Developing New Cleaning Strategies of Cultural Heritage Stones: Are Synergistic Combinations of a Low-Toxic Solvent Ternary Mixtures Followed by Laser the Solution?
Abstract
1. Introduction
2. Materials and Methods
2.1. Stones
2.2. Graffiti Paints
- -
- a violet spray paint, the MTN94 Ultraviolet RV173;
- -
- a green dabber paint, the MTN Street Paint Dabber UFO Green.
2.3. Cleaning Procedures
2.4. Analytical Techniques
- -
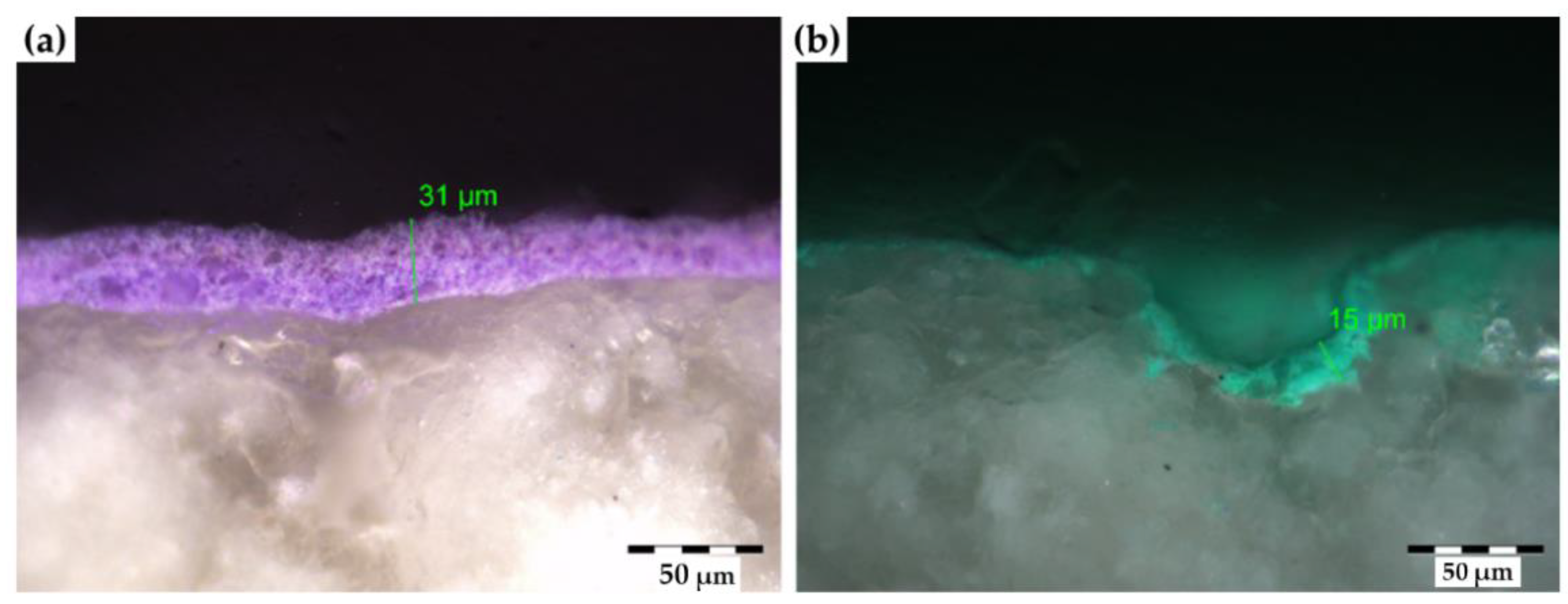
- Stereomicroscopy (OLYMPUS SZ X10, with digital camera OLYMPUS Color View I) was used to evaluate the appearance of painted surfaces and the thickness and penetration of the paint layer using a cross section sample from each painted stone (a fragment of 1 cm × 1 cm × 1 cm) embedded in resin. Moreover, stereomicroscopy was also used to identify the presence of visible paint remains on the surface
- -
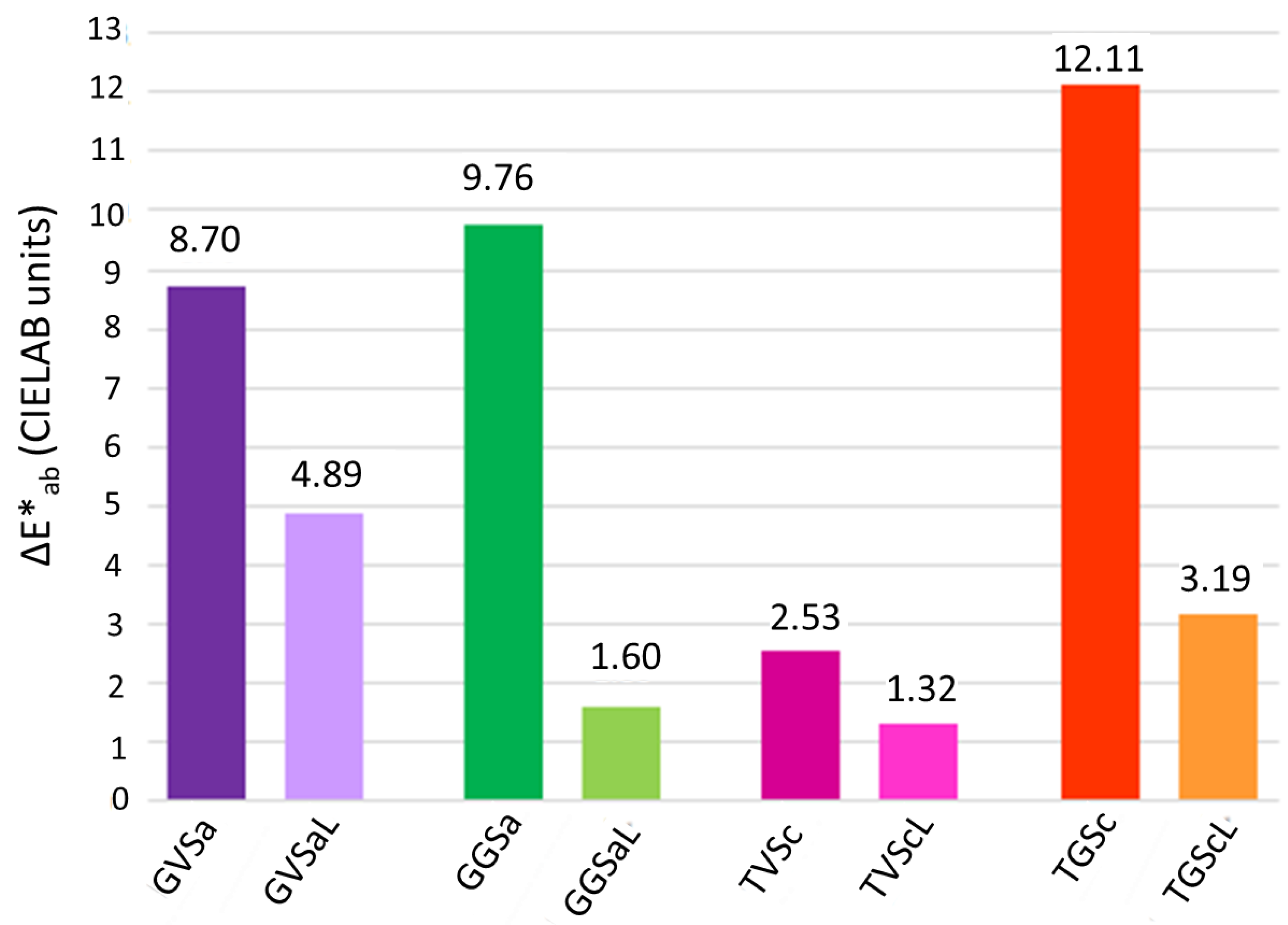
