Abstract
This study developed a biopreservation method for ready-to-eat (RTE) fresh salmon fillets based on the use of bacteriocin EFL4 produced by bacteriocinogenic Enterococcus faecalis L04 previously isolated from Chinese sea bass (Lateolabrax maculatus). Bacteriocin EFL4 has the ability to inhibit the growth of several fish-spoilage bacteria and foodborne pathogens, including Staphylococcus aureus, Escherichia coli, Shewanella putrefaciens, Pseudomonas fluorescens and Listeria monocytogenes, and the minimal inhibitory concentration (MIC) for S. putrefaciens was 0.32 μg/mL. The biopreservation potential of bacteriocin EFL4 for RTE fresh salmon fillets during cold storage at 4 °C was tested for the first time on a laboratory scale. Microbiological and physicochemical properties, as well as organoleptic evaluations, have been done during the biopreservation trials. The results show that RTE fresh salmon fillets treated with 0.64 μg/mL bacteriocin EFL4 could significantly (p < 0.05) reduce the total viable count (TVC), total volatile basic nitrogen (TVB-N), K value and maintain the quality of RTE fresh salmon fillets during 8-day storage on the basis of the organoleptic evaluation results.
1. Introduction
Fresh fish can easily deteriorate after being captured. Most fish are degraded by microbial spoilage, digestive enzymes and lipases, and the oxidation of surface bacteria [1,2]. Fish decay could result in lipid oxidation, protein degradation, as well as changes in fish flavor, odor and texture. Therefore, it is necessary to develop effective processing technologies to extend the shelf life. In addition, the consumers’ demand for high-quality but minimally processed products is increasing [3].
A soft or mushy texture of fish could limit the shelf-life, thereby impeding its marketing. The holding temperature, endogenous, oxygen, moisture or microbial proteases can result in detrimental changes in the color, odor, flavor and texture of fish during postmortem handling and storage [4,5]. To slow the quality loss, the fish should be refrigerated immediately after capture. The newly caught fish should be cooled and stored in the ice slurries, refrigerated sea water, flake ice or preserved by chemical preservatives. And the fishery industry is always searching for new preservatives to prolong the shelf life [6].
Research studies have demonstrated that bacteriocin are a group of potent antimicrobial peptides containing about 30–60 amino acids forming amphiphilic helices and they differ in genetic origin, molecular weight, biochemical characteristics, activity spectrum, and mode of action [7,8]. Bacteriocin-producing lactic acid bacteria (LAB) strains could protect themselves by expressing a specific immune protein, which is encoded in the bacteriocin [9,10].
Until recently, the use of bacteriocins for the biopreservation of salmon fillets has been reported with the control of L. monocytogenes [11,12]. In the present study, we first applied bacteriocinogenic Enterococcus faecalis L04 (EFL4) to preserve ready-to-eat (RTE) fresh salmon fillets and investigate the effect of bacteriocin EFL4 on the microbiological, physicochemical qualities and organoleptic evaluation of RTE fresh salmon fillets during cold storage at 4 °C for 8 days.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
E. faecalis L04, a bacteriocin-producing strain, was previously isolated from the intestine of Chinese sea bass (Lateolabrax maculatus) by the serial dilution method [13]. It was selected for this study due to the inhibitory activity against several food-borne pathogens and fish-spoilage bacteria, including S. aureus, E. coli, S. putrefaciens and P. fluorescens, shown in previous research. This strain was cultivated in De Man, Rogosa, Sharpe (MRS) broth (Hangwei-media, Hangzhou, China) at 37 °C for 24 h and then centrifugated at 10744× g at 4 °C for 15 min to obtain the cell free supernatant (CFS).
2.2. Purification of Bacteriocin
A total of 20 mL of overnight seed culture of strain E. faecalis L04 and 1 L MRS broth were mixed together and statically cultivated at 37 °C for 24 h. After removing the cells by centrifugation (10744× g at 4 °C for 15 min), the CFS was extracted with an equal volume of ethyl acetate by a separatory funnel [14]. The ethyl acetate was removed by rotary vacuum evaporation and concentrated to 150 mL. The Sephadex G50 column was balanced with phosphate buffer solution (PBS, pH 6.0) and then the concentrated sample was eluted at 0.5 mL/min with PBS (pH 6.0). Each 4 mL fraction was collected and the anti-microbial activity was tested. The fractions containing bacteriocin were merged, vacuum freeze-dried and redissolved in 1 mL of PBS (pH 6.0). In order to further purify and concentrate the active fraction, a Prep-HPLC system (Prep 150, Waters, Milford, DE, USA) was used. A total of 20 mL of concentrated active fraction was injected into a DIKMA Bio-bond C18 column (250 mm × 4.6 mm, 5 μm), and the elution was carried out with 60% methanol and 40% ultrapure water at 0.5 mL/min for 30 min. The elution peaks were monitored at 280 nm by spectrophotometry. The antimicrobial activity of the collected active fractions were tested and the purified active fractions were vacuum freeze-dried at −60 °C, 10 Pa and kept at 4 °C before use.
2.3. Antimicrobial Activity Assay of Bacteriocin EFL4
The antimicrobial activity of bacteriocin EFL4 was measured by the Oxford cup method [15]. In brief, 200 μL of suspended target bacteria (OD600 = 0.1, Table 1) was uniformly coated on a dish using the coating rod after solidification of the corresponding medium. After 1 h of inverted incubation at 30 °C, the Oxford cups (6mm, inner diameter) were placed on the dish and 200 μL of sample was added to each Oxford cup. After diffusing at 4 °C for 12 h, the dish was cultivated at 30 °C for 24 h, and the inhibition zone was determined.

