The Effect of Ti-6Al-4V Alloy Surface Structure on the Adhesion and Morphology of Unidirectional Freeze-Coated Gelatin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
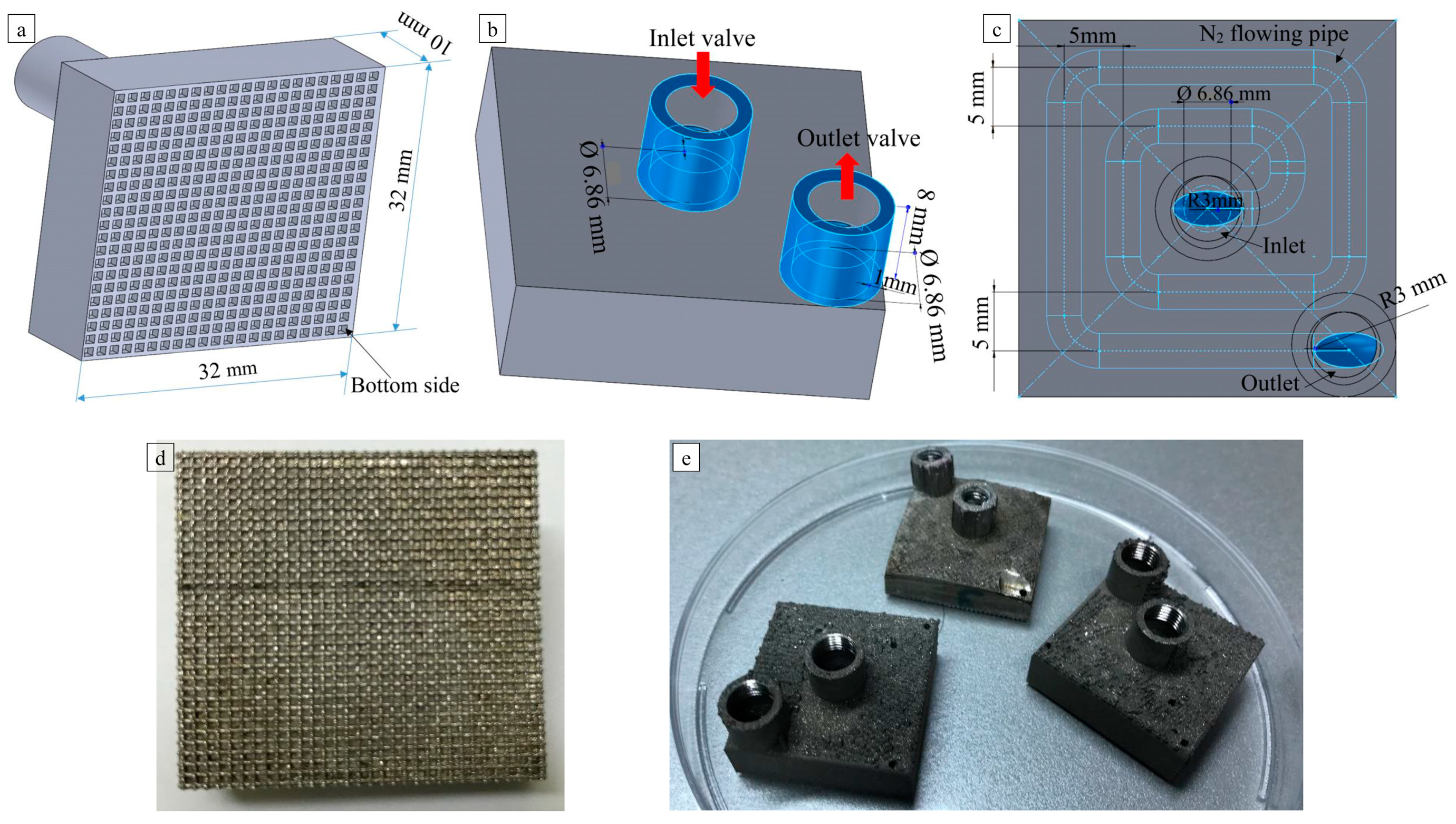
2.2.1. Modeling and Manufacturing of Ti-6Al-4V Substrates
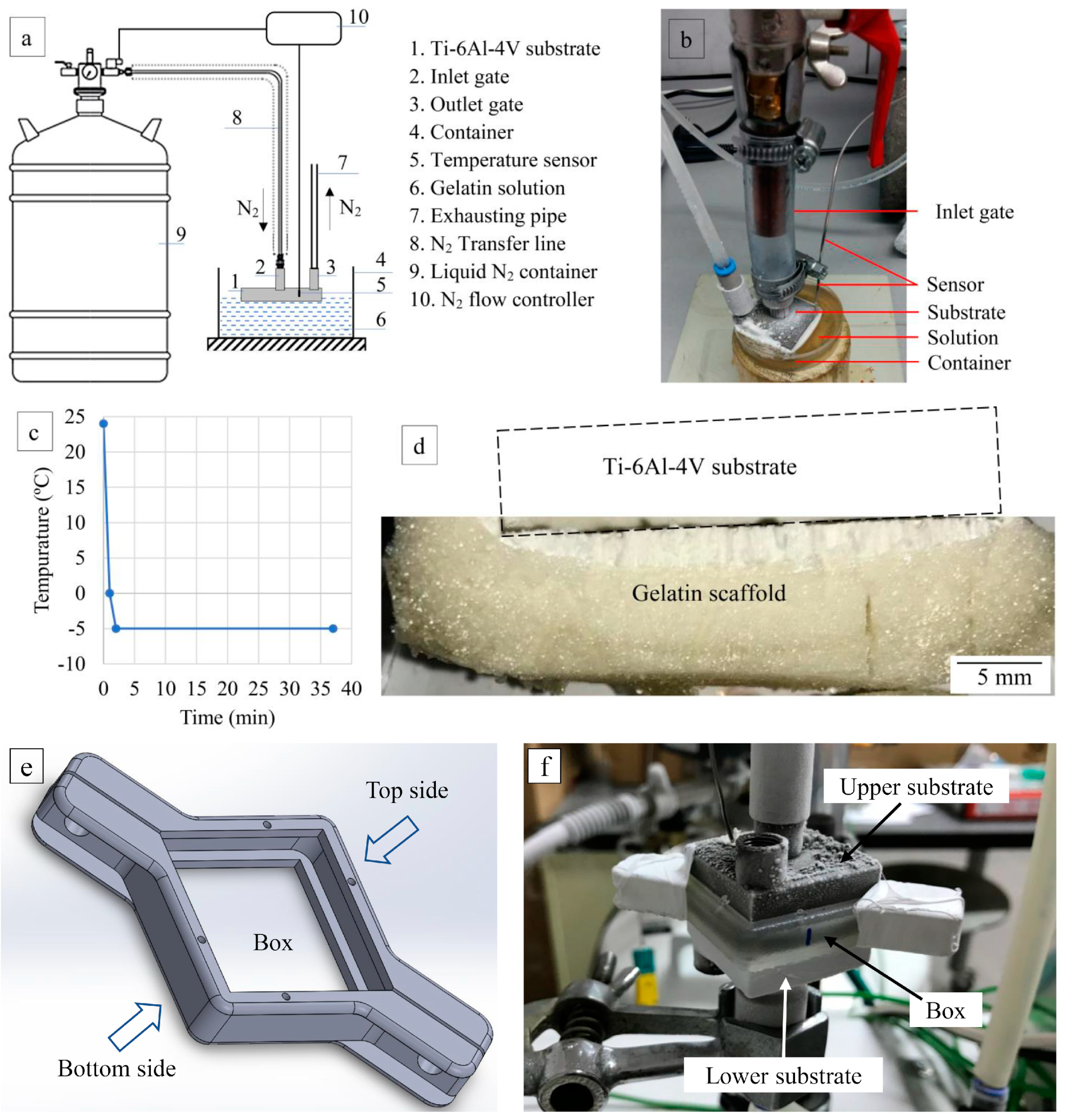
2.2.2. Performing of GEL Freeze-Coating on Ti-6Al-4V Substrate Surfaces
2.2.3. Measuring of Pore Size and Pore-Size Distribution of the GEL Coating
2.2.4. Scanning Electron Microscopy (SEM) analysis of GEL coatings
2.2.5. Determination of Surface Tension (ST) Properties of Test Liquids and GEL Solutions
2.2.6. Measurement of Contact Angle (CA) of Test Liquids and GEL Solutions on Ti-6Al-4V Substrates
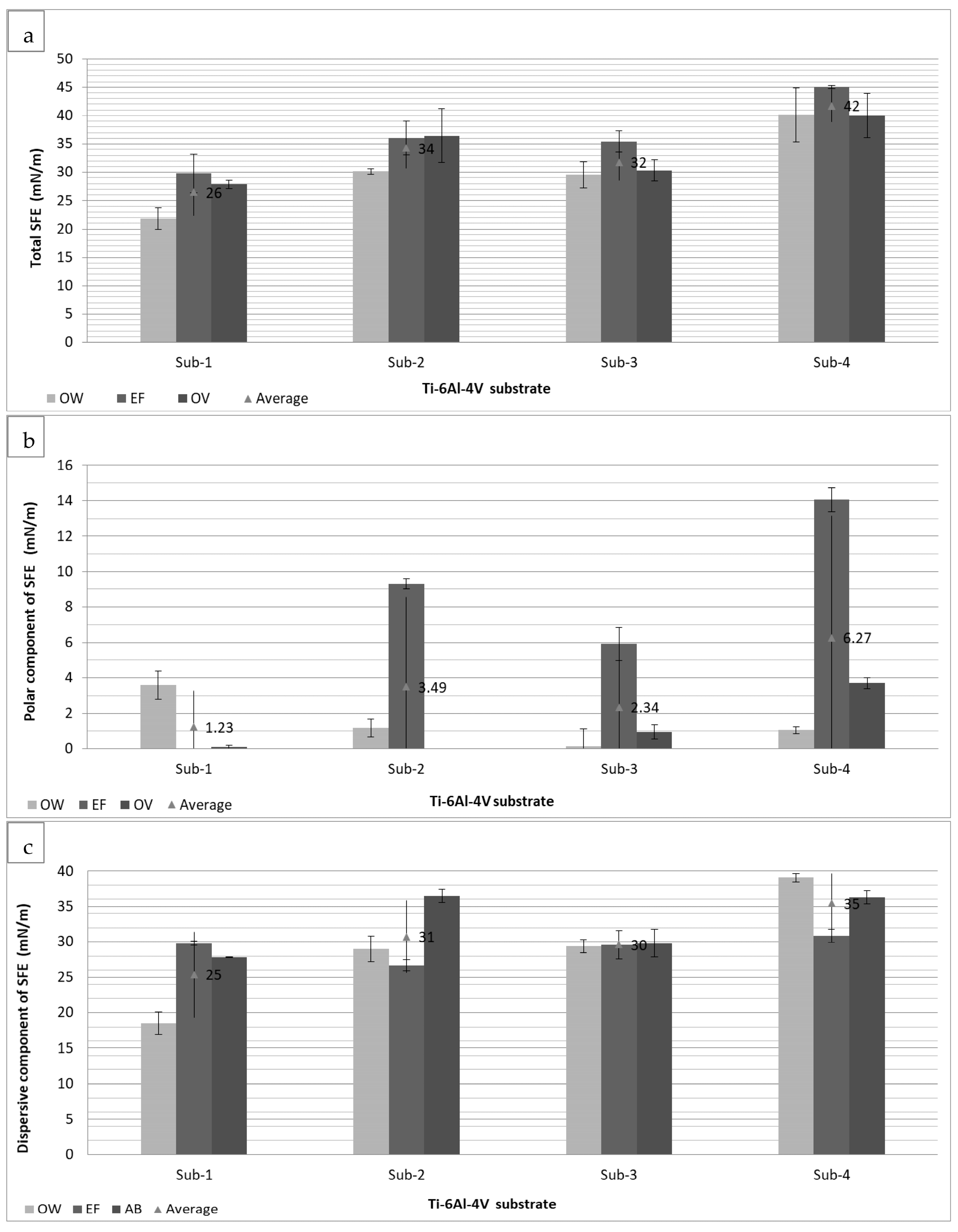
2.2.7. Determination of Surface Polarities and Free Energy of Immersional Wetting
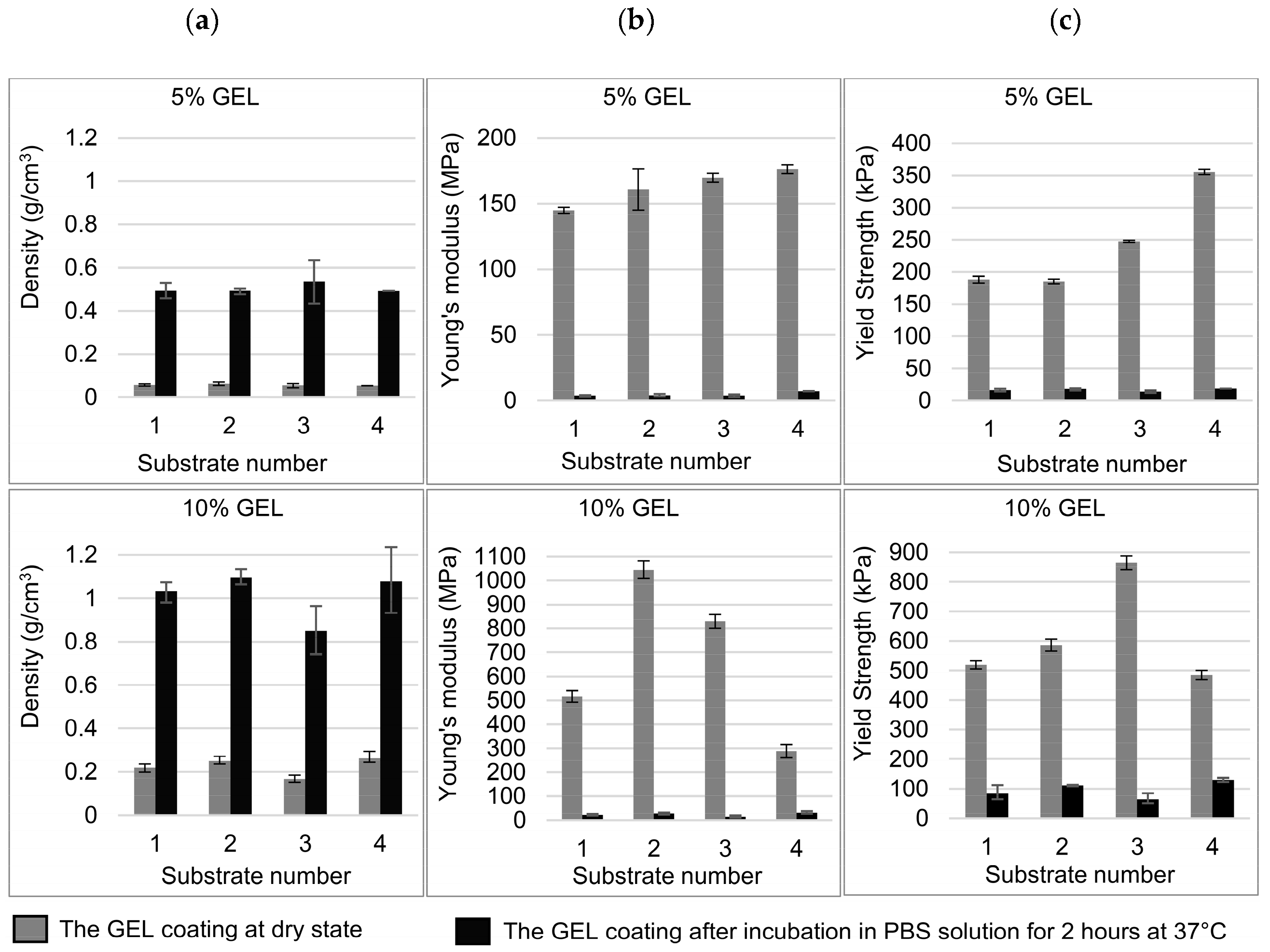
2.2.8. Compression Testing of GEL Coatings
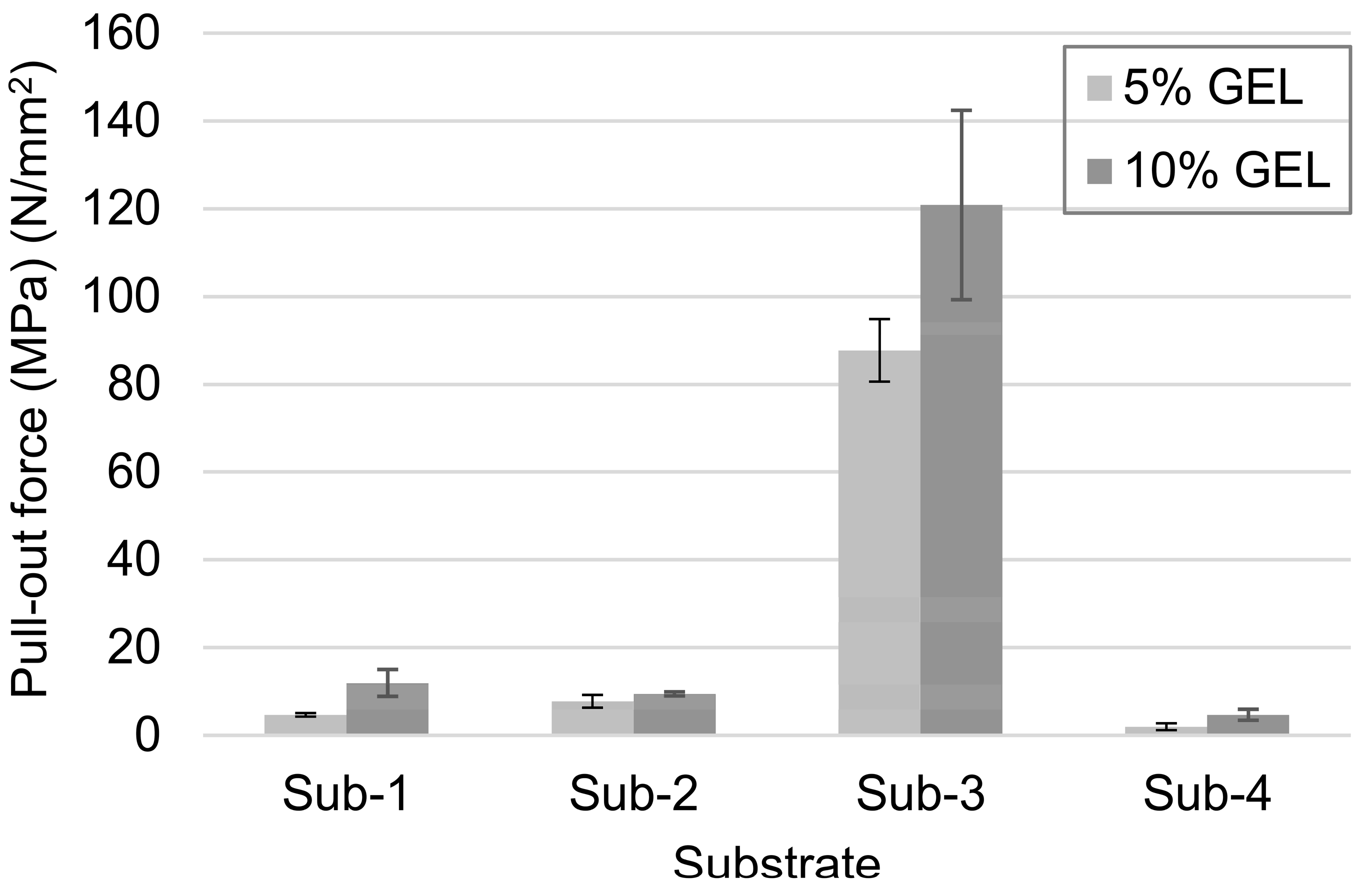
