The Effects of the Addition of Ti3SiC2 on the Microstructure and Properties of Laser Cladding Composite Coatings
Abstract
1. Introduction
2. Experimental Procedures
2.1. Preparation of Coatings
2.2. Microstructure Characterization and Property Measurements
3. Results and Discussion
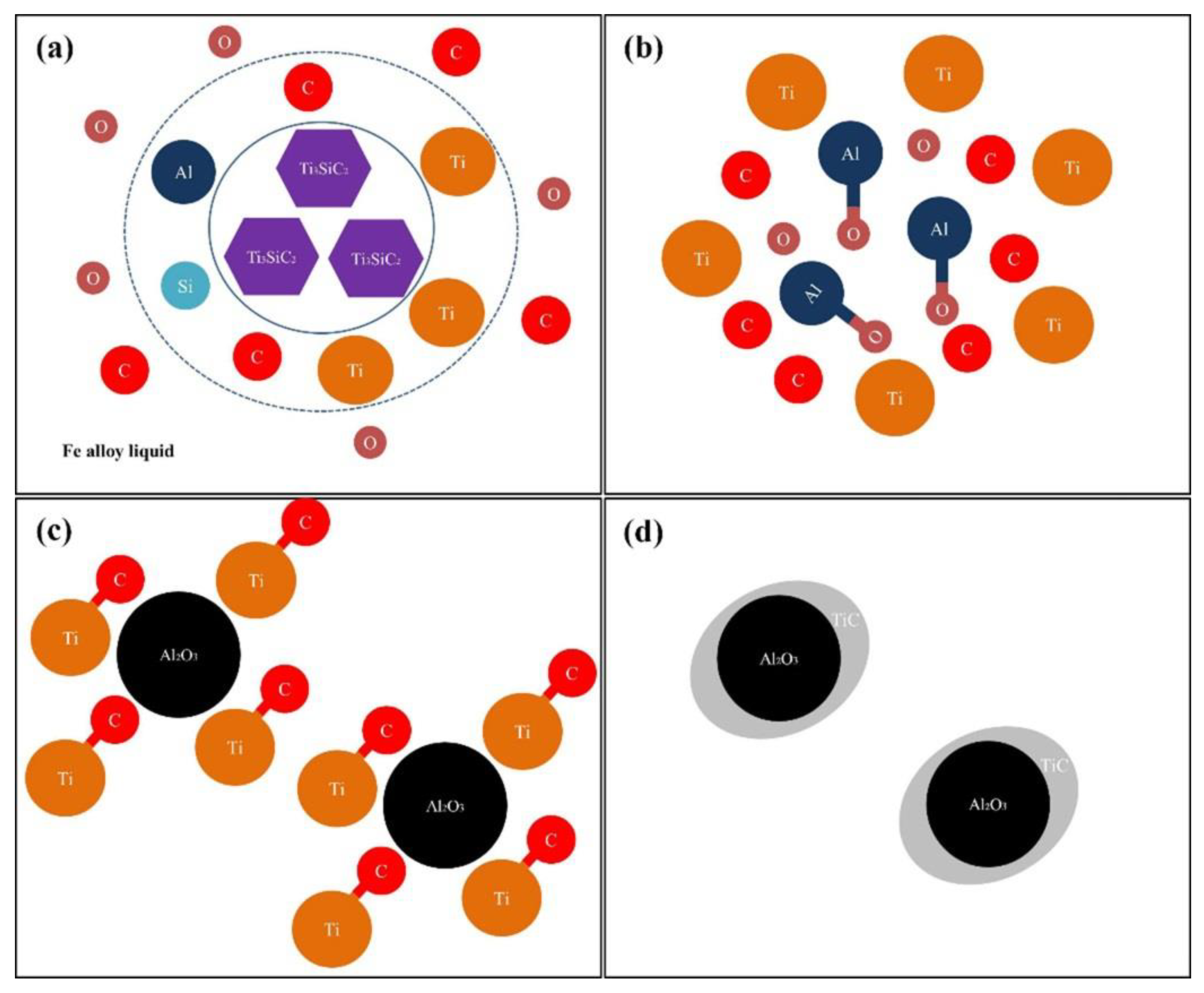