- Color spectrophotometry, using a CM-700d spectrophotometer (Minolta, Osaka, Japan) to measure the color surface of the samples before to be painted and after the cleaning. The color was measured in CIELAB color space [36]. The parameter L* (lightness) and the polar coordinates a* (+a*: red; −a*: green) and b* (+b*: yellow; −b*: blue), were measured in specular component included (SCI) mode, with a spot diameter of 8 mm under the CIE standard daylight illuminant D65 at a viewing angle of 10°. In accordance with [37], 10 measurements were randomly taken. In order to determine the color change after the cleaning, ΔL*, Δa* and Δb* color differences were computed considering the color of the surface before to be painted as the reference color. Then, the global color change (ΔE*ab) was obtained [36].
- -
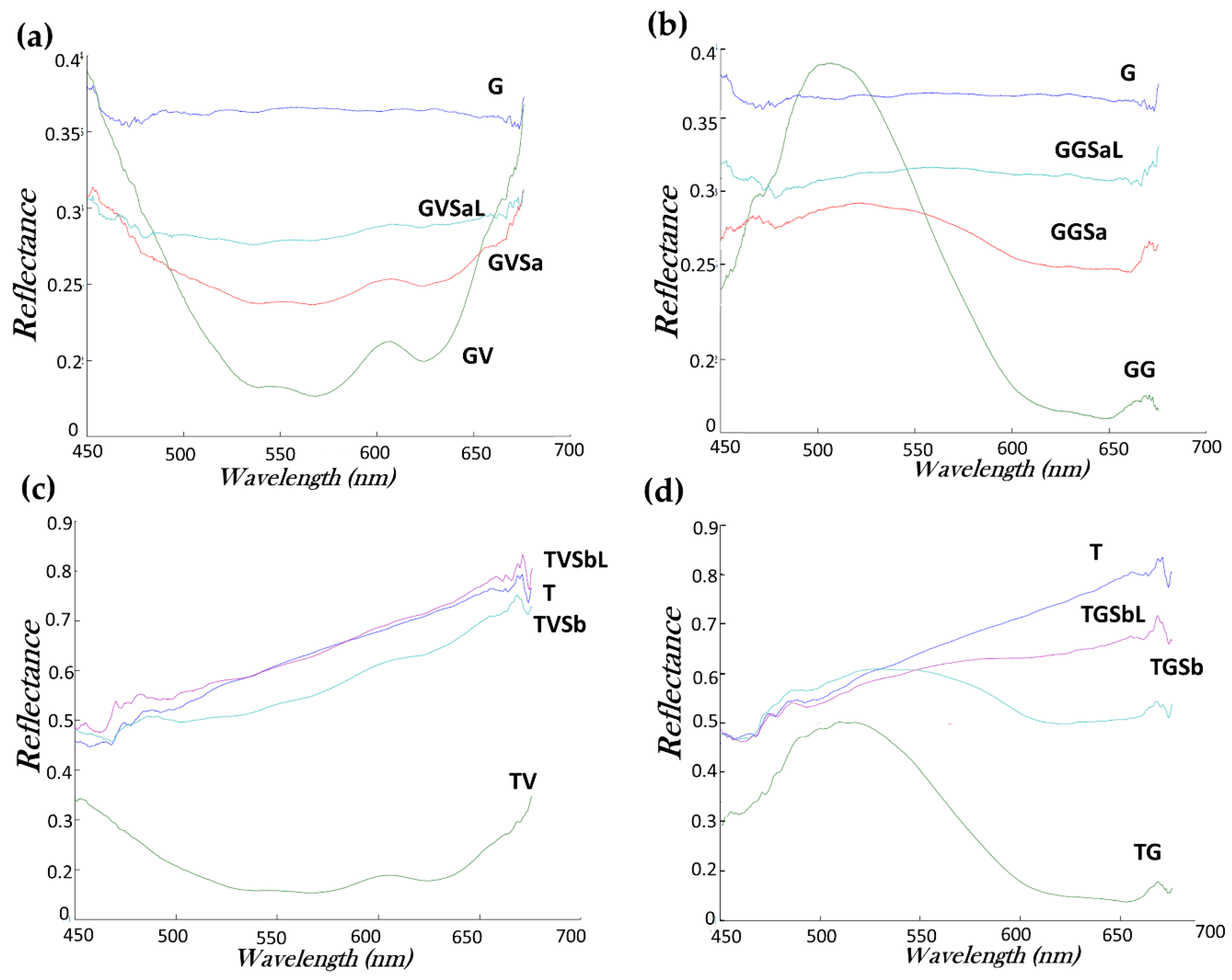
- Hyperspectral imaging was applied to determine the reflectance change of the surfaces after the cleaning. This system was applied to the unpainted stones, painted stones and the surfaces after being cleaned. The system as was reported