Table 1.
Antimicrobial activity of bacteriocin EFL4 from Enterococcus faecalis L04.
2.4. The Minimum Inhibitory Concentration (MIC) of Bacteriocin EFL4 for S. Putrefaciens
The MIC of bacteriocin EFL4 for against S. putrefaciens was evaluated by the double dilution method according to Cui et al. [16]. Different concentrations of bacteriocin EFL4 were made as 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8 and 25.6 μg/mL, respectively, with broth medium. The MIC of bacteriocin EFL4 was defined as the minimum concentration of the bacteriocin EFL4 that demonstrated no visible S. putrefaciens growth and completely inhibited for 16 h [17].
2.5. RTE Fresh Salmon Fillets Preparation and Sampling
Sodium alginate (food-grade, viscosity of 200 ± 20 mpa·s) was used as the active coating matrix and prepared by dissolving 1.5% of sodium alginate (w/v) in deionized water at 50 °C and stirred for 6 h with the addition of glycerol as a plasticiser in a 20% (w/w) ratio relative to sodium alginate. To obtain active coatings, 1× and 2× MIC of bacteriocin EFL4 for S. putrefaciens were added to each film-forming solution, respectively. RTE fresh salmon (Salmo salar) fillets with an average weight of 50 ± 5 g were purchased from a local aquatic product market in Luchao Port town (Pudong New District, Shanghai). The RTE fresh salmon fillets were surface sterilized by exposure to UV-C light (265 nm) for 10 min at room temperature [18]. The salmon fillets were randomized into three groups: Treatment 1(T1) and 2 (T2) were dipped in active coating solutions containing 1× and 2× MIC of bacteriocin EFL4 for S. putrefaciens at 4 °C for 10 min, respectively, and the control (CK) was dipped in a sterile normal saline solution without bacteriocin EFL4. All the samples were allowed to drain at 4 °C around 45 min to form the active coatings on the RTE fresh salmon fillets’ surface. Then, they were put into sterile plastic bags and stored at 4 °C for 8 days. The RTE fresh salmon fillets samples were taken randomly for analysis on the 0, 2nd, 4th, 6th, 7th, and 8th day, respectively.
2.6. Analysis of Bacterial Count
Representative 5-g salmon samples were fully homogenized with 45 mL sterilized normal saline and then continuously diluted. The total viable counts (TVC) were incubated on plate count agar at 30 °C for 48 h [19].
2.7. pH Measurements and Electrical Conductivity
A total of 2.5 g of each sample was homogenized with 25 mL of distilled water and centrifuged at 9150× g at 4 °C for 10 min. pH value and electrical conductivity were measured with an FE-20 pH meter (Mettler Toledo, Switzerland) and an FE30K conductivity meter (Mettler Toledo, Greifensee, Switzerland) of the supernatant at ambient temperature, respectively.
2.8. ATP-Related Compounds
ATP-related compounds were extracted with 0.6 M perchloric acid solution and were determined by a RP-HPLC (Waters 2695, Milford, DE, USA) described by Li et al. [20]. The K value was calculated as follows:
where ATP, ADP, AMP, IMP, HxR, Hx stand for adenosine triphosphate, adenosine diphosphate, adenosine monophosphate, inosine monophosphate, inosine and hypoxanthine, respectively.
2.9. Determination of Total Volatile Basic Nitrogen (TVB-N)
The TVB-N was measured by the microtitration method illustrated by Li et al. [21]. A total of 5 mL of obtained supernatant in pH test and 5 mL of magnesium oxide (10 g·L−1) were mixed homogeneously and then distilled using Foss 8400 Kjeldahl apparatus (Copenhagen, Denmark). The distillate was collected with 20 mL boric acid solution containing a mixed indicator of methyl red and methylene blue. Then, the absorption solution was titrated with 0.01 mol/L hydrochloric acid standard solution and TVB-N was indicated as mg N/100 g of salmon fillets samples.
2.10. Texture Profile Analysis (TPA)
The salmon fillets samples were cut into small pieces (2 × 2 × 1 cm3) and were subjected to a texture analyzer (TA. XT Plus, Surrrey, UK) using P/5 cylindrical probe with a uniaxial compression test [22]. The condition of testing was two consecutive cycles at 50% compression. The pre-test and post-test constant speed were 5 and 1 mm/s, respectively. The rest period between cycles was 15 s and the probe was allowed to return to its initial position after the second cycle. The parameters, including hardness, chewiness and springiness, were tested and calculated. Each sample was tested at least in six points.
2.11. Microstructure
The salmon fillets were cut into blocks (3 × 3 × 1 mm3) and fixed at 4 °C for 24 h with 2.5% glutaraldehyde. Then, the fixed salmon fillets samples were washed with PBS (pH 6.0) for three times and dehydrated by series concentration of ethanol. The microstructure of the salmon fillets samples was observed with scanning electron microscope (Hitachi S3400, Tokyo, Japan) on the basis of the gold coating treatment.
2.12. Organoleptic Evaluation
The Quality Index Method (QIM), containing color, smell, mucus, elasticity and muscular tissue, developed by Bonilla et al. [23] and Ozogul et al. [24] was conducted with some modifications. For each parameter, the scheme had five simple descriptors with 5 indicating the best quality and a lower score representing poorer quality. Twelve trained panelists participated in the organoleptic evaluation and performed within the required time. The panelists were asked to state whether the RTE salmon fillets samples were acceptable or not to determine the shelf life.