2.2.9. Pull-Out Mechanical Testing
3. Results and Discussion
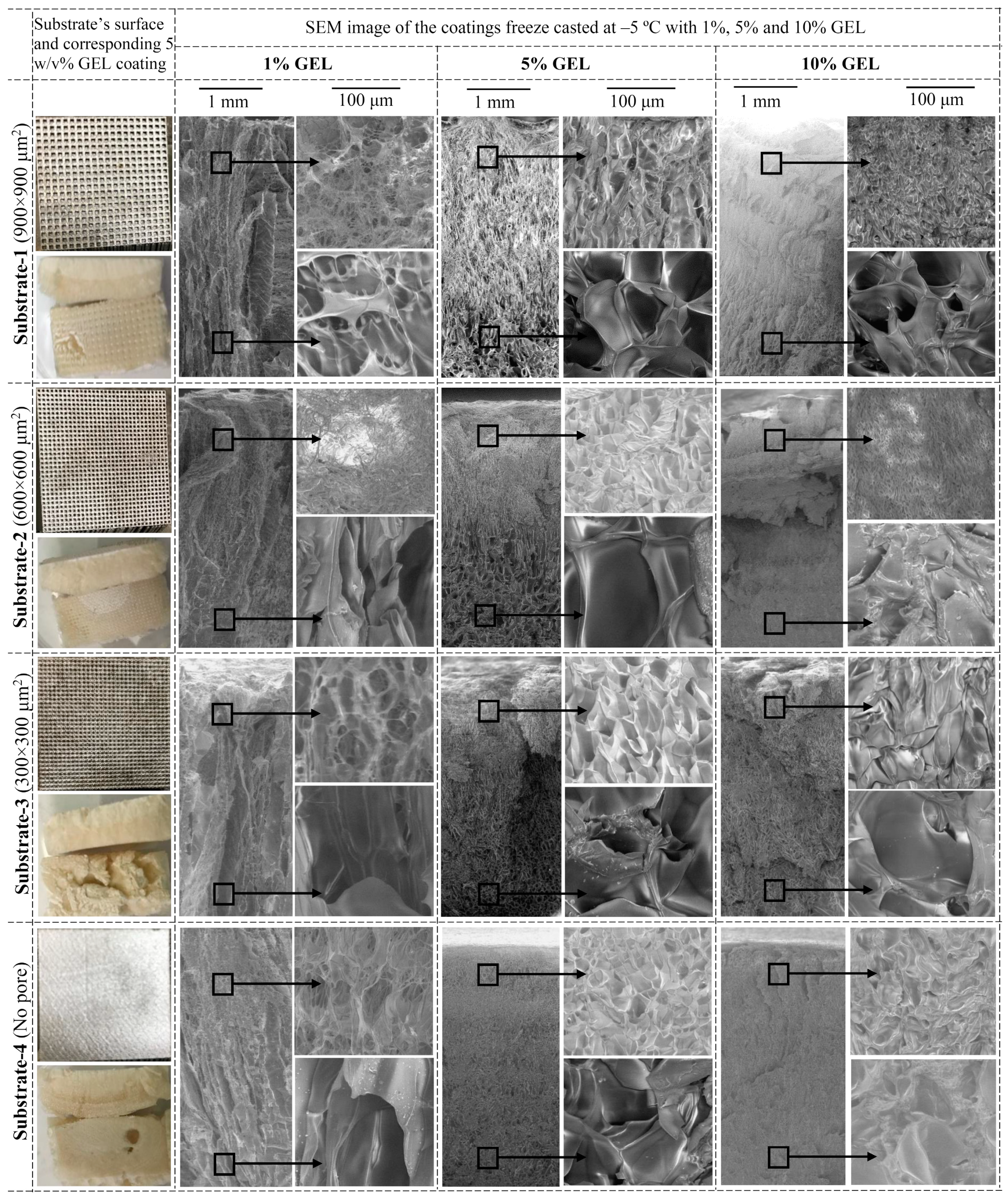
3.1. The Influence of GEL Concentration and Ti-6Al-4V Substrate’s Surface Texture on Coating Morphology
3.2. The Adhesion of GEL Coatings on Different Ti-6Al-4V Substrates
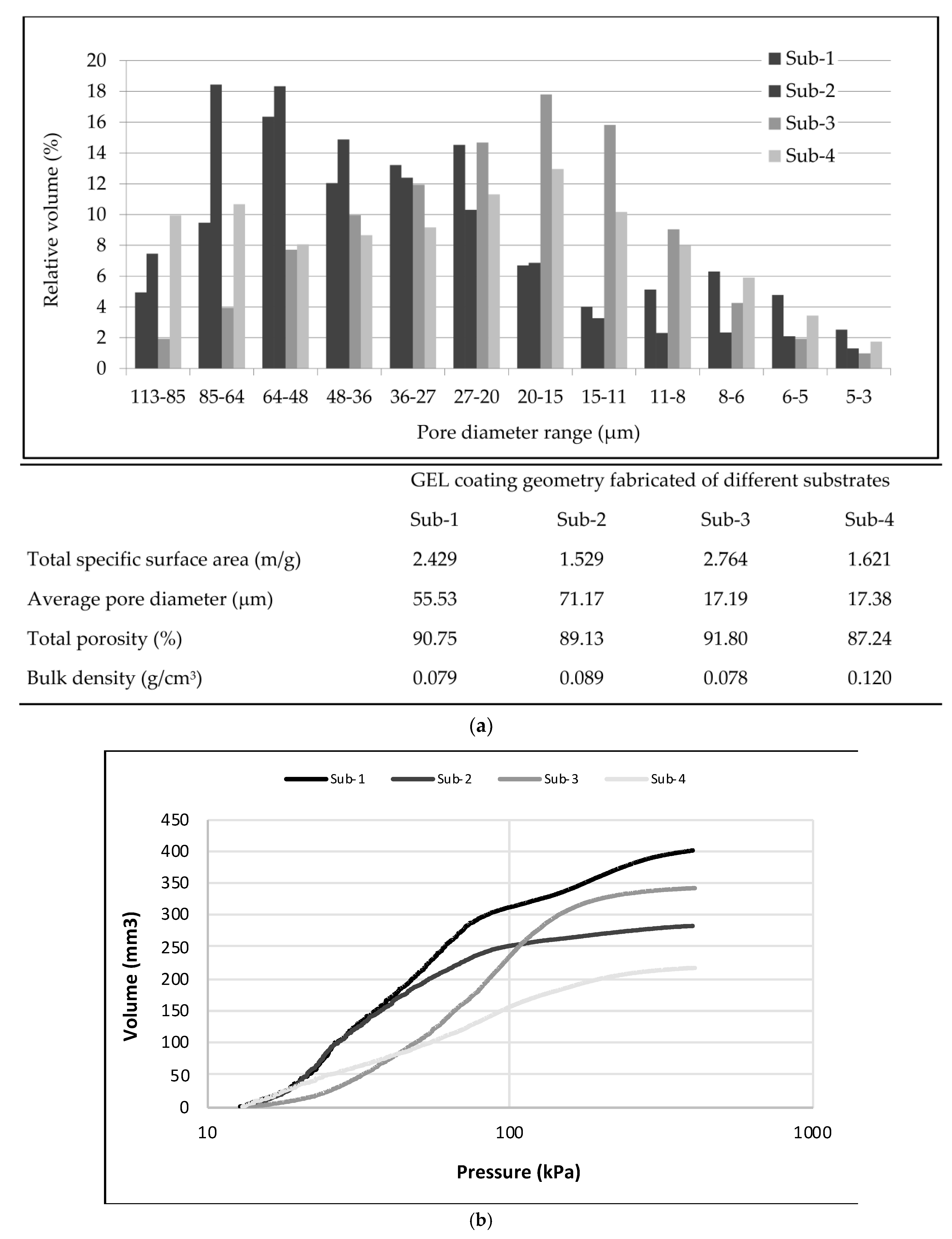
3.3. Compression Strength and Density Properties of GEL Coatings on Different Ti-6Al-4V Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benedetti, M.; Torresani, E.; Leoni, M.; Fontanari, V.; Bandini, M.; Pederzolli, C.; Potrich, C. The effect of post-sintering treatments on the fatigue and biological behavior of Ti-6Al-4V ELI parts made by selective laser melting. J. Mech. Behav. Biomed. Mater. 2017, 71, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Lek, J.Y.; Bhowmik, A.; Tan, A.W.Y.; Sun, W.; Song, X.; Zhai, W.; Buenconsejo, P.J.; Li, F.; Liu, E.; Lam, Y.M.; et al. Understanding the microstructural evolution of cold sprayed Ti-6Al-4V coatings on Ti-6Al-4V substrates. Appl. Surf. Sci. 2018, 459, 492–504. [Google Scholar] [CrossRef]