in [16] consisted in a CCD sensor Pulnix TM-1327 GE (PULNiX America Inc., Sunnyvale, CA, USA) with an objective lens (10 mm focal length) and a spectrograph ImSpector V10 with a spectral range of 400–1000 nm and a spectral resolution of 4.55 nm. The camera scans the surface line by line to obtain an image at each of the 1040 wavelengths. A Schott DCR® III (SCHOTT-FOSTEC, LLC, NY, USA) incandescent lamp with a rectangular head of 51 mm long and 0.89 mm wide was used as a light source. A cylindrical lens placed in front of the lamp focused the light to produce an illuminated area that was 15 cm long and 1 cm wide. The sample was placed on a motorized XYZ translation stage in which the Z- axis was perpendicular to the sample surface. The 5 cm × 5 cm samples were fully scanned. Once the hyperspectral images were acquired, the data were processed in a MATLAB programming environment in order to display the respective reflectance graphs.
- -
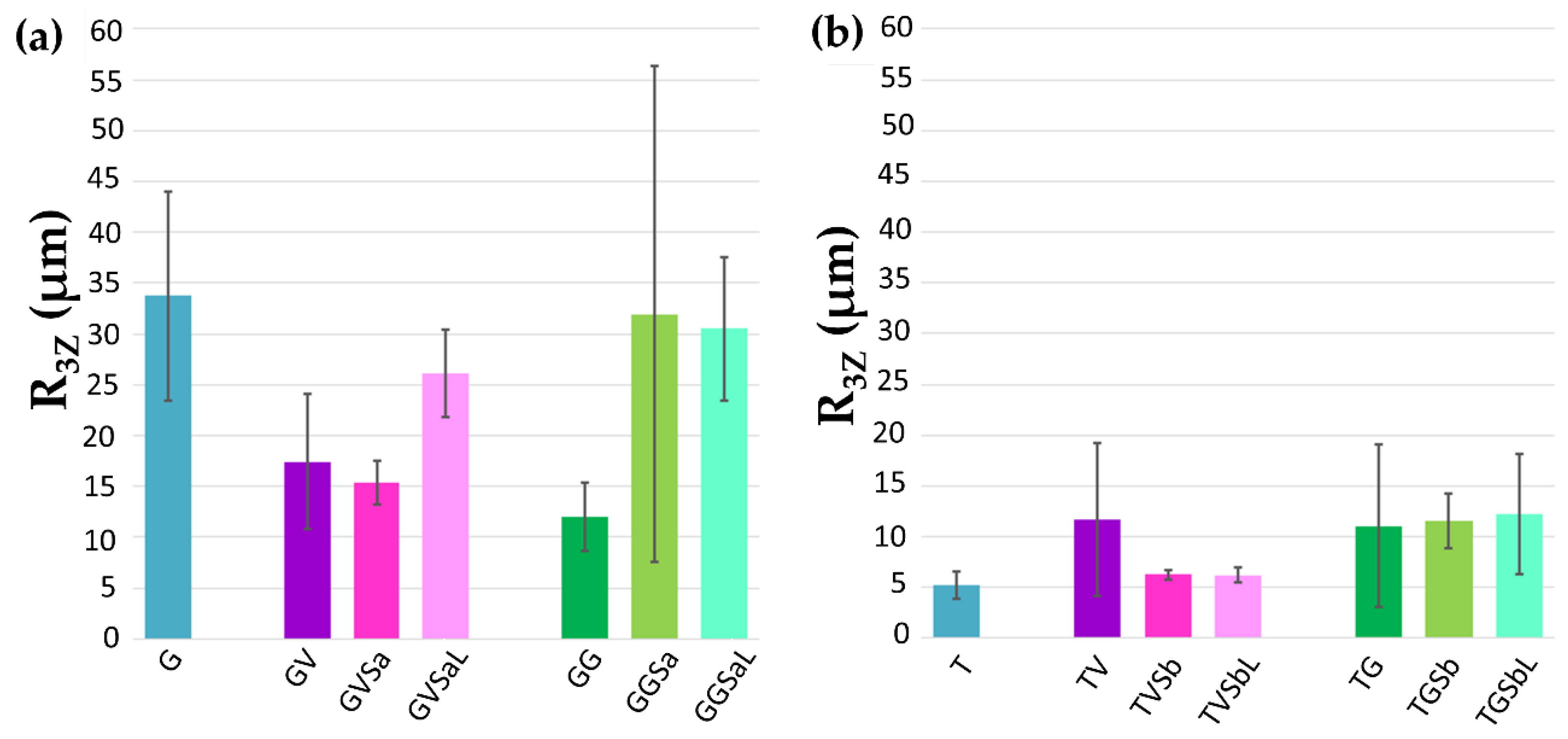