2.13. Statistical Analysis
Each measurement was repeated at least three times in three different samples. The data were presented as means ± SD by a one-way ANOVA procedure followed by Duncan’s test (SPSS 22.0).
3. Results and Discussions
3.1. Detection of Antimicrobial Activity of Bacteriocin EFL4
Purificated bacteriocin EFL4 from E. faecalis L04 could inhibit the growth of Gram-positive bacteria, including Streptococcus and Listeria (Table 1). The antilisterial activity of bacteriocins has been widely reported [25,26,27]. Moreover, it has been reported that bacteriocins could also inhibit some Gram-negative bacteria genera, including Escherichia, Shewanella and Pseudomonas. However, no inhibitory activity against Lactobacillus was found in the present research. It should be noted that purified bacteriocin EFL4 displayed good antimicrobial activity against S. putrefaciens and P. fluorescens, which were considered as the specific spoilage organisms linked to the occurrence of unpleasant flavors associated with seafood spoilage [28,29,30]. Previously, Hwanhlem et al. [31] and H-Kittikun et al. [32] reported that some bacteriocins produced by E. faecalis showed activities against food-borne pathogens and food-spoilage bacteria. Furthermore, Liu et al. [33] reported that bacteriocins produced by different subspecies of E. faecalis displayed strong antimicrobial activity against S. aureus, L. monocytogenes, B. subtilis, E. faecalis, B. cereus and Candida albicans. Regarding bacteriocins produced by other LAB genus, Gómez-Sala et al. [34,35] observed that bacteriocins produced by Lb. curvatus BCS35 isolated from dry-salted cod had antimicrobial activity against f food-borne pathogens and food-spoilage bacteria, such as Clostridium spp., L. monocytogenes, S. putrefaciens, Brochothrix thermosphacta, and P. fluorescens. The obtained antimicrobial results suggest that the application of the bacteriocin produced by E. faecalis L04 as a bio-preservative may be effective against food-spoilage and food-borne pathogens.
3.2. MIC of Bacteriocin EFL4 for S. Putrefaciens
The MIC of bacteriocin EFL4 against S. putrefaciens was 3.2 μg/mL. For most bacteriocins, MIC values against tested bacteria were 1–100 μg/mL [36,37,38,39]. The MIC value of bacteriocin EFL4 against S. putrefaciens was at a relatively low concentration, which demonstated bacteriocin EFL4 had excellent antimicrobial activity and promising potential as antimicrobial agent. Therefore, the concentrations of bacteriocin EFL4 for T1 and T2 were 3.2 and 6.4 μg/mL, respectively.
3.3. Microbiological Results for RTE Fresh Salmon Fillets’ Preservation
It is known that fish quality deterioration is mainly caused by microbial activity, and the TVC variations were well correlated with the changes of or ganoleptic evaluation results, K value and TVB-N [40]. Figure 1a displays the corresponding data for the TVC growth of RTE fresh salmon fillets during storage at 4 °C. The initial low count of TVC (3.2 log CFU/g) signified the excellent quality for fresh fish [41]. Compared with CK, T1 and T2 samples significantly inhibited the growth of microorganisms during cold storage (p < 0.05). The TVC counts of all the samples increased throughout the refrigeration period and CK, T1 and T2 exceeded the upper acceptability limit (5 log CFU/g) on 6th, 7th and 8th day, respectively, for RTE fresh salmon fillets.
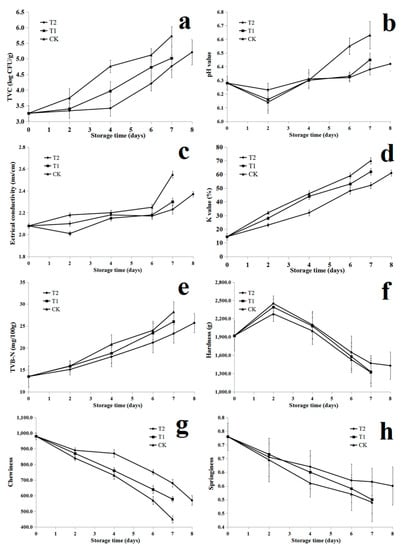
Figure 1.
Change in the total viable counts (TVC, a), pH value (b), electrical conductivity (c), K value (d), total volatile basic nitrogen (TVB-N, e), hardness (f), chewiness (g) and springiness (h) of salmon treated with bacteriocin EFL4 during cold storage at 4 °C (CK: control; T1: salmon treated with 1MIC bacteriocin EFL4; T2: salmon treated with 2MIC bacteriocin EFL4).
3.4. pH and Electrical Conductivity Values
Microbial activity could also be determined indirectly through pH value changes [42]. The pH values decreased initially and then increased in T1 and T2 samples (Figure 1b). The pH decreasing at the beginning due to the accumulation of lactic acid in the glycolysis reactions and the increased pH values resulted from the production of volatile basic components including ammonia, biogenic amines, and trimethylamine (TMA) on account of bacterial reproduction [43]. CK samples showed significantly (p < 0.05) higher average pH values comparing with T1 and T2 samples during cold storage. In addition, smaller pH changes were observed in T2 samples and it could be concluded that 0.64 μg/mL bacteriocin EFL4 combined with storage at 4 °C could have a positive combined effect on inhibiting bacterial spoilage growth.
