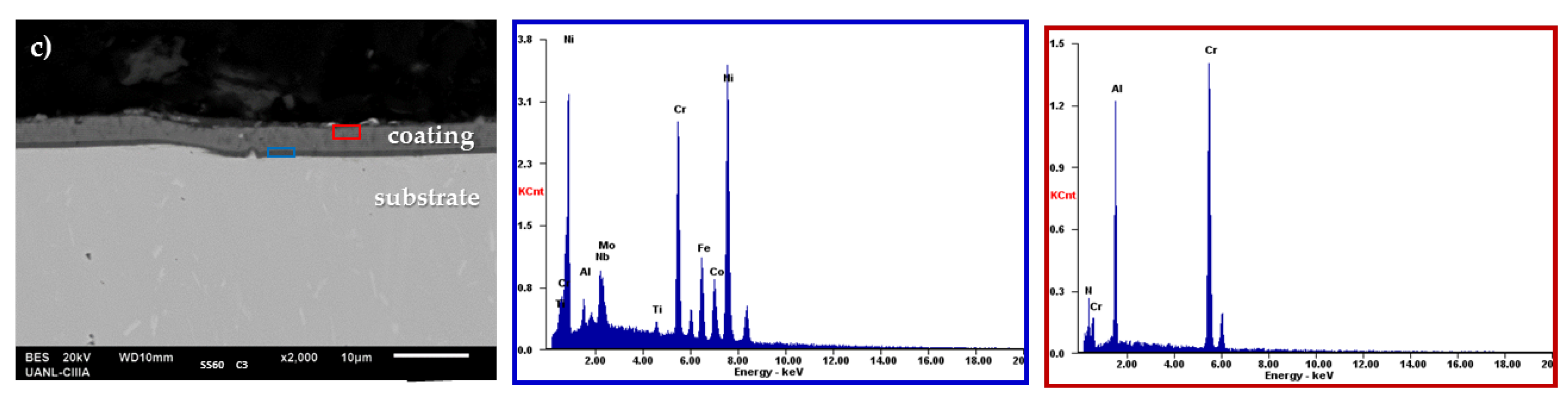
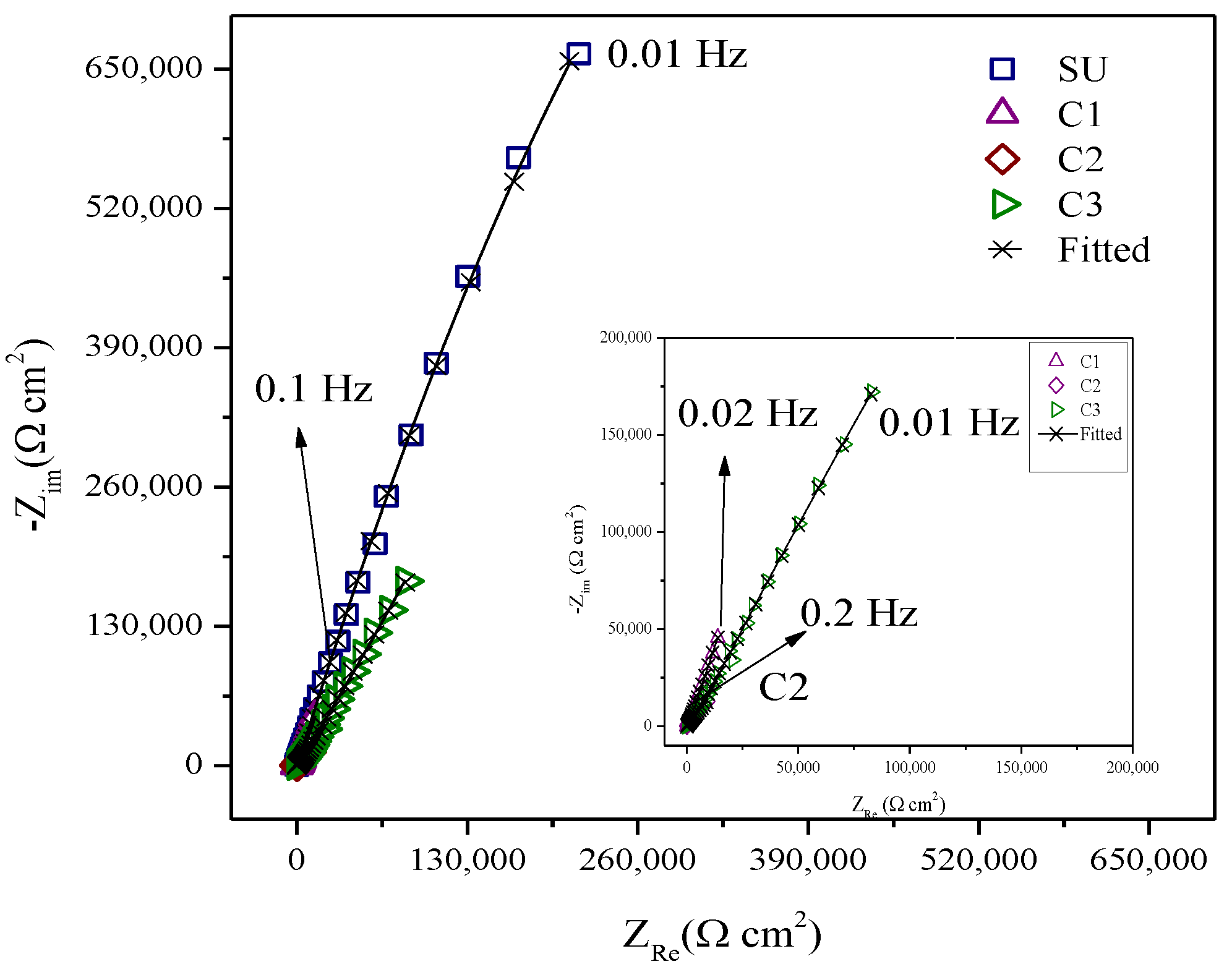
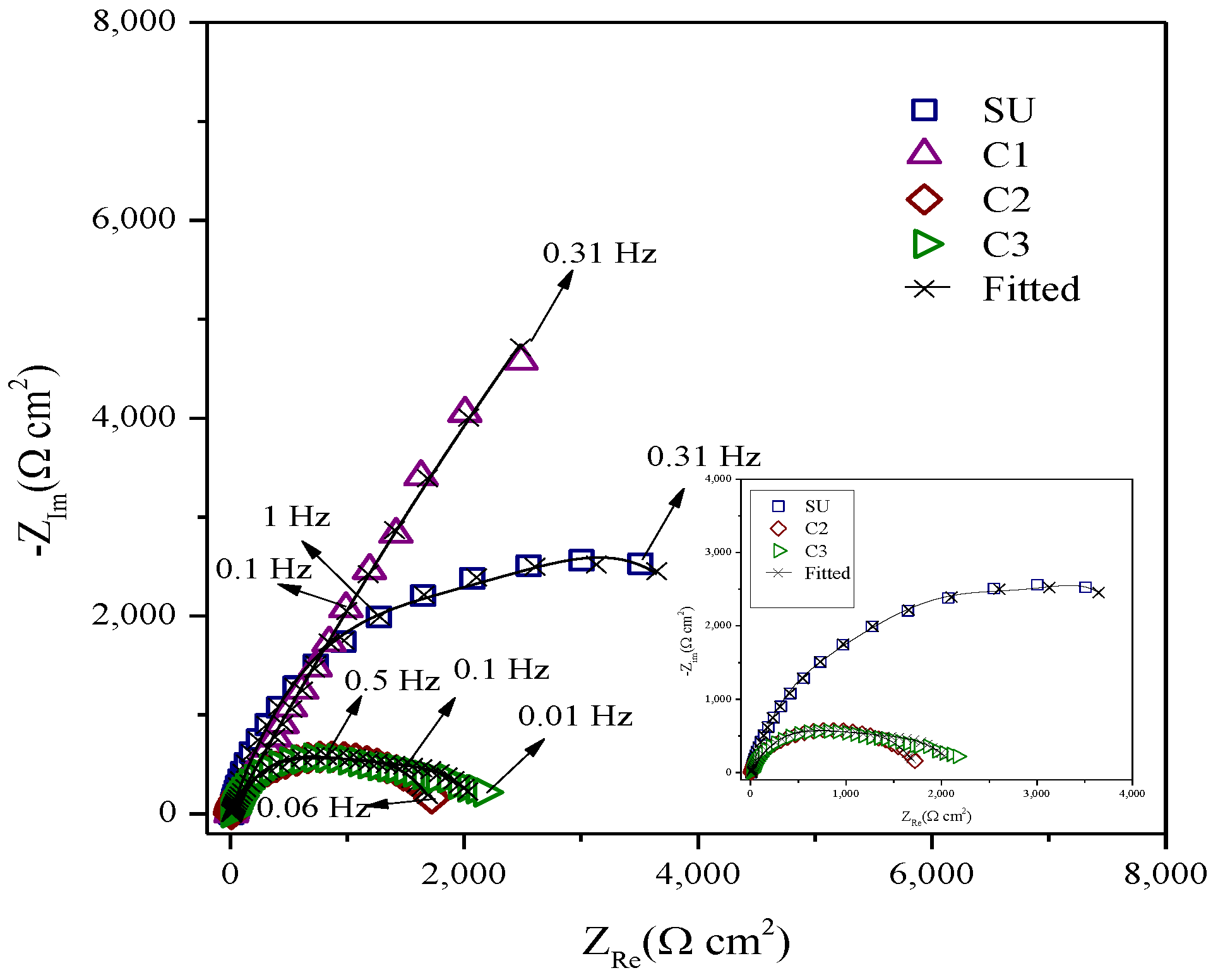

| Samples | Coating |
|---|---|
| SU | Inconel 718 |
| C1 | AlCrN/TiSiN |
| C2 | AlCrN/TiCrSiN |
| C3 | AlCrN/AlCrN + CrN |
| Sample | EIS Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CPEc (F cm−2) | n1 | CPEs (F cm−2) | ndl | Re (Ω cm2) | Rpore (Ω cm2) | Rs (Ω cm2) | Error % | χ2 | |
| SU | 0.000014 | 0.857 | - | - | 12.28 | 6,100,000 | - | <16.10 | 0.0036 |
| C1 | 0.000086 | 0.805 | 0.000030 | 0.88 | 13.17 | 11,000 | 7,940,000 | <2.73 | 0.00061 |
| C2 | 0.000024 | 0.774 | 0.000036 | 0.545 | 12.28 | 3200 | 763,000 | <5.91 | 0.000084 |
| C3 | 0.000017 | 0.826 | 0.000023 | 0.696 | 12.35 | 10,000 | 9,220,000 | <4.19 | 0.00059 |
| Sample | EIS Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CPEc (F cm−2) | n1 | CPE2 (F cm−2) | ndl | Re (Ω cm2) | Rpore (Ω cm2) | Rs (Ω cm2) | Error % | χ2 | |