- Confocal microscopy was used to determine the roughness of the references, the painted surfaces and the treated surfaces (2 cm × 2 cm), using a PLu 2300 Sensofar® optical imaging profiler (SENSOFAR Group, Terrassa, Barcelona, Spain). The images with the optical imaging profiler were collected with an EPI 10X-N objective, an overlapping of 25%, a depth resolution of 2 mm and a lateral resolution of 1 nm. The system allowed to obtain a 3D images of the surfaces and therefore, the roughness parameter [38], R3z (third maximum peak-to-valley height) was obtained using the Gwyddion 2.47 software.
- -
- SEM-EDX (two equipment: a Philips XL30- Philips, and a FEI Quanta 200) in BSE and SE modes was used to evaluate the chemical composition and microtexture of the painted surfaces and also, the damages induced on the surfaces after treatment. Optimal conditions of observation were the same used to characterize the stones.
3. Results
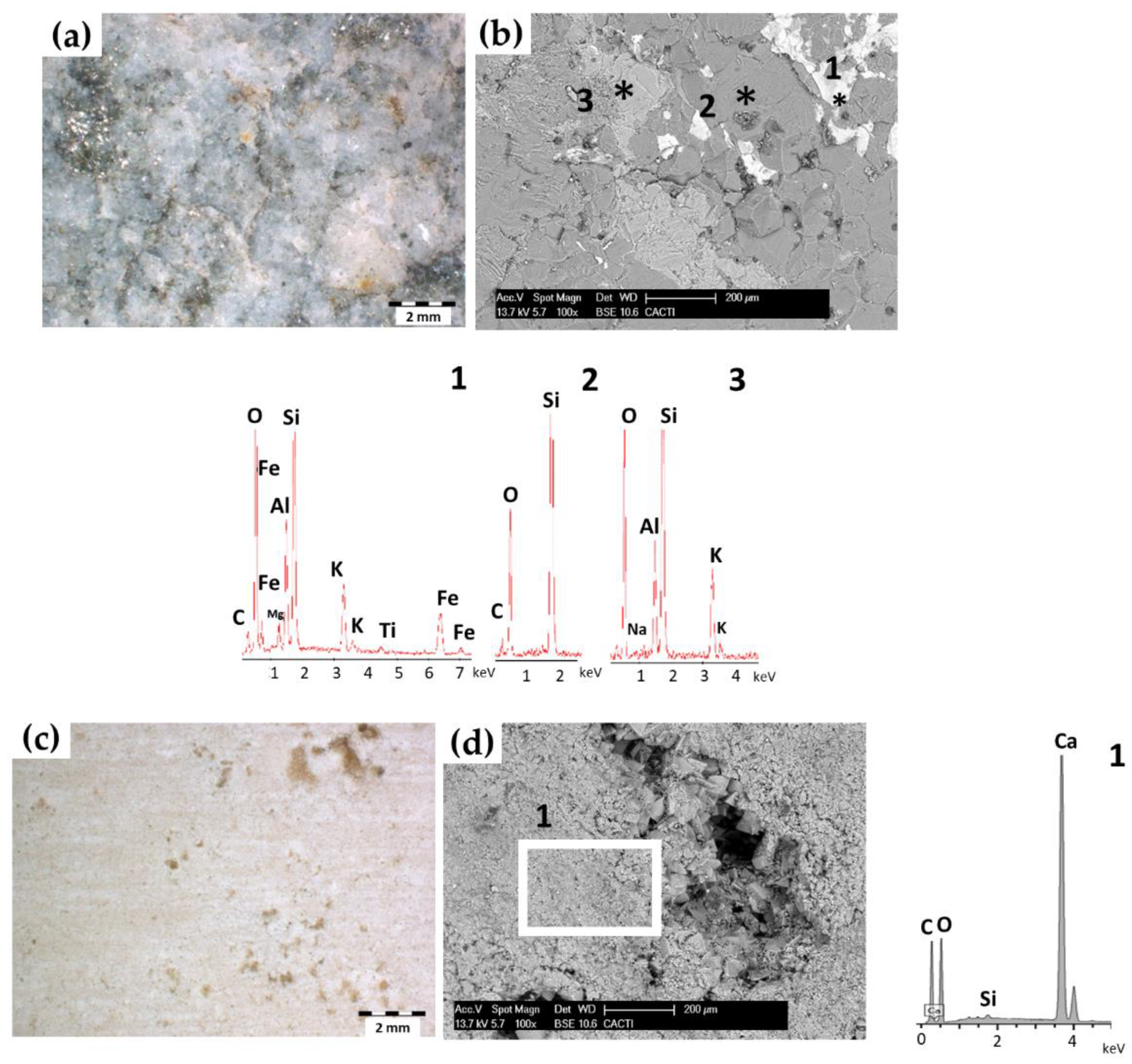
3.1. Graffiti Paint Characterization