- Vanderleyden, E.; Van Bael, S.; Chai, Y.C.; Kruth, J.P.; Schrooten, J.; Dubruel, P. Gelatin functionalised porous titanium alloy implants for orthopaedic applications. Mater. Sci. Eng. C 2014, 42, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.C.; Hsieh, M.C.; Yang, S.P.; Chuang, P.K.; Lin, J.C.; Yang, C.Y.; Lee, T.M. Characteristics and cyto-compatibility of collagen/Ca–P coatings on Ti6Al4V substrate. Surf. Coat. Technol. 2011, 205, 4683–4689. [Google Scholar] [CrossRef]
- Xiu, P.; Jia, Z.; Lv, J.; Yin, C.; Cai, H.; Song, C.; Leng, H.; Zheng, Y.; Liu, Z.; Cheng, Y. Hierarchical micropore/nanorod apatite hybrids in-situ grown from 3-D Printed macroporous Ti6Al4V implants with improved bioactivity and osseointegration. J. Mater. Sci. Technol. 2017, 33, 179–186. [Google Scholar] [CrossRef]
- Wauthle, R.; Van Der Stok, J.; Yavari, S.A.; Van Humbeeck, J.; Kruth, J.P.; Zadpoor, A.A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef]
- Duan, K.; Wang, R. Surface modifications of bone implants through wet chemistry. J. Mater. Chem. 2006, 16, 2309–2321. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Gorbyk, P.P.; Petranovska, A.L.; Korduban, O.M.; Markovsky, P.E.; Ivasyshyn, O.M. Chapter 7—Formation of biomimetic hydroxyapatite coatings on the surface of titanium and Ti-containing alloys: Ti–6Al–4V and Ti–Zr–Nb. In Surface Chemistry of Nanobiomaterials, 1st ed.; Grumezescu, A., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 193–229. ISBN 978-0-323-42861-3. [Google Scholar]
- Vanderleyden, E.; Van Hoorebeke, L.; Schacht, E.; Dubruel, P. Comparative study of collagen and gelatin coatings on titanium surfaces. Macromol. Symp. 2011, 309–310, 190–198. [Google Scholar] [CrossRef][Green Version]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering - A mini