The electrical conductivity value was determined as 2.08 mS/cm for RTE fresh salmon fillets and increased in all the samples during cold storage (Figure 1c). After 7 days of storage, the electrical conductivity values of samples for CK presented 2.56 mS/cm. Corresponding reductions of 0.20 and 0.13 mS/cm were observed in comparison with CK for T1 and T2 on the 7th day, respectively. Shi et al. [44] reported that the increase in electrical conductivity value was mainly attributed to the release of ionic substances caused by bacterial metabolism and biochemical reactions. The above results effectively suggested that bacteriocin EFL4 could effectively inhibit the growth of spoilage microorganisms and the decomposition of nutrients.
3.5. K Values
The sequence of nucleotide degradation in fish muscle is ATP→ADP→AMP→IMP→HxR→Hx [45]. IMP imparts a sweet and meaty taste, improving the quality of fish, whereas its degradation products (HxR and Hx) lead to an unpleasant bitterness [46]. The concentrations of HxR and Hx increased with IMP consumption during cold storage with the action of microbial and autolytic microbial enzymes [47]. As to the K value, the rejection limit is generally considered as 60% and the fish muscle shall lose its edible potential once the K value exceeds the rejection limit [48]. The intinial K value was 15.31% in RTE fresh salmon fillets and increased in all samples during cold storage (Figure 1d); however, the RTE fresh salmon fillets treated with bacteriocin EFL4 significantly delayed the increase of K-values, which was similar with that of TVC results. CK and T1 exceeded the rejection limit on the 6th and 7th day, respectively; however, T2 did not exceed the maximum permissible level at the end of cold storage. These results indicated that RTE fresh salmon fillets treated with 6.4 μg/mL bacteriocin EFL4 could effectively inhibit the ATP degradation and maintain excellent quality of RTE fresh salmon fillets during storage at 4 °C for 8 days.
3.6. TVB-N Analysis
The TVB-N value of RTE fresh salmon fillets was 13.65 mg/100 g fish muscle (Figure 1e), indicating the relatively fresh nature of salmon fillets at the very start. The TVB-N values in all salmon fillets increased during cold storage, especially obvious in CK samples; however, salmon samples treated with bacteriocin EFL4 (T1 and T2) significantly reduced the formation of TVB-N comparing with the CK samples during cold storage. The TVB-N values of CK, T1 and T2 samples reached to 27.91, 26.35 and 25.96 N/100 g fish muscle on the 7th, 7th and 8th day, respectively, exceeding the upper limit value (25 mg N/100 g) of TVB-N in marine fish [49]. The high value of TVB-N in fish preservation signifies that the accumulation of nitrogen containing molecules (such as proteins and nucleic acids) are degraded by proteolytic bacteria.
3.7. TPA Results
TPA can reflect the masticatory sensation caused by the persistent elastic resistance in fish under the influence of the cross-linked network [50]. The texture changes in fish are related to the density of muscle fibers and depend on some intrinsic biological factors, such as collagen and fat contents, as well as autolytic and microbiological processes attributed to fish death, which result in the degradation of myofibril and the softening of fish muscle [51]. TPA parameters (hardness, chewiness and springiness) were used to evaluate the RTE fresh salmon fillets, and the results are shown in Figure 1f–h. The hardness of RTE fresh salmon fillets on 0 day was 1910 g, and increased at the beginning and then decreased as the activity of autolytic enzymes that hydrolyze myofibril and break down the connective tissue took place, making the muscle less elastic and softer [51]. Hardness decreased in all RTE fresh salmon fillets during cold storage, in keeping with the results of Feng et al. [52] and Yadav et al. [53]. Chewiness showed a decreased behavior as the storage time increased, in agreement with the results of others [54]. The decrease in chewiness values demonstated that fish samples became softer during cold storage [55]. CK samples showed significantly lower (p < 0.05) chewiness values compared with T1 and T2 samples during cold storage. Springiness is used to describe the ability of muscles to return to its original form when the deformation force is removed, and their resistance to subsequent deformation [51]. Bacteriocin EFL4 treatments had a significant (p < 0.05) effect on the springiness of RTE fresh salmon fillets. The average value of the springiness of RTE fresh salmon fillets was 0.73 on day 0, and decreased significantly (p < 0.05) to 0.47, 0.48 and 0.57 for CK, T1 and T2 salmon fillets on the 7th day, respectively. Previous studies have shown that the deterioration of texture is closely related to the degradation and disintegration of myofibril attributed to the decrease of endogenous proteolytic activity and then softening after death, affecting the organoleptic acceptability of consumers [56,57,58]. The results also demonstrate that bacteriocin EFL4 treatments for RTE fresh salmon fillets could effectively minimize the changes in salmon muscle.
3.8. Microstructure Variations
Figure 2 shows the microstructure changes in muscle tissues of RTE fresh salmon fillets stored at 4 °C on the 7th day. In the images, the myofibril could be clearly visible in the T2 sample with rod-like structure. Cao et al. [59] indicated that the shrinkage and fracture of myofibrils may be due to the decreased water holding capacity of the fish fillets. The destruction degree of microstructure in CK was higher than that of the T1 and T2 samples on the 7th day, indicating that bacteriocin EFL4 treatments have better performances in maintaining the integrity of myofibril in the salmon fillets, probably caused by the deterioration and degradation of myofibril. This behavior that the disordered arrangement of myofibril enhanced during cold storage was similar with the TPA results. Previous researches have shown that the reduced mechanical constraints of fiber nets lead to the conversion of secondary structure by reducing the hydrogen bonds’ force and result in the immobilized water loss [60].