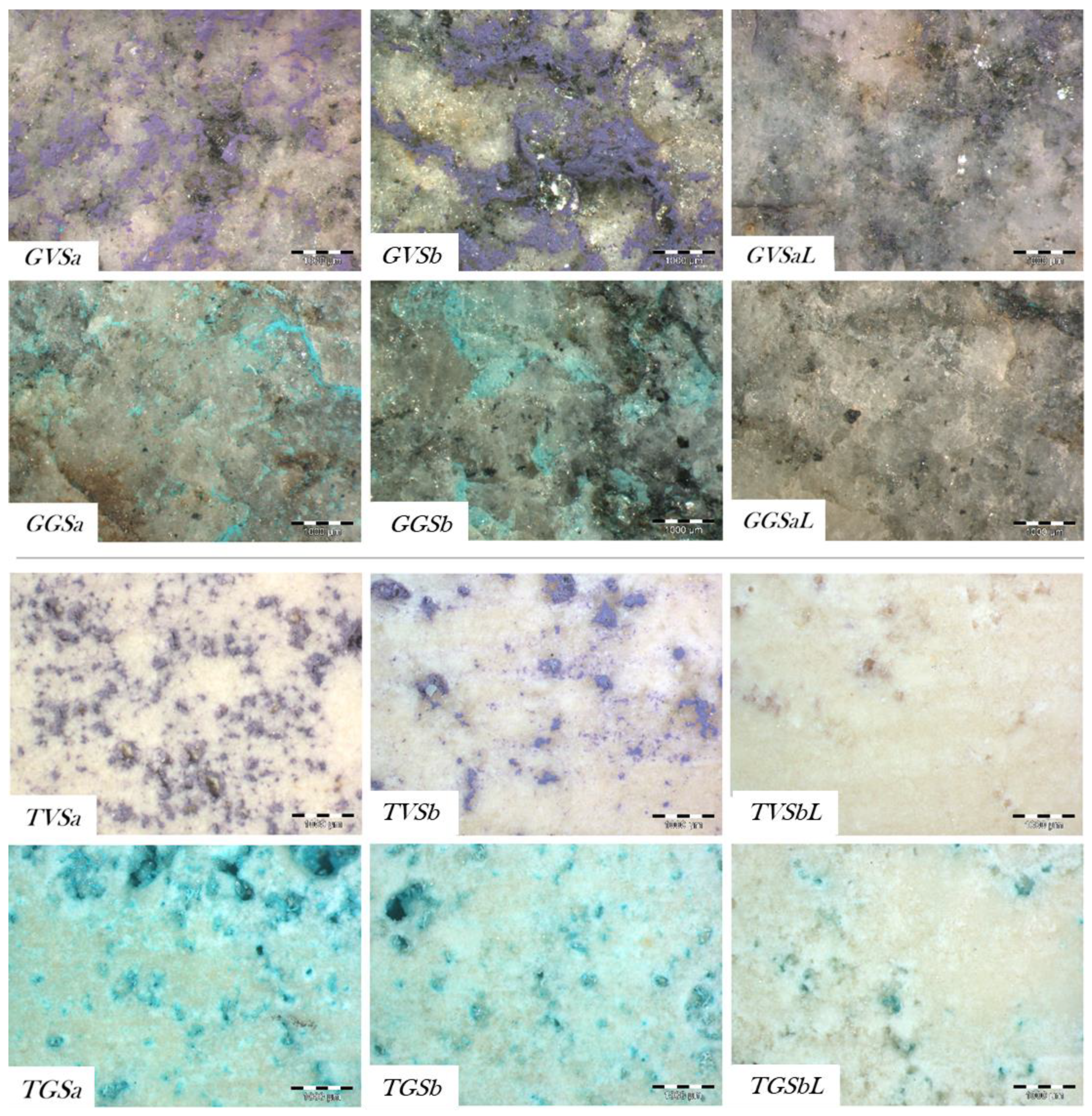
3.2. Cleaning Evaluation
3.2.1. Selection of the Ternary Solvent Mixtures
3.2.2. Evaluation of the Surfaces Treated with the Ternary Solvent Mixtures followed by 532 nm
- -
- For the removal of the violet graffiti from the gneiss, a surface smoother than the reference stone was obtained after the application of the chemical procedure (GVSa), while for the combined treatment (GVSaL) the measured roughness resulted closer to that of the reference (G) (Figure 7a).
- -
- For the cleaning of both the green graffiti from the gneiss and the violet spray from the travertine, all the cleaning methodologies tested (GGSa, GGSaL, TVSb, and TVSbL) produced a roughness closer to the value of the stone reference (G, T respectively) than to the painted stone (Figure 7a,b). However, in the case of the gneiss cleaned from the green graffiti with the combined methodology (GGSaL), a high standard deviation suggested the high variability of the surface roughness.