3.1. Macroscopic Appearance and Phase Composition
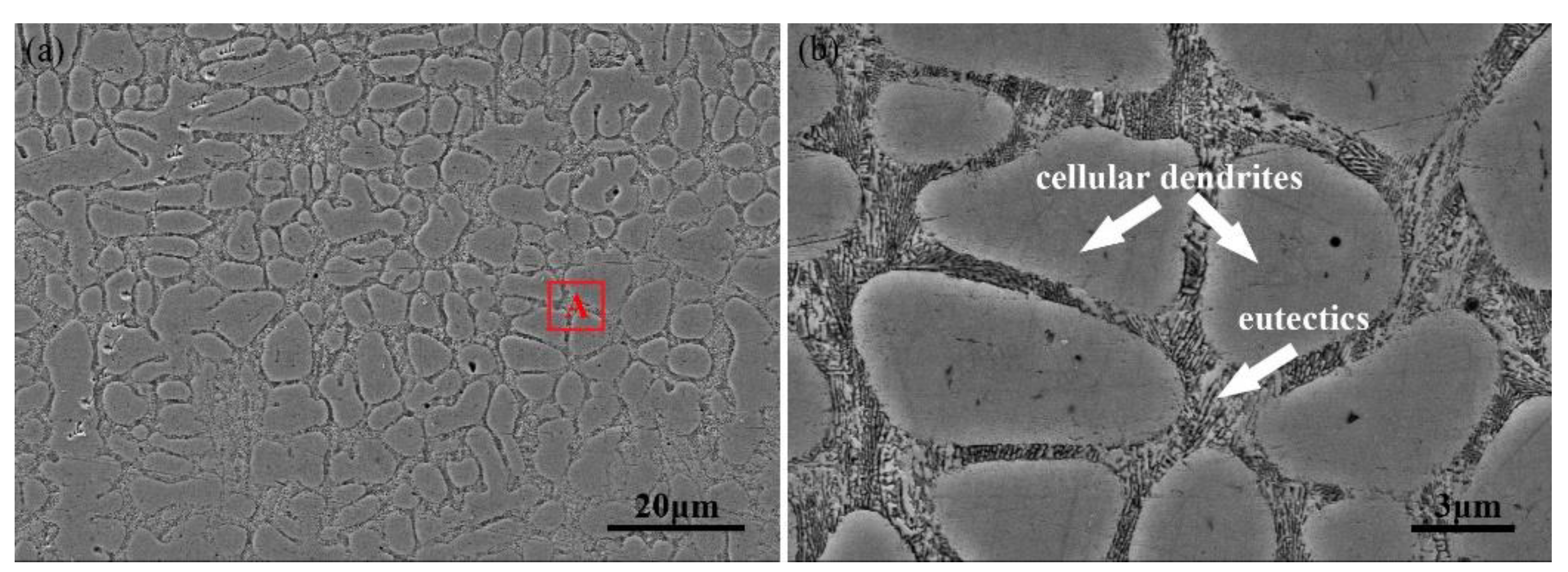
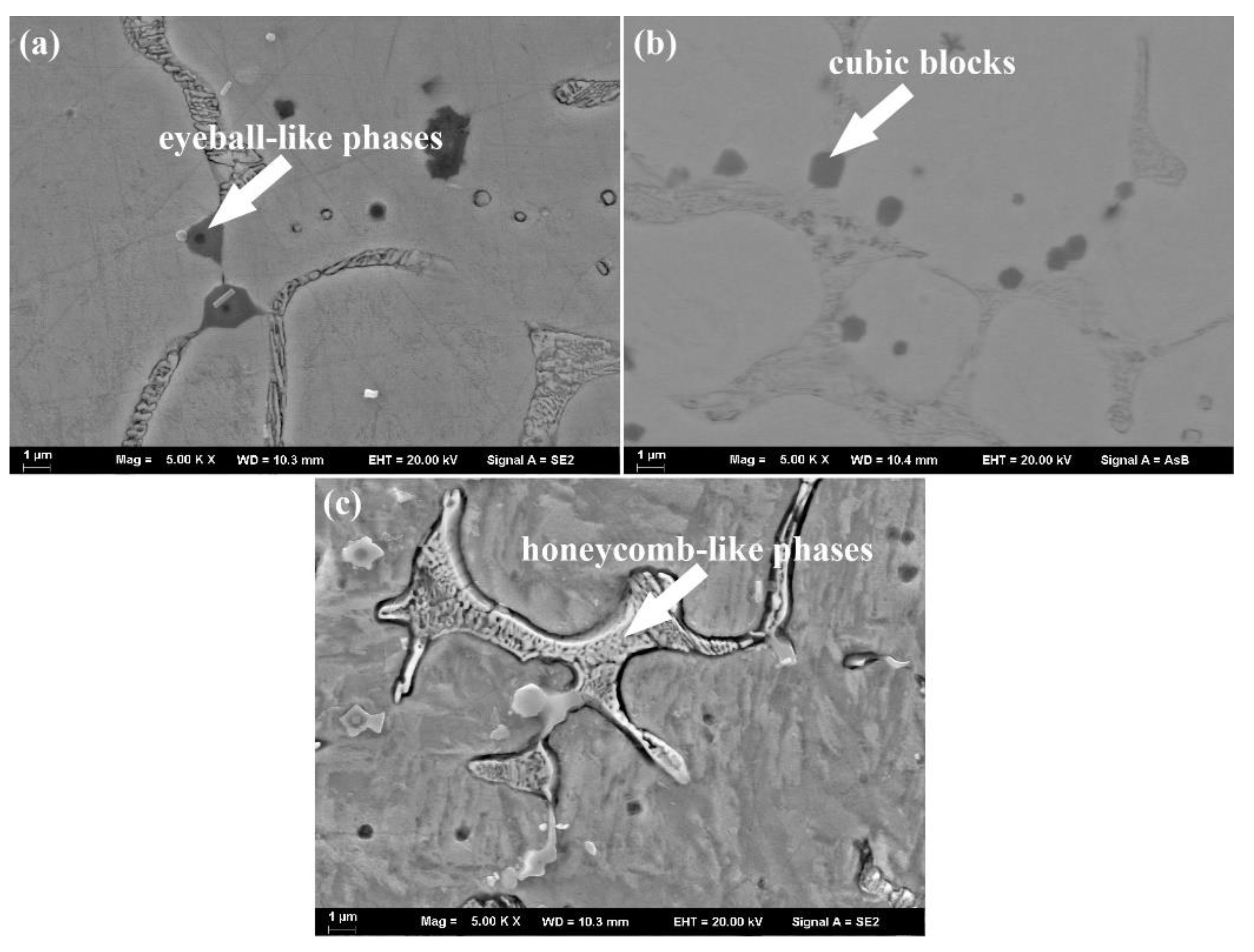
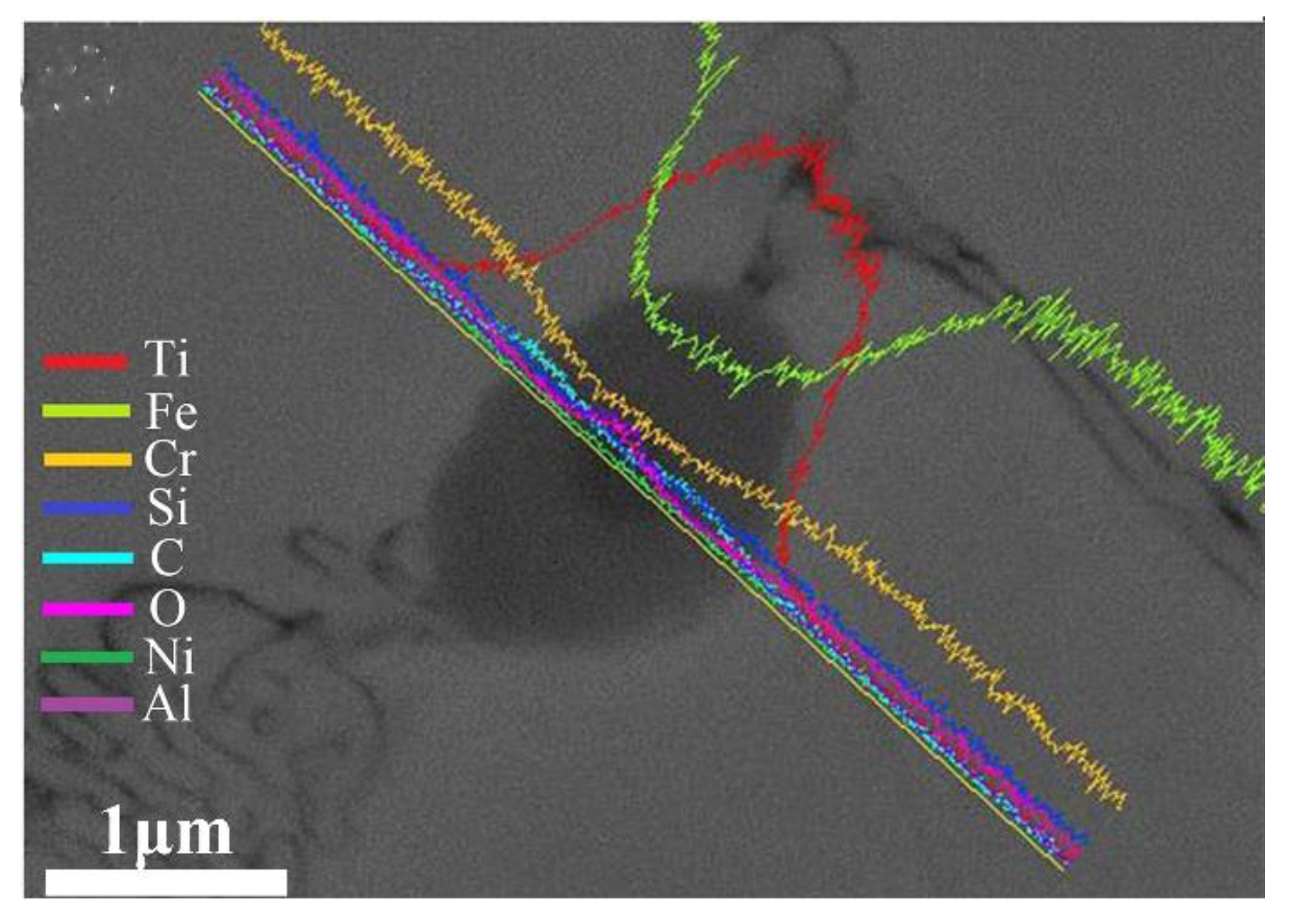
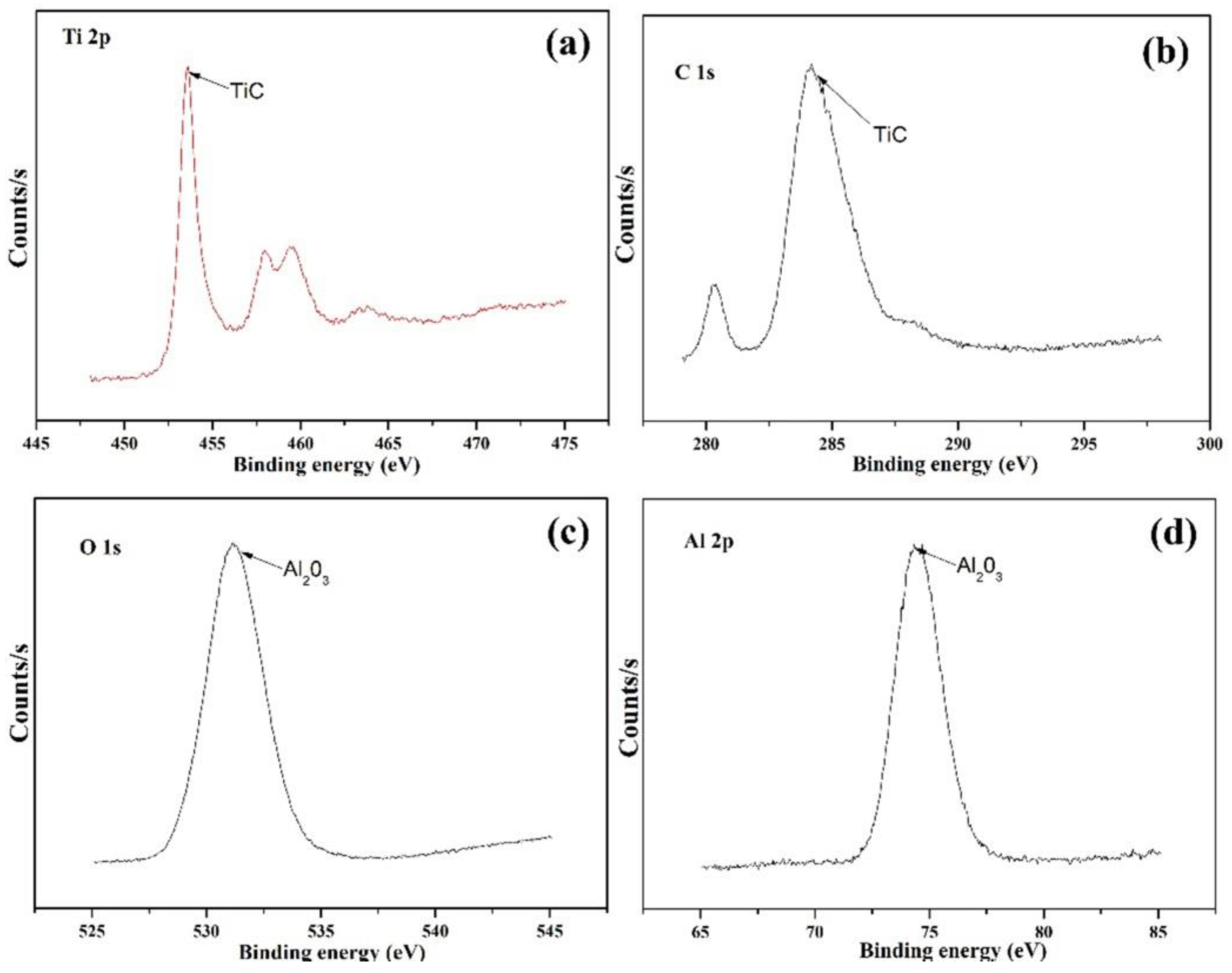
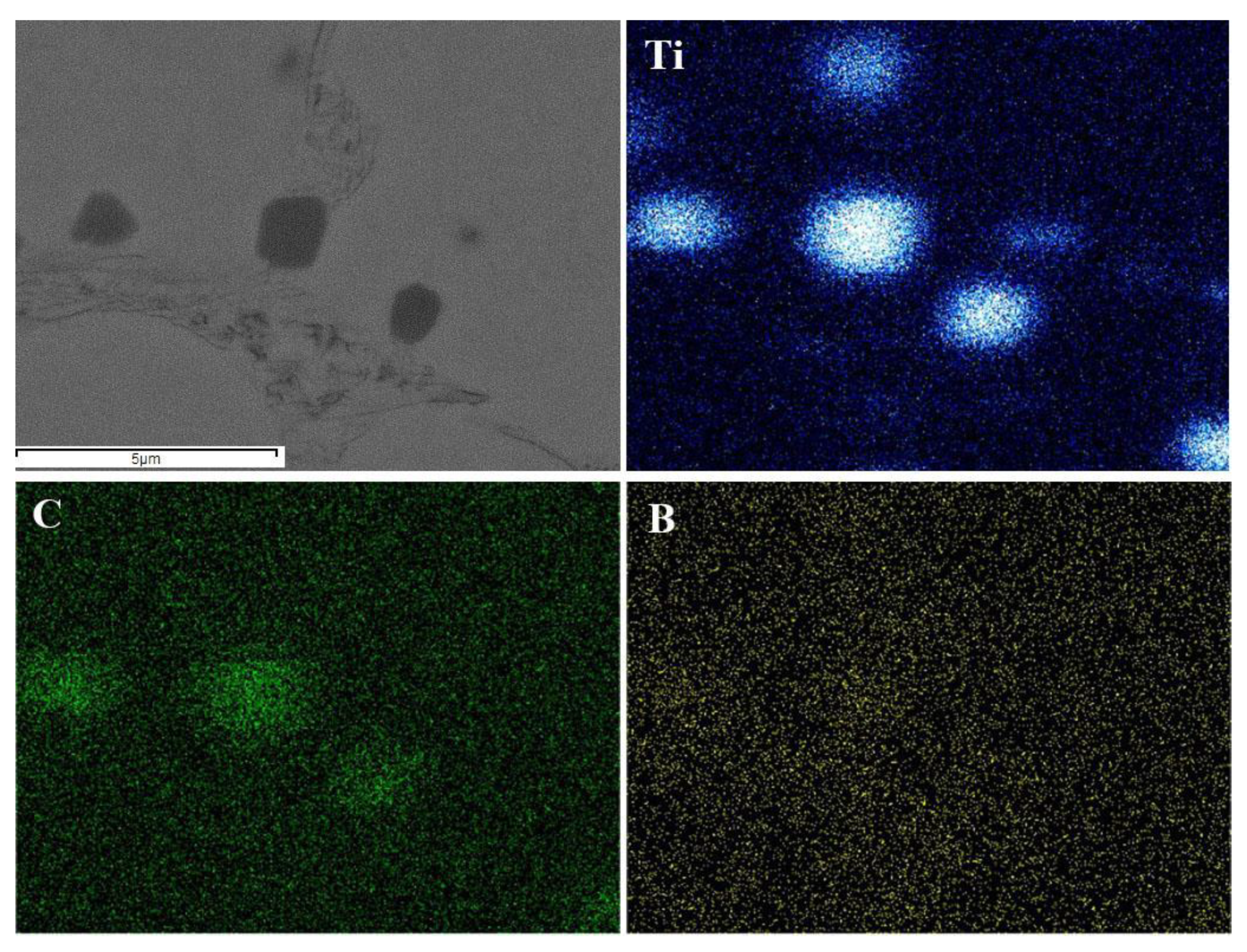
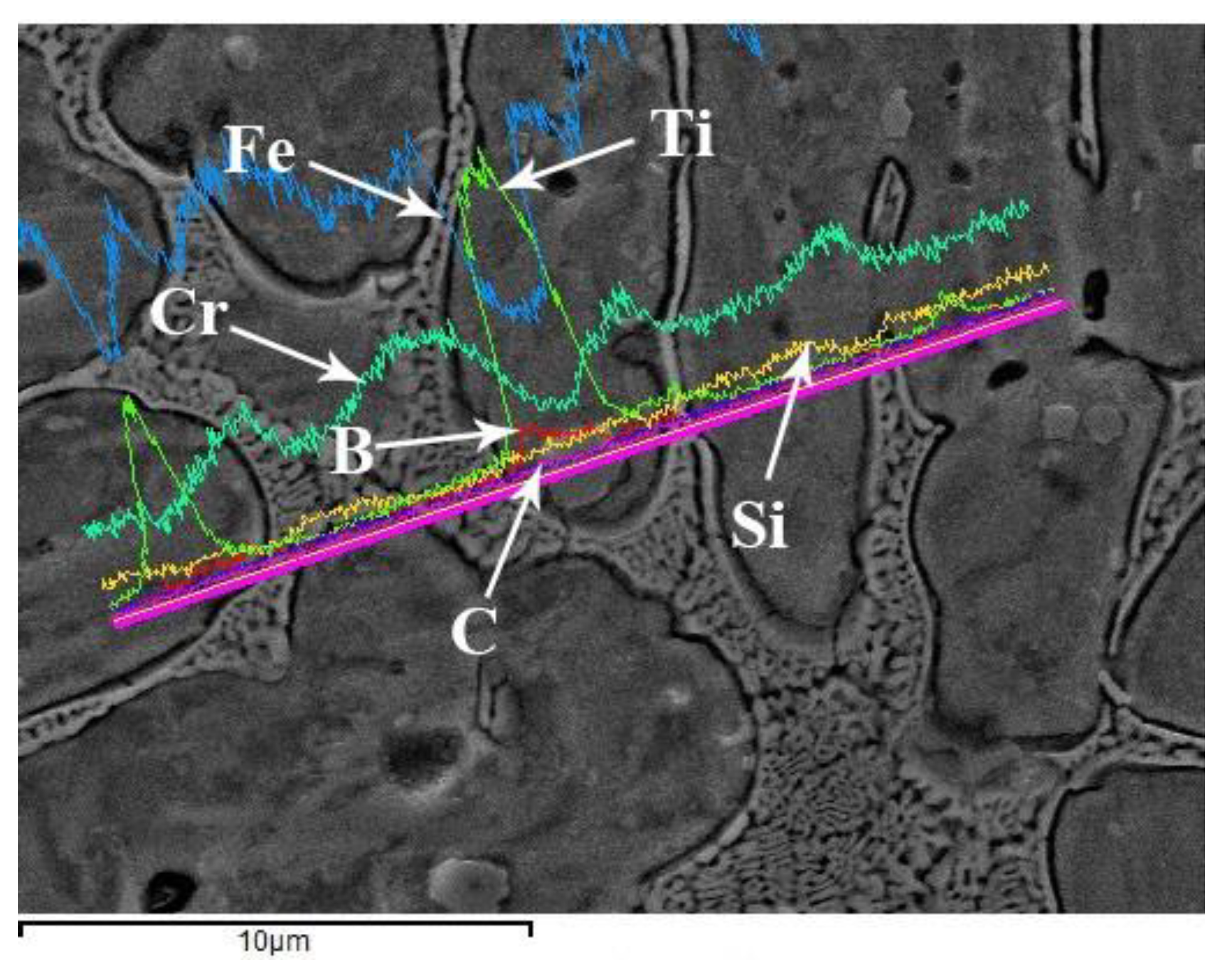
3.2. Microstructure Analysis