Figure 2.
Changes in the microstructure of salmon treated with bacteriocin EFL4 during storage at 4 °C (CK: control; T1: salmon treated with 1MIC bacteriocin EFL4; T2: salmon treated with 2MIC bacteriocin EFL4).
3.9. Organoleptic Evaluation Results
Figure 3 shows the organoleptic evaluation results containing smell, color, mucus, muscular tissue and elasticity of RTE fresh salmon fillets during cold storage. On day, all RTE fresh salmon fillets had higher scores, indicting excellent quality. The organoleptic evaluation results of all RTE fresh salmon fillets decreased significantly (p < 0.05) with the extension of storage time; however, T2 had significantly higher scores than those of CK and T1. On the 6th day, the score of CK was lower than the rejection limit of 3, which was taken for the unacceptable value for RTE fresh salmon fillets in our research; however, T2 could be accepted on the 8th day. Bacteriocin-EFL4-treated salmon fillets kept relatively organoleptic evaluation qualities; thus, the way of treating with bacteriocin EFL4 could be a potential way to slow the deterioration and keep the organoleptic quality of salmon fillets. Comparing with chemical and microbiological analysis, organoleptic evaluation showed some retardation in evaluating the quality of RTE fresh salmon fillets. This phenomenon may be due to the organoleptic evaluation being subjective and the quality deterioration occurring inside of the RTE fresh salmon fillets first, which was usually difficult to detect promptly by visual observation [61,62]. Therefore, the quality of RTE fresh salmon fillets should be more sensitively detected by the comprehensive analysis of microbiological, chemical, and organoleptic evaluation.
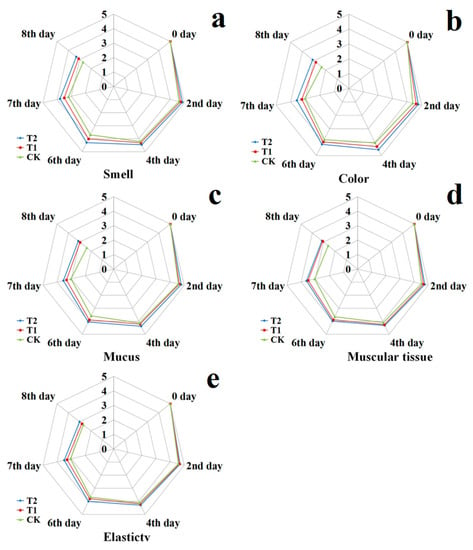
Figure 3.
Changes in the organoleptic evaluation results (smell: a; color: b; mucus: c; muscular tissue: d; elasticity: e) of salmon fillets treated with bacteriocin EFL4 during cold storage at 4 °C (CK: control; T1: salmon treated with 1MIC bacteriocin EFL4; T2: salmon treated with 2MIC bacteriocin EFL4).
4. Conclusions
The present study demonstrates the potential of bacteriocinogenic E. faecalis L04 strain and its bacteriocin EFL4 as natural biopreservatives for RTE fresh salmon fillets during cold storage. This study indicates that bacteriocin EFL4 could inhibit some spoilage bacteria growth and the MIC for S. putrefaciens was 0.32 μg/mL. This ingredient was purified and applied in improving the preservation of RTE fresh salmon fillets. The results of microbiological, physicochemical, and organoleptic properties demonstrated that the RTE fresh salmon fillets treated with 0.64 μg/mL maintained better quality during cold storage, which is probably because that bacteriocin EFL4 could effectively inhibit the growth of fish-spoilage bacteria and foodborne pathogens. In general, bacteriocin EFL4 can be considered a suitable and effective fish biopreservative; however, further studies should be focused on optimizing bacteriocin production and large-scale processes to enhance the fish industry’s economic profitability to be used as part of hurdle-based preservation technology.
Author Contributions
Conceptualization, J.M.; Data curation, Y.S.; Formal analysis, Y.S., W.L. (Wenru Liu), W.L. (Weiqing Lan) and N.L.; Funding acquisition, J.X.; Investigation, W.L. (Wenru Liu); Methodology, J.M. and N.L.; Project administration, J.M., Y.S. and J.X.; Resources, W.L. (Weiqing Lan); Software, J.M. and W.L. (Wenru Liu); Supervision, J.X.; Validation, Y.S.; Writing—original draft, J.M.; Writing—review & editing, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NSFC (31972142, 31571914, 31601414) and CARS-47-G26.