review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.D.; Wen, C. Biomimetic porous titanium scaffolds for orthopaedic and dental applications; InTech: Milton, Australia, 2010. [Google Scholar]
- Li, J.P.; Li, S.H.; Van Blitterswijk, C.A.; de Groot, K. A novel porous Ti6Al4V: Characterization and cell attachment. J. Biomed. Mater. Res. A 2005, 73, 223–233. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Z.; Zhou, Y.; Liu, Y.; Wang, Z.; Tong, H.; Shen, X.; Wang, Y. Surface functionalization of titanium with chitosan/gelatin via electrophoretic deposition: Characterization and cell behavior. Biomacromolecules 2010, 11, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Kiran, A.S.K.; Sampath Kumar, T.S.; Perumal, G.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Dual nanofibrous bioactive coating and antimicrobial surface treatment for infection resistant titanium implants. Prog. Org. Coat. 2018, 121, 112–119. [Google Scholar] [CrossRef]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Li, C.; Qin, Y.; Zhong, L.; Chen, B.; Li, Z.; Liu, H.; Chang, F.; Wang, J. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: A review. J. Alloys Compd. 2017, 717, 271–285. [Google Scholar] [CrossRef]
- Van Cleynenbreugel, T.; Schrooten, J.; Van Oosterwyck, H.; Vander Sloten, J. Micro-CT-based screening of biomechanical and structural properties of bone tissue engineering scaffolds. Med. Biol. Eng. Comput. 2006, 44, 517–525. [Google Scholar] [CrossRef]
- Vehof, J.W.M.; Fisher, J.P.; Dean, D.; van der Waerden, J.P.C.M.; Spauwen, P.H.M.; Mikos, A.G.; Jansen, J.A. Bone formation in transforming growth factor beta-1-coated porous poly(propylene fumarate) scaffolds. J. Biomed. Mater. Res. 2002, 60, 241–251. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Esen, Z.; Bor, Ş. Processing of titanium foams using magnesium spacer particles. Scr. Mater. 2007, 56, 341–344. [Google Scholar] [CrossRef]