- -
- For the travertine cleaned from the green graffiti, both cleaning procedures (TGSb, TGSbL) induced surfaces rougher than the reference (T) (Figure 7b).
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. About the Sustainable Development Goals (Agenda 2030). Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 20 March 2020).
- ICOMOS. Concept Note for the United Nations Agenda 2030 and the Third United Nations Conference on Housing and Sustainable Urban Development (HABITAT). 2016. Prepared by Hosagrahar, J.; Soule, J.; Fusco Girard, L.; Potts, A.. Available online: http://www.usicomos.org/wp-content/uploads/2016/05/Final-Concept-Note.pdf (accessed on 20 March 2020).
- GRAFFITAGE Project. Development of a New Anti-graffiti System, Based on Traditional Concepts, Preventing Damage of Architectural Heritage Materials. Final Report of the SSP (Policy Oriented Research) of the Sixth European Programme of the European Commission. European Union: Brussels, Belgium, 2008; FP6-2003-SSP 3–513718. Available online: https://cordis.europa.eu/project/id/513718/reporting (accessed on 20 March 2020).
- GRAFFOLUTION Project. Awareness and Prevention Solutions against Graffiti Vandalism in Public Areas and Transport. Final Report of the SSP (Policy Oriented Research) of the Seventh European Programme of the European Commission. European Union: Brussels, Belgium, 2016; FP7-SEC-2013-1. 2016. Available online: https://cordis.europa.eu/project/id/608152 (accessed on 20 March 2020).
- Sanmartín, P.; Cappitelli, F.; Mitchell, R. Current methods of graffiti removal: A review. Constr. Build. Mater. 2014, 71, 363–374. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Rivas, T.; López, A.J.; Fiorucci, M.P.; Ramil, A. Effectiveness of granite cleaning procedures in cultural heritage: A review. Sci. Total Environ. 2016, 571, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.; Dionisio, A.; Pozo-Antonio, J.S. Conservation strategies against graffiti vandalism on Cultural Heritage stones: Protective coatings and cleaning methods. Prog. Org. Coat. 2017, 113, 90–109. [Google Scholar] [CrossRef]
- Rivas, T.; Pozo, S.; Fiorucci, M.P.; López, A.J.; Ramil, A. Nd:YVO4 laser removal of graffiti from granite Influence of paint and rock properties on cleaning efficacy. Appl. Surf. Sci. 2012, 263, 563–572. [Google Scholar] [CrossRef]
- Venice Charter for the Conservation and Restoration of Monuments and Sites, ICOMOS. 1964. Available online: http://www.icomos.org/venicecharter2004/index.html (accessed on 20 March 2020).
- Grimmer, A.E. Keeping It Clean: Removing Exterior Dirt, Paint, Stains and Graffiti from Historic Masonry Buildings. In Library of Congress Cataloging in Publication Data; Diane Pub Co: Collingdale, PA, USA, 1988. [Google Scholar] [CrossRef]
- Historic England. Graffiti on Historic Buildings and Monuments. Methods of Removal and Prevention. 1999, pp. 1–12. Available online: https://historicengland.org.uk/images-books/publications/graffiti-on-historic-buildings-and-monuments/ (accessed on 20 March 2020).
- Urquhart, D. The treatment of graffiti on historic surfaces. Advice on graffiti removal procedures, anti-graffiti coatings and alternative strategies. In Historic Scotland Technical Advice Note; no.18; TCRE Division/Scottish Conservation Bureau: Edinburgh, Scotland, 1999. [Google Scholar]
- Samolik, S.; Walczak, M.; Plotek, M.; Sarzynski, A.; Pluska, I.; Marczak, J. Investigation into the removal of graffiti on mineral supports: Comparison of nano-second Nd:YAG laser cleaning with traditional mechanical and chemical methods. Stud. Conserv. 2015, 60, 58–64. [Google Scholar] [CrossRef]
- Careddu, N.; Akkoyun, O. An investigation on the efficiency of water-jet technology for graffiti cleaning. J. Cult. Herit. 2016, 19, 426–434. [Google Scholar] [CrossRef]
- Gomes, V.; Dionísio, A.; Pozo-Antonio, J.S. The influence of the SO2 aging on the graffiti cleaning effectiveness with chemical procedures on a granite substrate. Sci. Total Environ. 2018, 625, 233–245. [Google Scholar] [CrossRef]
- Gomes, V.; Dionísio, A.; Pozo-Antonio, J.S.; Rivas, T.; Ramil, A. Mechanical and laser cleaning of spray graffiti paints on a granite subjected to a SO2-rich atmosphere. Constr. Build. Mater. 2018, 188, 621–632. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; López, L.; Dionísio, A.; Rivas, T. A Study on the Suitability of Mechanical Soft-Abrasive Blasting Methods to Extract Graffiti Paints on Ornamental Stones. Coatings 2018, 8, 335. [Google Scholar] [CrossRef]