Acknowledgments
The authors express their profound gratitude to Weiqiang Qiu for his technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food 2018, 17, 1595–1612. [Google Scholar] [CrossRef]
- DeWitt, C.A.M.; Oliveira, A.C.M. Modified atmosphere systems and shelf life extension of fish and fishery products. Foods 2016, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.A.; Castro, M.P.; Aubourg, S.P.; Velázquez, J.B. Novel Technologies in Food Science; Springer: New York, NY, USA, 2012; pp. 325–360. [Google Scholar]
- Sriket, C.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Collagenolytic serine protease in fresh water prawn (Macrobrachium rosenbergii): Characteristics and its impact on muscle during iced storage. Food Chem. 2010, 124, 29–35. [Google Scholar] [CrossRef]
- Sriket, C. Proteases in fish and shellfish: Role on muscle softening and prevention. Int. Food Res. J. 2014, 21, 433–445. [Google Scholar]
- Gil, B.; Handel, A.P. Effect of a natural organic acid-icing system on the microbiological quality of commercially relevant chilled fish species. LWT Food Sci. Technol. 2012, 46, 217–223. [Google Scholar]
- Vijayakumar, P.P.; Muriana, P.M. Inhibition of Listeria monocytogenes on ready-to-eat meats using bacteriocin mixtures based on mode-of-action. Foods 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Anacarso, I.; Messi, P.; Condò, C.; Iseppi, R.; Bondi, M.; Sabia, C.; de Niederhäusern, S. A bacteriocin-like substance produced from Lactobacillus pentosus 39 is a natural antagonist for the control of Aeromonas hydrophila and Listeria monocytogenes in fresh salmon fillets. LWT Food Sci. Technol. 2014, 55, 604–611. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Todorov, S.D.; LeBlanc, J.G.; de Melo Franco, B.D.G.; Vaz-Velho, M. Lactobacillus: Classification, Uses and Health Implications; Nova Biomedical: Waltham, MA, USA, 2012; pp. 65–92. [Google Scholar]
- Concha-Meyer, A.; Schöbitz, R.; Brito, C.; Fuentes, R. Lactic acid bacteria in an alginate film inhibit Listeria monocytogenes growth on smoked salmon. Food Control 2011, 22, 485–489. [Google Scholar] [CrossRef]
- Zuckerman, H.; Avraham, R.B. Control of growth of L. monocytogenes in fresh salmon using MicrogardTM and Nisin. LWT Food Sci. Technol. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Yi, L.; Dang, J.; Zhang, L.; Wu, Y.; Liu, B.; Lü, X. Purification, characterization and bactericidal mechanism of a broad spectrum bacteriocin with antimicrobial activity against multidrug-resistant strains produced by Lactobacillus coryniformis XN8. Food Control 2016, 67, 53–62. [Google Scholar] [CrossRef]
- Lv, X.; Du, J.; Jie, Y.; Zhang, B.; Bai, F.; Zhao, H.; Li, J. Purification and antibacterial mechanism of fish-borne bacteriocin and its application in shrimp (Penaeus vannamei) for inhibiting Vibrio parahaemolyticus. World J. Microb. Biot. 2017, 33, 156. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, C.; Ruan, X.; Li, G.; Zhao, Y.; Wang, Y. Preservation of large yellow croaker (Pseudosciaena crocea) by Coagulin L1208, a novel bacteriocin produced by Bacillus coagulans L1208. Int. J. Food Microbiol. 2018, 266, 60–68. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Zhao, F.; Xiao, H.; Han, Y.; Zhou, Z. The mode of action of bacteriocin CHQS, a high antibacterial activity bacteriocin produced by Enterococcus faecalis TG2. Food Control 2019, 96, 470–478. [Google Scholar] [CrossRef]
- Sarika, A.R.; Lipton, A.P.; Aishwarya, M.S. Biopreservative efficacy of bacteriocin GP1 of Lactobacillus rhamnosus GP1 on stored fish filets. Front. Nutr. 2019, 6, 29. [Google Scholar] [CrossRef]
- Zheng, X.; Peng, Y.; Wang, W. A nondestructive real-time detection method of total viable count in pork by hyperspectral imaging technique. Appl. Sci.-Basel 2017, 7, 213. [Google Scholar] [CrossRef]
- Li, D.; Qin, N.; Zhang, L.; Li, Q.; Prinyawiwatkul, W.; Luo, Y. Degradation of adenosine triphosphate, water loss and textural changes in frozen common carp (Cyprinus carpio) fillets during storage at different temperatures. Int. J. Refrig. 2019, 98, 294–301. [Google Scholar] [CrossRef]
- Li, N.; Shen, Y.; Liu, W.; Mei, J.; Xie, J. Low-field NMR and MRI to analyze the effect of edible coating incorporated with map on qualities of half-smooth tongue sole (Cynoglossus semilaevis Günther) fillets during refrigerated storage. Appl. Sci.-Basel 2018, 8, 1391. [Google Scholar] [CrossRef]
- Li, N.; Liu, W.; Shen, Y.; Mei, J.; Xie, J. Coating effects of ε-polylysine and rosmarinic acid combined with chitosan on the storage quality of fresh half-smooth tongue sole (Cynoglossus semilaevis Günther) fillets. Coatings 2019, 9, 273. [Google Scholar] [CrossRef]
- Bonilla, A.C.; Sveinsdottir, K.; Martinsdottir, E. Development of Quality Index Method (QIM) scheme for fresh cod (Gadus morhua) fillets and application in shelf life study. Food Control 2007, 18, 352–358. [Google Scholar] [CrossRef]