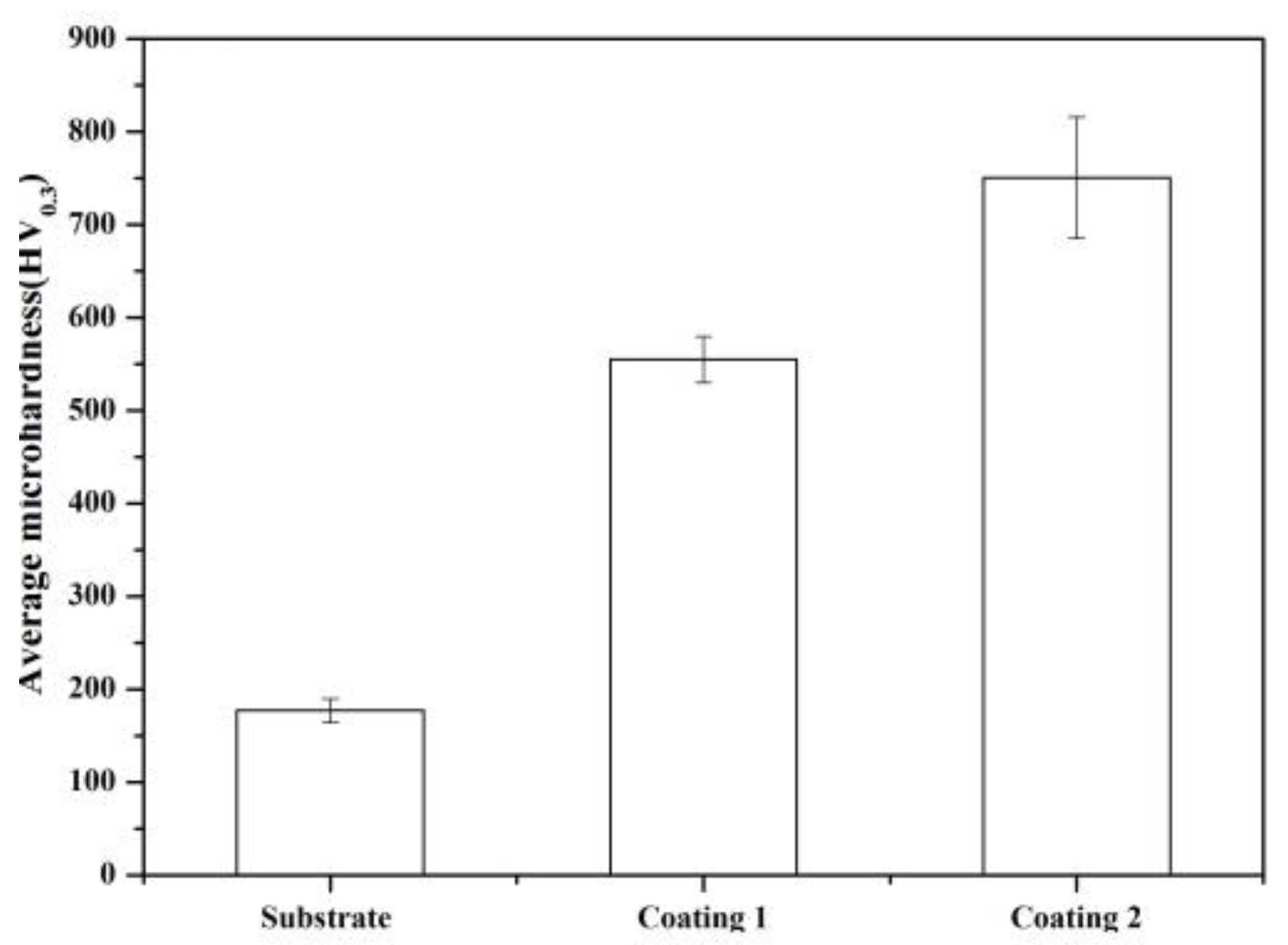
3.3. Microhardness
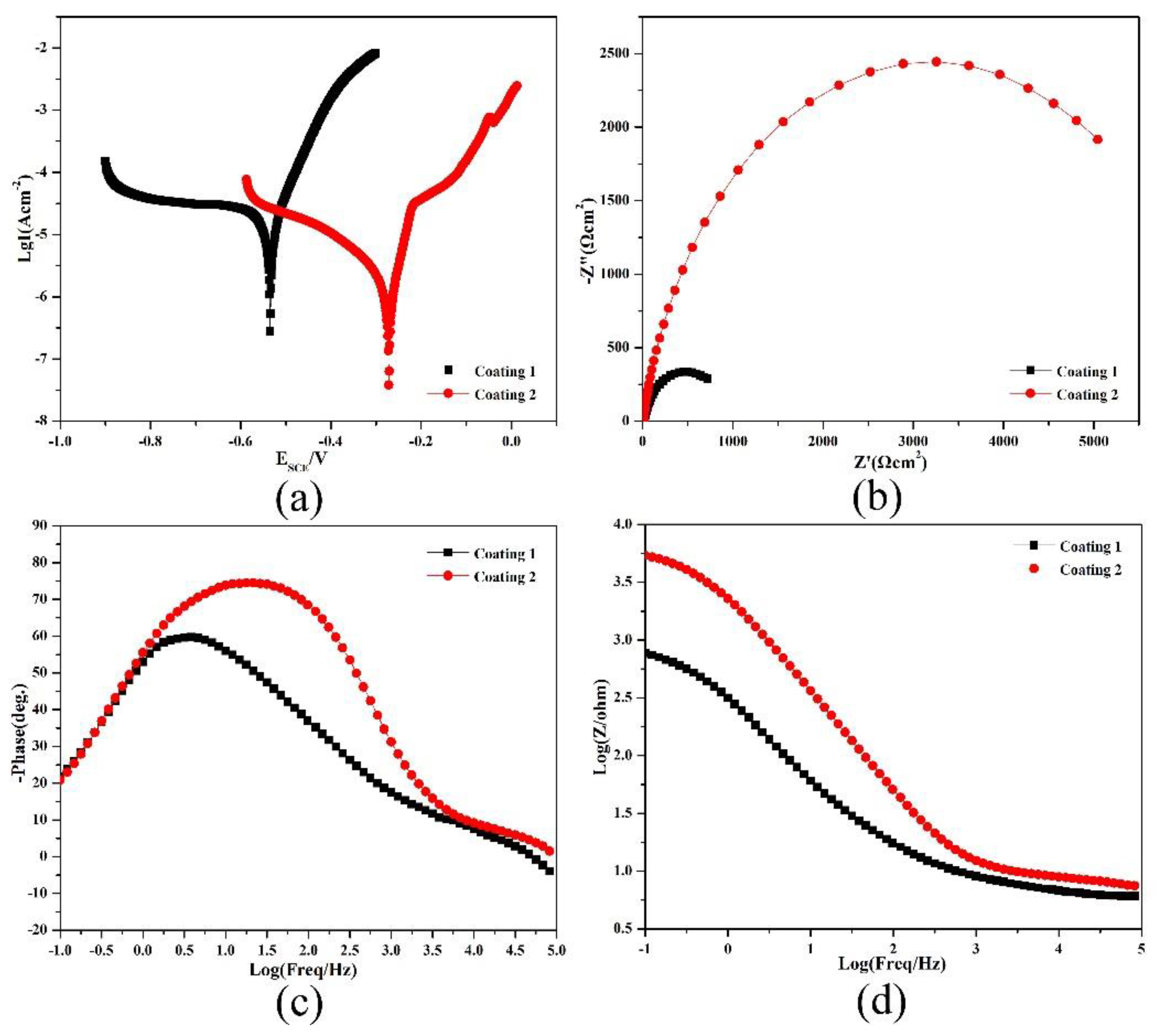
3.4. Electrochemical Properties
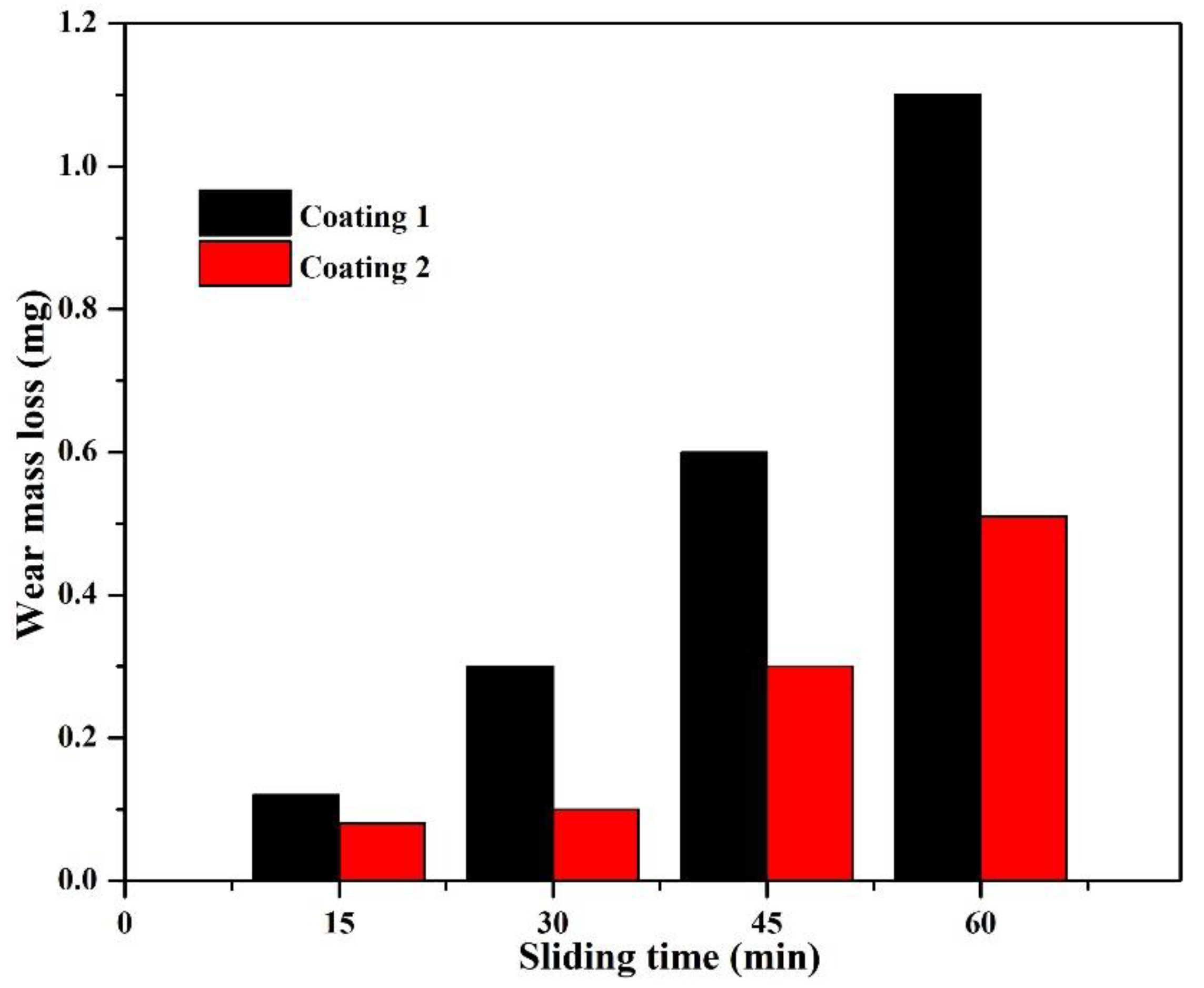
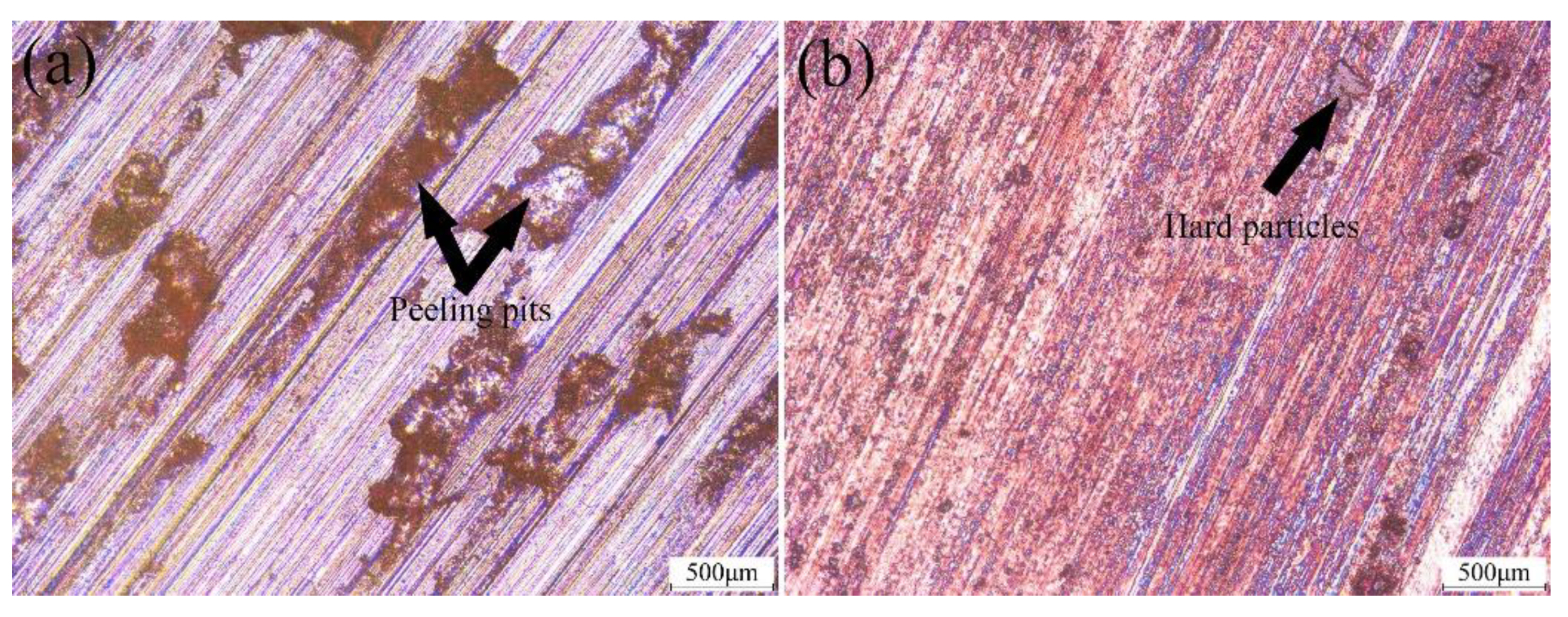
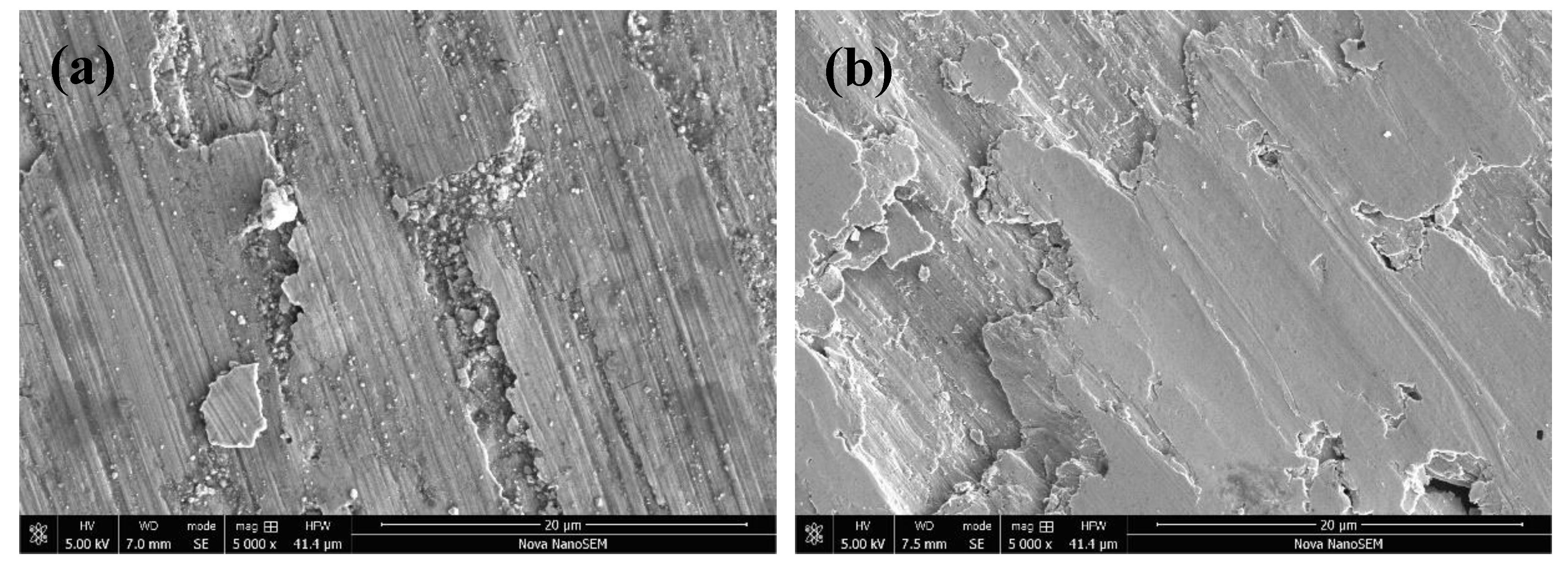
3.5. Tribological Properties and Mechanisms
4. Conclusions
- (1)
- With the addition of Ti3SiC2, many additional reinforcements, such as (Cr, Fe)23C6 carbides, Ti(B,C)2, and Al2O3/TiC composite ceramics, were formed in situ in the composite coatings;
- (2)
- The microhardness, corrosion, and wear resistance of composite coating with 10 wt.% Ti3SiC2 were about 1.35, 4.3, and 2 times the values of the original coating, respectively, which was mainly attributed to the formation of additional reinforcements;
- (3)
- Severe fatigue spalling and microcutting were the main wear mechanisms of the original coating, while severe slight microcutting and the formation of the transferred film were the main mechanisms of the composite coating.