- Fotakis, C.; Anglos, D.; Zafiropulos, V.; Georgiou, S.; Tornari, V. Lasers in the Preservation of Cultural Heritage: Principles and Applications; CRC Press, Taylor & Francis Group: Abingdon, Oxfordshire, UK, 2006. [Google Scholar]
- Pozo-Antonio, J.S.; Rivas, T.; Fiorucci, M.P.; López, A.J.; Ramil, A. Effectiveness and harmfulness evaluation of graffiti cleaning by mechanical, chemical and laser procedures on granite. Microchem. J. 2016, 125, 1–9. [Google Scholar] [CrossRef]
- Giusti, C.; Colombini, M.P.; Lluveras-Tenorio, A.; La Nasa, J.; Striova, J.; Salvadori, B. Graphic vandalism: Multi-analytical evaluation of laser and chemical methods for the removal of spray paints. J. Cult. Herit. 2020, in press. [Google Scholar] [CrossRef]
- Ortiz, P.; Antúnez, V.; Ortiz, R.; Martín, J.M.; Gómez, M.A.; Hortal, A.R.; Martínez-Haya, B. Comparative study of pulsed laser cleaning applied to weathered marble surfaces. Appl. Surf. Sci. 2013, 283, 193–201. [Google Scholar] [CrossRef]
- Costela, A.; García-Moreno, I.; Gómez, C.; Caballero, O.; Sastre, R. Cleaning graffitis on urban buildings by use of second and third harmonic wavelength of a Nd:YAG laser: A comparative study. Appl. Surf. Sci. 2003, 207, 86–99. [Google Scholar] [CrossRef]
- Gómez, C.; Costela, A.; García-Moreno, I.; Sastre, R. Comparative study between IR and UV laser radiation applied to the removal of graffitis on urban buildings. Appl. Surf. Sci. 2006, 252, 2782–2793. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Papanikolaou, A.; Melessanaki, K.; Rivas, T.; Pouli, P. Laser-Assisted Removal of Graffiti from Granite: Advantages of the Simultaneous Use of Two Wavelengths. Coatings 2018, 8, 124. [Google Scholar] [CrossRef]
- Chapman, S. Laser technology for graffiti removal. J. Cult. Herit. 2000, 1, 75–78. [Google Scholar] [CrossRef]
- Langworth, S.; Anundi, H.; Friis, L.; Johanson, G.; Lind, M.L.; Söderman, E.; Åkesson, B.A. Acute health effects common during graffiti removal. Int. Arch. Occup. Environ. Health 2001, 74, 213–218. [Google Scholar] [CrossRef]
- TriSolv Software, ISCR. Triangolo interattivo dei solventi e delle solubilità®. Available online: http://icr.beniculturali.it/flash/progetti/TriSolv/TriSolv.html (accessed on 31 January 2018).
- Coladonato, M.; Scarpitti, P. Note sul Triangolo interattivo dei solvent e delle solubilità©. 2008. Available online: http://www.icr.beniculturali.it/flash/progetti/TriSolv/dati/IT/TriSolv_IT.pdf (accessed on 31 January 2018).
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press, Taylor & Francis Group: Abingdon, Oxfordshire, UK, 2007. [Google Scholar]
- Handbook for Critical Cleaning: Cleaning Agents and Systems; Kanegsberg, B., Kanegsberg, E., Eds.; CRC Press, Taylor & Francis Group: Abingdon, Oxfordshire, UK, 2011. [Google Scholar]
- Gambino, F.; Borghi, A.; D’Atri, A.; Gallo, L.M.; Ghiraldi, L.; Giardino, M.; Martire, L.; Palomba, M.; Perotti, L. TOURinSTONES: A free mobile application for promoting geological heritage in the city of Turin (NW Italy). Geoheritage 2018, 11, 3–17. [Google Scholar] [CrossRef]
- Sandrone, R.; Colombo, A.; Fiora, A.; Fornaro, L.; Lovera, E.; Tunesi, A.; Cavallo, A. Contemporary natural stones from the Italian western Alps (Piedmont and Aosta Valley Regions). Per. Mineral. 2004, 73, 211–226. [Google Scholar]
- ASTM-D4404-10. Standard Test Method for Determination of Pore Volume and Pore Volume Distribution of Soil and Rock by Mercury Intrusion Porosimetry; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Montana Colors. Available online: www.montanacolors.com (accessed on 31 January 2017).
- U.S. National Library of Medicine. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 31 January 2018).
- CIE0144 S–S/E:2007 Colorimetry Part 4: CIE 1976 L*a*b* Color Space; CIE Central Bureau: Vienna, Austria, 2007.
- Prieto, B.; Sanmartín, P.; Silva, B.; Martinez-Verdú, F. Measuring the color of granite rocks. A proposed procedure. Color Res. Appl. 2010, 35, 368–375. [Google Scholar] [CrossRef]
- UNI-EN ISO 4288:1996 Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Rules and Procedures for the Assessment of Surface Texture; International Organization for Standardization: Geneva, Switzerland, 1996.