- Ozogul, Y.; Yuvka, İ.; Ucar, Y.; Durmus, M.; Kösker, A.R.; Öz, M.; Ozogul, F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT Food Sci. Technol. 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Castellano, P.; Peña, N.; Ibarreche, M.P.; Carduza, F.; Soteras, T.; Vignolo, G. Antilisterial efficacy of Lactobacillus bacteriocins and organic acids on frankfurters. Impact on sensory characteristics. J. Food Sci. Technol. 2018, 55, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.C.; Ross, R.P.; Stanton, C.; Silva, C.C.G. Characterization and application of antilisterial enterocins on model fresh cheese. J. Food Prot. 2017, 80, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, T.; Szakmár, K.; Kiskó, G.; Mohácsi-Farkas, C.; Reichart, O. Combined effect of NaCl and low temperature on antilisterial bacteriocin production of Lactobacillus plantarum ST202Ch. LWT Food Sci. Technol. 2018, 89, 104–1097. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Beninati, C.; Ziino, G.; Giuffrida, A.; Panebianco, A. Activity of R(+) limonene on the maximum growth rate of fish spoilage organisms and related effects on shelf-life prolongation of fresh gilthead sea bream fillets. Int. J. Food Microbiol. 2016, 237, 109–113. [Google Scholar] [CrossRef]
- Jääskeläinen, E.; Jakobsen, L.M.A.; Hultman, J.; Eggers, N.; Bertram, H.C.; Björkroth, J. Metabolomics and bacterial diversity of packaged yellowfin tuna (Thunnus albacares) and salmon (Salmo salar) show fish species-specific spoilage development during chilled storage. Int. J. Food Microbiol. 2019, 293, 44–52. [Google Scholar] [CrossRef]
- Bono, G.; Okpala, C.O.R.; Vitale, S.; Ferrantelli, V.; Noto, A.D.; Costa, A.; Di Bella, C.; Monaco, D.L. Effects of different ozonized slurry-ice treatments and superchilling storage (−1 °C) on microbial spoilage of two important pelagic fish species. Food Sci. Nutr. 2017, 5, 1049–1056. [Google Scholar] [CrossRef]
- Hwanhlem, N.; Ivanova, T.; Biscola, V.; Choiset, Y.; Haertlé, T. Bacteriocin producing Enterococcus faecalis isolated from chicken gastrointestinal tract originating from Phitsanulok, Thailand: Isolation, screening, safety evaluation and probiotic properties. Food Control 2017, 78, 187–195. [Google Scholar] [CrossRef]
- H-Kittikun, A.; Biscola, V.; El-Ghaish, S.; Jaffrès, E.; Dousset, X.; Pillot, G.; Haertlé, T.; Chobert, J.-M.; Hwanhlem, N. Bacteriocin-producing Enterococcus faecalis KT2W2G isolated from mangrove forests in southern Thailand: Purification, characterization and safety evaluation. Food Control 2015, 54, 126–134. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Li, X.; Hao, X.; Xu, D.; Zhou, Y.; Mehmood, A.; Wang, C. Genetic and biochemical evidence that Enterococcus faecalis Gr17 produces a novel and sec-dependent bacteriocin, Enterocin Gr17. Front. Microbiol. 2019, 10, 1806. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sala, B.; Herranz, C.; Díaz-Freitas, B.; Hernández, P.E.; Sala, A.; Cintas, L.M. Strategies to increase the hygienic and economic value of fresh fish: Biopreservation using lactic acid bacteria of marine origin. Int. J. Food Microbiol. 2016, 223, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sala, B.; Muñoz-Atienza, E.; Sánchez, J.; Basanta, A.; Herranz, C.; Hernández, P.E.; Cintas, L.M. Bacteriocin production by lactic acid bacteria isolated from fish, seafood and fish products. Eur. Food Res. Technol. 2015, 241, 341–356. [Google Scholar] [CrossRef]
- Daba, G.M.; Ishibashi, N.; Gong, X.; Taki, H.; Yamashiro, K.; Lim, Y.Y.; Zendo, T.; Sonomoto, K. Characterisation of the action mechanism of a Lactococcus-specific bacteriocin, lactococcin Z. J. Biosci. Bioeng. 2018, 126, 603–610. [Google Scholar] [CrossRef]
- Yi, L.; Luo, L.; Lü, X. Efficient exploitation of multiple novel bacteriocins by combination of complete genome and peptidome. Front. Microbiol. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Zhang, Y.; Wu, R.; Li, P. Antibacterial mechanism of plantaricin LPL-1, a novel class IIa bacteriocin against Listeria monocytogenes. Food Control 2019, 97, 87–93. [Google Scholar] [CrossRef]
- Al-Seraih, A.; Belguesmia, Y.; Baah, J.; Szunerits, S.; Boukherroub, R.; Drider, D. Enterocin B3A-B3B produced by LAB collected from infant faeces: Potential utilization in the food industry for Listeria monocytogenes biofilm management. Anton. Leeuw. Int. J. Gener. 2016, 110, 1–15. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ozyurt, G.; Ozogul, F.; Kuley, E.; Polat, A. Freshness assessment of European eel (Anguilla anguilla) by sensory, chemical and microbiological methods. Food Chem. 2005, 92, 745–751. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G.; Alpas, H.; Selçuk, A.; Özden, Ö.; Buzrul, S. The effect of different high pressure conditions on the quality and shelf life of cold smoked fish. Innov. Food Sci. Emerg. 2011, 12, 104–110. [Google Scholar] [CrossRef]
- Jia, S.; Liu, X.; Huang, Z.; Li, Y.; Zhang, L.; Luo, Y. Effects of chitosan oligosaccharides on microbiota composition of silver carp (Hypophthalmichthys molitrix) determined by culture-dependent and independent methods during chilled storage. Int. J. Food Microbiol. 2018, 268, 81–91. [Google Scholar] [CrossRef]