| SU | 0.000069 | 0.894 | - | - | 7.205 | 5968 | - | <0.56 | 0.00021 |
| C1 | 0.00036 | 0.891 | 0.00036 | 0.75 | 7.292 | 1347 | 36377 | <3.08 | 0.00025 |
| C2 | 0.000091 | 0.876 | 0.00075 | 0.851 | 7.976 | 1240 | 511.3 | <2.33 | 0.00067 |
| C3 | 0.00020 | 0.849 | 0.0038 | 0.842 | 9.242 | 1428 | 704.7 | <1.84 | 0.00083 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaona-Tiburcio, C.; Montoya-Rangel, M.; Cabral-Miramontes, J.A.; Estupiñan-López, F.; Zambrano-Robledo, P.; Orozco Cruz, R.; Chacón-Nava, J.G.; Baltazar-Zamora, M.Á.; Almeraya-Calderón, F. Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique. Coatings 2020, 10, 521. https://doi.org/10.3390/coatings10060521
Gaona-Tiburcio C, Montoya-Rangel M, Cabral-Miramontes JA, Estupiñan-López F, Zambrano-Robledo P, Orozco Cruz R, Chacón-Nava JG, Baltazar-Zamora MÁ, Almeraya-Calderón F. Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique. Coatings. 2020; 10(6):521. https://doi.org/10.3390/coatings10060521
Chicago/Turabian StyleGaona-Tiburcio, Citlalli, Marvin Montoya-Rangel, José A. Cabral-Miramontes, Francisco Estupiñan-López, Patricia Zambrano-Robledo, Ricardo Orozco Cruz, José G. Chacón-Nava, Miguel Ángel Baltazar-Zamora, and Facundo Almeraya-Calderón. 2020. "Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique" Coatings 10, no. 6: 521. https://doi.org/10.3390/coatings10060521
APA StyleGaona-Tiburcio, C., Montoya-Rangel, M., Cabral-Miramontes, J. A., Estupiñan-López, F., Zambrano-Robledo, P., Orozco Cruz, R., Chacón-Nava, J. G., Baltazar-Zamora, M. Á., & Almeraya-Calderón, F. (2020). Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique. Coatings, 10(6), 521. https://doi.org/10.3390/coatings10060521