Author Contributions
Funding
Conflicts of Interest
References
- Aussavy, D.; Costil, S.; El Kedim, O.; Montavon, G.; Bonnot, A.-F. Metal matrix composite coatings manufactured by thermal spraying: Influence of the powder preparation on the coating properties. J. Therm. Spray Technol. 2014, 23, 190–196. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Raghupathy, Y.; Srinivasan, D.; Suwas, S.; Srivastava, C. Microstructural evolution of cold-sprayed Inconel 625 superalloy coatings on low alloy steel substrate. Acta Mater. 2017, 129, 11–25. [Google Scholar] [CrossRef]
- Nolan, D.; Huang, S.W.; Leskovsek, V.; Braun, S. Sliding wear of titanium nitride thin films deposited on Ti–6Al–4V alloy by PVD and plasma nitriding processes. Surf. Coat. Technol. 2006, 200, 5698–5705. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Hafnway, A.; Khaled, A. Electrodeposition and corrosion behavior of nano-structured Ni-WC and Ni-Co-WC composite coating. J. Alloys Compd. 2017, 695, 1505–1514. [Google Scholar] [CrossRef]
- Zeisig, J.; Schädlich, N.; Giebeler, L. Microstructure and abrasive wear behavior of a novel FeCrMoVC laser cladding alloy for high-performance tool steels. Wear 2017, 382, 107–112. [Google Scholar] [CrossRef]
- Wang, K.; Du, D.; Liu, G.; Chang, B. Microstructure and properties of WC reinforced Ni-based composite coatings with Y2O3 addition on titanium alloy by laser cladding. Sci. Technol. Weld. Join. 2019, 24, 517–524. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.H.; Zhang, S. Manufacturing of Ti3SiC2 lubricated Co-based alloy coatings using laser cladding technology. Opt. Laser Technol. 2019, 114, 209–215. [Google Scholar] [CrossRef]
- Zhai, L.L.; Ban, C.Y.; Zhang, J.W. Microstructure, microhardness and corrosion resistance of NiCrBSi coatings under electromagnetic field auxiliary laser cladding. Surf. Coat. Technol. 2019, 358, 531–538. [Google Scholar] [CrossRef]
- Liu, X.B.; Meng, X.J.; Liu, H.Q.; Shi, G.L.; Wu, S.H.; Sun, C.F.; Wang, M.D.; Qi, L.H. Effects of aging treatment on microstructure and tribological properties of nickel-based high-temperature self-lubrication wear resistant composite coatings by laser cladding. Mater. Chem. Phys. 2014, 143, 616–621. [Google Scholar] [CrossRef]
- Zhai, L.L.; Ban, C.Y.; Zhang, J.W. Investigation on laser cladding Ni-base coating assisted by electromagnetic field. Opt. Laser Technol. 2019, 114, 81–88. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C.; Dai, J. Microstructures and wear properties of laser cladding Co-based composite coatings on Ti–6Al–4V. Mater. Des. 2015, 80, 174–181. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Wang, J. Investigation on cored-eutectic structure in Ni60/WC composite coatings fabricated by wide-band laser cladding. J. Alloys Comp. 2015, 645, 151–157. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.; Zhao, J. Effect of ZrB2 on the microstructure and wear resistance of Ni-based composite coating produced on pure Ti by laser cladding. Tribol. Trans. 2010, 54, 80–86. [Google Scholar] [CrossRef]