- Zhe, W.; Hu, S.; Gao, Y.; Chen, Y.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT Food Sci. Technol. 2017, 75, 59–64. [Google Scholar]
- Shi, C.; Han, L.; Cui, J.; Shen, H.; Luo, Y. Study on the predictive models of the quality of silver carp (Hypophthalmichthys Molitrix) fillets stored under variable temperature conditions. J. Food Process. Pres. 2014, 38, 356–363. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shi, W.; Zhou, S.; Qu, Y.; Wang, Z. Research on the changes of water-soluble flavor substances in grass carp during steaming. J. Food Biochem. 2019, 43, e12993. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, X.; Jia, S.; Luo, Y. Antimicrobial effects of cinnamon bark oil on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Food Control 2017, 82, 316–324. [Google Scholar] [CrossRef]
- Laly, S.J.; Ashok Kumar, K.N.; Sankar, T.V.; Lalitha, K.V.; Ninan, G. Quality of monosex tilapia under ice storage: Gutting effects on the formation of biogenic amines, biochemical, and microbiological characteristics. Int. J. Food Prop. 2017, 20, 1368–1377. [Google Scholar] [CrossRef]
- Aghaei, Z.; Emadzadeh, B.; Ghorani, B.; Kadkhodaee, R. Cellulose acetate nanofibres containing alizarin as a halochromic sensor for the qualitative assessment of rainbow trout fish spoilage. Food Bioprocess Tech. 2018, 11, 1087–1095. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Thavaraj, P.; Yang, X.; Guo, Y. Effects of thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) surimi during cold storage. Food Chem. 2017, 224, 372–381. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Monitoring changes in whiting (Merlangius merlangus) fillets stored under modified atmosphere packaging by front face fluorescence spectroscopy and instrumental techniques. Food Chem. 2016, 200, 343–353. [Google Scholar] [CrossRef]
- Feng, X.; Ng, K.V.; MikšKrajnik, M.; Yang, H. Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioprocess Tech. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Yadav, U.; Singh, R.R.B.; Arora, S. Evaluation of quality changes in nutritionally enriched extruded snacks during storage. J. Food Sci. Technol. 2018, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Liao, L.; Qiao, Y.; Xiong, G.; Li, D.; Wang, C.; Hu, J.; Wang, L.; Wu, W.; Ding, A. The effects of vacuum package combined with tea polyphenols (V+TP) treatment on quality enhancement of weever (Micropterus salmoides) stored at 0 °C and 4 °C. LWT Food Sci. Technol. 2018, 91, 484–490. [Google Scholar] [CrossRef]
- Viji, P.; Tanuja, S.; Ninan, G.; Lalitha, K.V.; Zynudheen, A.A.; Binsi, P.K.; Srinivasagopal, T.K. Biochemical, textural, microbiological and sensory attributes of gutted and ungutted sutchi catfish (Pangasianodon hypophthalmus) stored in ice. J. Food Sci. Technol. 2015, 52, 3312–3321. [Google Scholar] [CrossRef] [PubMed]
- Mahto, R.; Ghosh, S.; Das, M.K.; Das, M. Effect of gamma irradiation and frozen storage on the quality of fresh water prawn (Macrobrachium rosenbergii) and tiger prawn (Penaeus monodon). LWT Food Sci. Technol. 2015, 61, 573–582. [Google Scholar] [CrossRef]
- Chaparrohernández, S.; Ruízcruz, S.; Márquezríos, E.; Ocañohiguera, V.M.; Valenzuelalópez, C.C.; Ornelaspaz, J.D.J.; Deltorosánchez, C.L.; Chaparrohernández, S.; Ruízcruz, S.; Márquezríos, E. Effect of chitosan-carvacrol edible coatings on the quality and shelf life of tilapia (Oreochromis niloticus) fillets stored in ice. Food Sci. Technol. 2015, 35, 734–741. [Google Scholar] [CrossRef]
- Yang, F.; Hu, S.; Ye, L.; Hui, Y.; Yong, Z.; Li, L. Effects of coatings of polyethyleneimine and thyme essential oil combined with chitosan on sliced fresh Channa argus during refrigerated dtorage. J. Food Process Eng. 2015, 38, 225–233. [Google Scholar] [CrossRef]
- Cao, L.; Rasco, B.A.; Tang, J.; Niu, L.; Lai, K.; Fan, Y.; Huang, Y. Effects of freshness on the cook loss and shrinkage of grass carp (Ctenopharyngodon idellus) fillets following pasteurization. Int. J. Food Prop. 2016, 19, 2297–2306. [Google Scholar] [CrossRef]
- Cao, M.; Cao, A.; Wang, J.; Cai, L.; Regenstein, J.; Ruan, Y.; Li, X. Effect of magnetic nanoparticles plus microwave or far-infrared thawing on protein conformation changes and moisture migration of red seabream (Pagrus major) fillets. Food Chem. 2018, 266, 498–507. [Google Scholar] [CrossRef]
- Xuan, X.T.; Fan, Y.F.; Ling, J.G.; Hu, Y.Q.; Liu, D.H.; Chen, S.G.; Ye, X.Q.; Ding, T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control 2017, 73, 1483–1489. [Google Scholar] [CrossRef]
- Lather, P.; Mohanty, A.K.; Jha, P.; Garsa, A.K.; Sood, S.K. Changes associated with cell membrane composition of Staphylococcus aureus on acquisition of resistance against class IIa bacteriocin and its in vitro substantiation. Eur. Food Res. Technol. 2015, 240, 101–107. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).