- Cremonesi, P. L’uso dei Solventi Organici nella Pulitura di Opere Policrome; IL PRATO PUBLISHING HOUSE SRL: Saonara, Italy, 2004. [Google Scholar]
- Mokrzycki, W.S.; Tatol, M. Color difference DE—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Abel, A. Pigments for paints. In Paints in Surface Coatings: Theory and Practice; Lambourne, R., Strivens, T.A., Eds.; Woodhead Publishing: Cambridge, UK, 1999. [Google Scholar]
- Diebold, M.P. Optimizing the benefits of TiO2 in paints. J. Coat. Technol. Res. 2020, 17, 1–17. [Google Scholar] [CrossRef]
- Zhou, D.; Ji, Z.; Jiang, X.; Dunphy, D.R.; Brinker, J.; Keller, A.A. Influence of material properties on TiO2 nanoparticle agglomeration. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Costa, D.; Mascalchi, M.; Osticioli, I.; Siano, S. Laser ablation of iron-rich black films from exposed granite surfaces. Appl. Phys. A 2014, 117, 365–370. [Google Scholar] [CrossRef]
- Ramil, A.; Pozo-Antonio, J.S.; Fiorucci, M.P.; López, A.J.; Rivas, T. Detection of the optimal laser fluence ranges to clean graffiti on silicates. Constr. Build. Mater. 2017, 148, 122–130. [Google Scholar] [CrossRef]
- Kliem, W.; Lehmann, G. A reassignment of the optical absorption bands in biotites. Phys. Chem. Miner. 1979, 4, 65–75. [Google Scholar] [CrossRef]



| Solvent | TLV-TWA (ppm) |
|---|---|
| n-butylacetate | 50 |
| MEK | 200 |
| 2,2,4-trimethylpentane | 300 |
| acetone | 500 |
| ethyl alcohol | 1000 |
| Mixture | Solvent Replaced | Ethyl Alcohol | Acetone | 2,2,4-Trimethylpentane |
|---|---|---|---|---|
| Mixture A | n-butyl acetate | 51% | 11% | 38% |
| Mixture B | MEK | 7% | 80% | 13% |
| Stone | Color Paint | Cleaning Method | Acronym |
|---|---|---|---|
| Gneiss | – | None (stone reference) | G |
| Violet | Solvent A | GVSa | |
| Solvent B | GVSb | ||
| Solvent A+ laser (532 nm) | GVSaL | ||
| Green | Solvent A | GGSa | |
| Solvent B | GGSb | ||
| Solvent A+ laser (532 nm) | GGSaL | ||
| Travertine | – | None (stone reference) | T |
| Violet | Solvent A | TVSa | |
| Solvent B | TVSc | ||
| Solvent B+ laser (532 nm) | TVSbL | ||
| Green | Solvent A | TGSa | |
| Solvent B | TGSb | ||
| Solvent B+ laser (532 nm) | TGSbL |
| GNEISS | L* | a* | b* | ΔL* | Δa* | Δb* |
| G | 62.45 ± 3.25 | −1.20 ± 0.44 | 2.69 ± 1.33 | – | – | – |
| GVSa | 57.36 ± 4.84 | 1.00 ± 0.83 | −4.01 ± 1.84 | −5.09 | 2.21 | −6.70 |
| GVSaL | 58.77 ± 2.85 | −0.48 ± 0.37 | −0.44 ± 0.59 | −3.68 | 0.72 | −3.13 |
| GGSa | 54.20 ± 4.39 | −6.36 ± 1.24 | 3.44 ± 0.17 | −8.25 | −5.16 | 0.74 |
| GGSaL | 61.46 ± 2.48 | −2.21 ± 0.09 | 1.95 ± 0.43 | −1.00 | −1.01 | −0.74 |
| TRAVERTINE | L* | a* | b* | ΔL* | Δa* | Δb* |
| T | 82.05 ± 1.11 | 2.01 ± 0.25 | 9.19 ± 1.75 | – | – | – |
| TVSb | 80.62 ± 0.12 | 3.65 ± 0.03 | 7.88 ± 0.36 | −1.43 | 1.64 | −1.31 |
| TVSbL | 81.63 ± 0.01 | 3.07 ± 0.01 | 9.86 ± 0.01 | −0.42 | 1.06 | 0.67 |
| TGSb | 78.44 ± 0.01 | −9.52 ± 0.01 | 8.33 ± 0.01 | −3.61 | −11.53 | −0.86 |
| TGSbL | 82.14 ± 0.07 | −1.12 ± 0.02 | 8.60 ± 0.02 | 0.09 | −3.13 | −0.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, C.; Gambino, F.; Nervo, M.; Piccirillo, A.; Scarcella, A.; Zenucchini, F.; Pozo-Antonio, J.S. Developing New Cleaning Strategies of Cultural Heritage Stones: Are Synergistic Combinations of a Low-Toxic Solvent Ternary Mixtures Followed by Laser the Solution? Coatings 2020, 10, 466. https://doi.org/10.3390/coatings10050466
Ricci C, Gambino F, Nervo M, Piccirillo A, Scarcella A, Zenucchini F, Pozo-Antonio JS. Developing New Cleaning Strategies of Cultural Heritage Stones: Are Synergistic Combinations of a Low-Toxic Solvent Ternary Mixtures Followed by Laser the Solution? Coatings. 2020; 10(5):466. https://doi.org/10.3390/coatings10050466
Chicago/Turabian StyleRicci, Chiara, Francesca Gambino, Marco Nervo, Anna Piccirillo, Arianna Scarcella, Francesca Zenucchini, and José Santiago Pozo-Antonio. 2020. "Developing New Cleaning Strategies of Cultural Heritage Stones: Are Synergistic Combinations of a Low-Toxic Solvent Ternary Mixtures Followed by Laser the Solution?" Coatings 10, no. 5: 466. https://doi.org/10.3390/coatings10050466
APA StyleRicci, C., Gambino, F., Nervo, M., Piccirillo, A., Scarcella, A., Zenucchini, F., & Pozo-Antonio, J. S. (2020). Developing New Cleaning Strategies of Cultural Heritage Stones: Are Synergistic Combinations of a Low-Toxic Solvent Ternary Mixtures Followed by Laser the Solution? Coatings, 10(5), 466. https://doi.org/10.3390/coatings10050466