- Wulin, S.; Echigoya, J.; Beidi, Z. Effects of Co on the cracking susceptibility and the microstructure of Fe–Cr–Ni laser-clad layer. Surf. Coat. Technol. 2001, 138, 291–295. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Gao, W. Microstructure and properties of laser cladding FeCrBSi composite powder coatings with higher Cr content. J. Mater. Process. Technol. 2014, 214, 899–905. [Google Scholar] [CrossRef]
- Yu, T.; Deng, Q.; Dong, G.; Yang, J. Effects of Ta on microstructure and microhardness of Ni based laser clad coating. Appl. Surf. Sci. 2011, 257, 5098–5103. [Google Scholar] [CrossRef]
- Qunshuang, M.; Yajiang, L.; Juan, W. Effects of Ti addition on microstructure homogenization and wear resistance of wide-band laser clad Ni60/WC composite coatings. Int. J. Refract. Met. Hard Mater. 2017, 64, 225–233. [Google Scholar] [CrossRef]
- Wulin, S.; Echigoya, J.; Beidi, Z.; Changsheng, X.; Kun, C. Effects of Co on the cracking susceptibility and the microstructure of Fe–Cr–Ni laser-clad layer. Wear 2014, 310, 72–82. [Google Scholar]
- Farahmand, P.; Liu, S.; Zhang, Z.; Kovacevic, R. Laser cladding assisted by induction heating of Ni–WC composite enhanced by nano-WC and La2O3. Ceram. Int. 2014, 40, 15421–15438. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y. Microstructure and properties of Fe-based composite coating by laser cladding Fe–Ti–V–Cr–C–CeO2 powder. Opt. Laser Technol. 2015, 65, 119–125. [Google Scholar] [CrossRef]
- Chen, D.; Tian, X.; Wang, H. Rapid synthesis of TiC/Ti3SiC2 composites by laser melting. Int. J. Refract. Met. Hard. 2014, 47, 102–107. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, X.; Wang, M. Effect of Ag and Ti 3 SiC 2 on tribological properties of TiAl matrix self-lubricating composites at room and increased temperatures. Tribol. Lett. 2014, 53, 617–629. [Google Scholar] [CrossRef]
- Gupta, S.; Barsoum, M.W. On the tribology of the MAX phases and their composites during dry sliding: A review. Wear 2011, 271, 1878–1894. [Google Scholar] [CrossRef]
- Lu, X.L.; Liu, X.B.; Yu, P.C. Efficient photocatalytic reduction of CO2 by amine-functionalized g-C3N4. Appl. Surf. Sci. 2015, 355, 350–358. [Google Scholar]
- Wang, A.H.; Zhang, X.L. Ni-based alloy/submicron WS2 self-lubricating composite coating synthesized by Nd: YAG laser cladding. Mater. Sci. Eng. A 2008, 475, 312–318. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C.; Liu, J.; Zhao, L.; Dai, J. Microstructure and property of composite coatings on titanium alloy deposited by laser cladding with Co42+TiN mixed powders. J. Alloys Compd. 2016, 686, 74–81. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Liu, J. Microstructure and wear property of the Ti5Si3/TiC reinforced Co-based coatings fabricated by laser cladding on Ti-6Al-4V. Opt. Laser Technol. 2017, 92, 156–162. [Google Scholar] [CrossRef]
- Lu, X.L.; Liu, X.B.; Yu, P.C. Synthesis and characterization of Ni60-hBN high temperature self-lubricating anti-wear composite coatings on Ti6Al4V alloy by laser cladding. Opt. Laser Technol. 2016, 78, 87–94. [Google Scholar] [CrossRef]
- Yang, M.S.; Liu, X.B.; Fan, J.W. Microstructure and wear behaviors of laser clad NiCr/Cr3C2–WS2 high temperature self-lubricating wear-resistant composite coating. Appl. Surf. Sci. 2012, 258, 3757–3762. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C. Microstructures and properties of TiN reinforced Co-based composite coatings modified with Y2O3 by laser cladding on Ti–6Al–4V alloy. J. Alloys Comp. 2015, 650, 178–184. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Zou, Z. Effects of chromium addition on microstructure and properties of TiC–VC reinforced Fe-based laser cladding coatings. J. Alloys Comp. 2014, 614, 107–112. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Selvi, V.E.; Rajam, K.S. Electrochemical behavior of low phosphorus electroless Ni–P–Si3N4 composite coatings. Mater. Chem. Phys. 2010, 120, 546–551. [Google Scholar] [CrossRef]
- Zhai, Y.J.; Liu, X.B.; Qiao, S.J. Surface nano-hardness and microstructure of a single crystal nickel base superalloy after laser shock peening. Opt. Laser Technol. 2017, 89, 97–107. [Google Scholar]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Zhang, X.; Ni, Q.; Chen, Y.; Wu, M.; Yang, C. Distinction in corrosion resistance of selective laser melted Ti-6Al-4V alloy on different planes. Corros. Sci. 2016, 111, 703–710. [Google Scholar] [CrossRef]
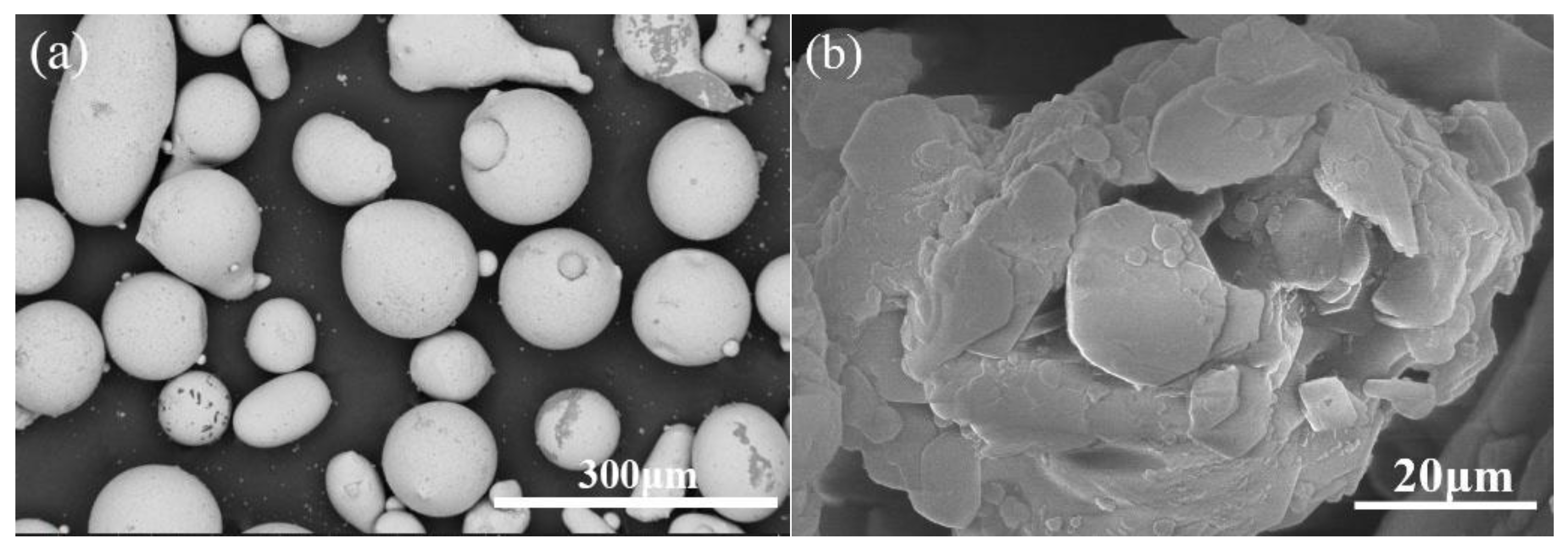

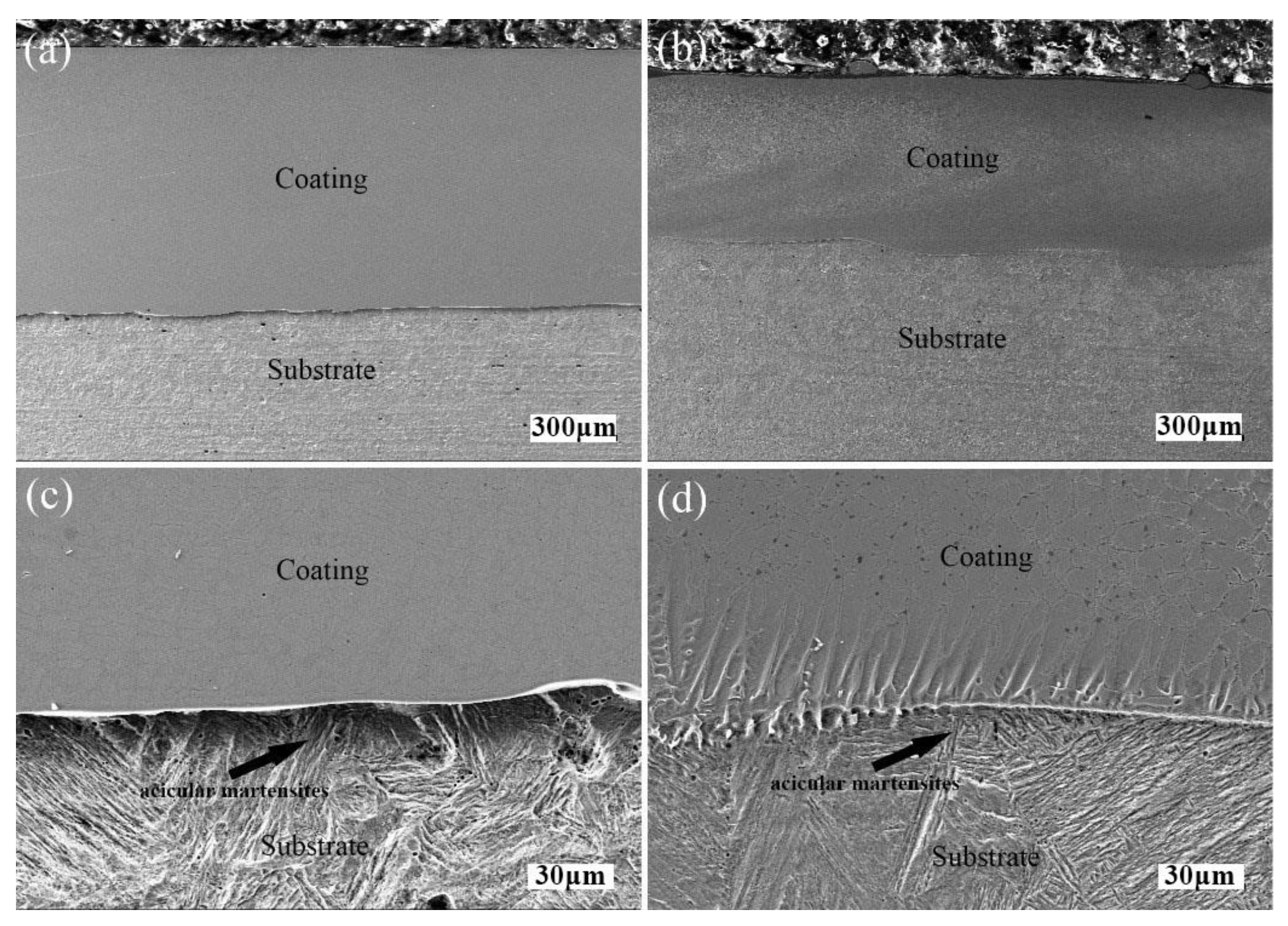
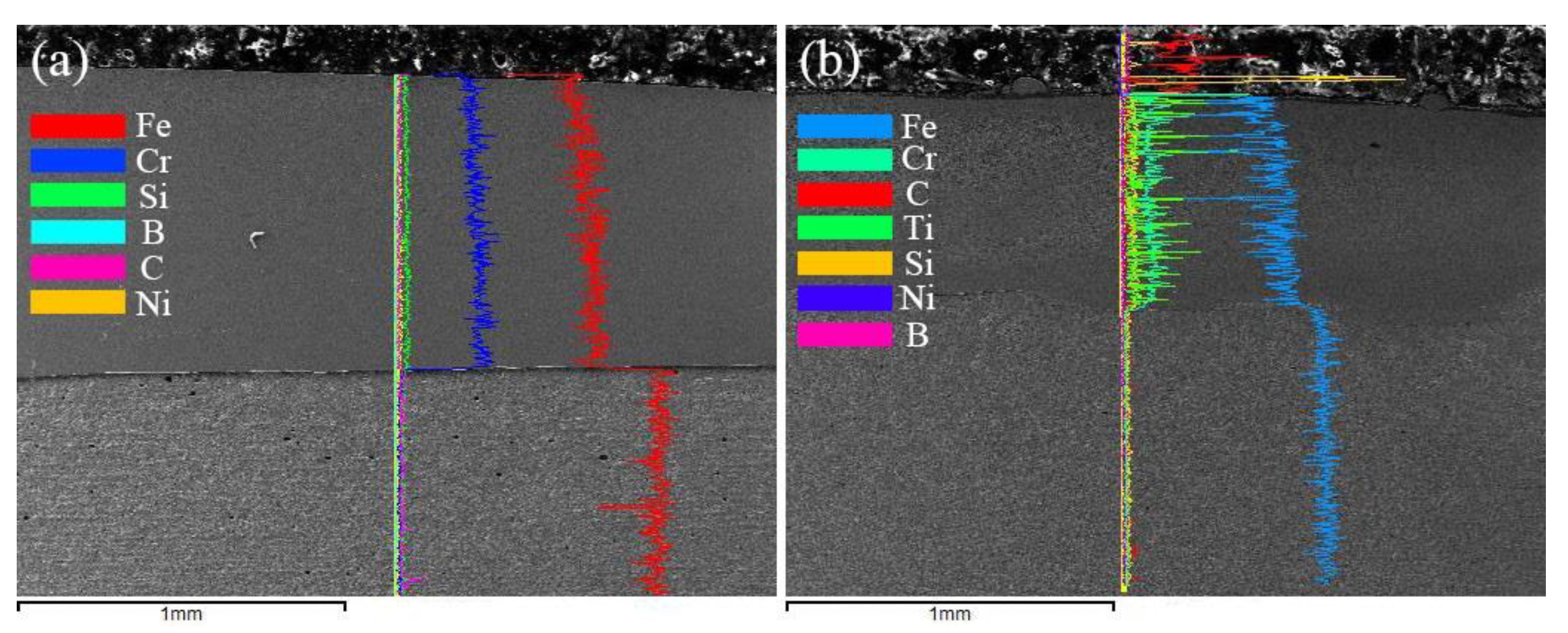
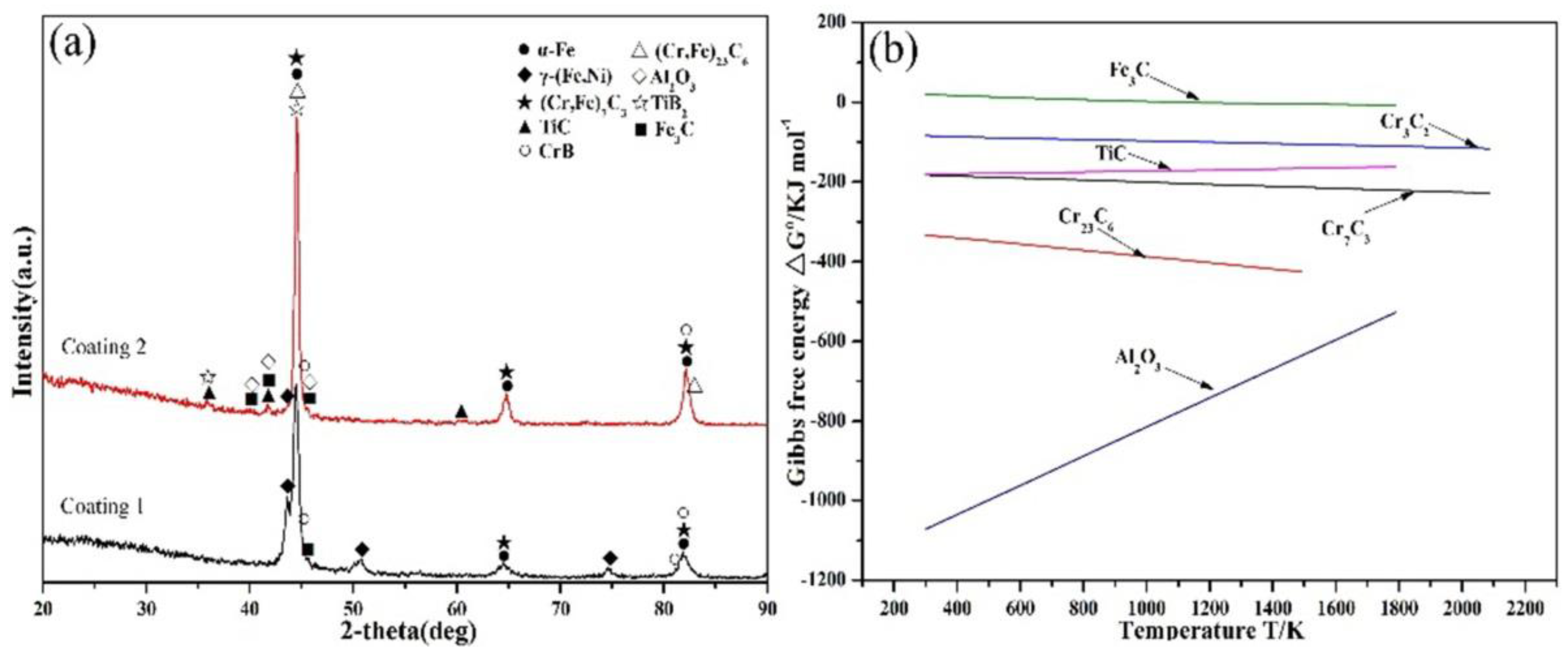

| Material | Fe | Mn | Ni | Cr | C | Si | Cu | P | S | B |
|---|---|---|---|---|---|---|---|---|---|---|
| 16Mn | Bal. | 1.2~1.6 | ≤0.3 | ≤0.3 | 0.1~0.2 | 0.2~0.6 | ≤0.25 | ≤0.03 | ≤0.03 | – |
| Fe60 | Bal. | – | 0.1~1 | 13~17 | 0.5~1.0 | 0.3~1.0 | – | – | – | 0.2~1.5 |
| Samples | Composition (wt.%) | Power (wk) | Scanning Speed (mm/s) |
|---|---|---|---|
| Coating 1 | Fe60 | 4.0 | 6 |
| Coating 2 | Fe60/10Ti3SiC2 |
| Phase | Fe | Ni | Cr | C | Si |
|---|---|---|---|---|---|
| Cellular dendrites | 52.47 | 1.12 | 12.88 | 31.64 | 1.89 |
| Eutectics | 52.30 | 1.24 | 24.06 | 21.39 | 1.0 |
| Samples | Peak I | Peak II | Peak III |
|---|---|---|---|
| Coating 1 | 0.614 | F1.016 | 0.819 |
| Coating 2 | 0.558 | 0.614 | 0.636 |
| Samples | Icorr (nA/cm2) | Rp (Ω) | Ecorr (V) | Corrosion Rate (mm/a) |
|---|---|---|---|---|
| Coating 1 | 24.26 | 1079.6 | −0.461 | 0.257 |
| Coating 2 | 5.631 | 8923.2 | −0.311 | 0.0602 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Zhu, H.; Li, C. The Effects of the Addition of Ti3SiC2 on the Microstructure and Properties of Laser Cladding Composite Coatings. Coatings 2020, 10, 498. https://doi.org/10.3390/coatings10050498
Shi Q, Zhu H, Li C. The Effects of the Addition of Ti3SiC2 on the Microstructure and Properties of Laser Cladding Composite Coatings. Coatings. 2020; 10(5):498. https://doi.org/10.3390/coatings10050498
Chicago/Turabian StyleShi, Qin, Hejun Zhu, and Changsheng Li. 2020. "The Effects of the Addition of Ti3SiC2 on the Microstructure and Properties of Laser Cladding Composite Coatings" Coatings 10, no. 5: 498. https://doi.org/10.3390/coatings10050498
APA StyleShi, Q., Zhu, H., & Li, C. (2020). The Effects of the Addition of Ti3SiC2 on the Microstructure and Properties of Laser Cladding Composite Coatings. Coatings, 10(5), 498. https://doi.org/10.3390/coatings10050498