- Bram, M.; Schiefer, H.; Bogdanski, D.; Köller, M.; Buchkremer, H.P.; Stöver, D. Implant surgery: How bone bonds to PM titanium. Met. Powder Rep. 2006, 61, 26–31. [Google Scholar] [CrossRef]
- Fukushima, M.; Ohji, T.; Hyuga, H.; Matsunaga, C.; Yoshizawa, Y.I. Effect of gelatin gel strength on microstructures and mechanical properties of cellular ceramics created by gelation freezing route. J. Mater. Res. 2017, 32, 3286–3293. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Processing of gelatin-based cryogels with improved thermomechanical resistance, pore size gradient, and high potential for sustainable protein drug release. J. Biomed. Mater. Res. A 2015, 103, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Gil, H.B.; Min, S.G.; Lee, S.K.; Choi, M.J. Effects of pressure-shift freezing on the structural and physical properties of gelatin hydrogel matrices. Korean J. Food Sci. Anim. Resour. 2014, 34, 33–39. [Google Scholar] [CrossRef]
- Fayyazbakhsh, F.; Solati-Hashjin, M.; Keshtkar, A.; Shokrgozar, M.A.; Dehghan, M.M.; Larijani, B. Novel layered double hydroxides-hydroxyapatite/gelatin bone tissue engineering scaffolds: Fabrication, characterization, and in vivo study. Mater. Sci. Eng. C 2017, 76, 701–714. [Google Scholar] [CrossRef]
- Huang, Z.M.; Qi, Y.Y.; Du, S.H.; Feng, G.; Unuma, H.; Yan, W.Q. Promotion of osteogenic differentiation of stem cells and increase of bone-bonding ability in vivo using urease-treated titanium coated with calcium phosphate and gelatin. Sci. Technol. Adv. Mater. 2013, 14, 55001. [Google Scholar] [CrossRef] [PubMed]
- Rohanizadeh, R.; Swain, M.V.; Mason, R.S. Gelatin sponges (Gelfoam®) as a scaffold for osteoblasts. J. Mater. Sci. Mater. Med. 2008, 19, 1173–1182. [Google Scholar] [CrossRef]
- Ulubayram, K.; Nur Cakar, A.; Korkusuz, P.; Ertan, C.; Hasirci, N. EGF containing gelatin-based wound dressings. Biomaterials 2001, 22, 1345–1356. [Google Scholar] [CrossRef]
- Goodman, S.; Toksvig-Larsen, S.; Aspenberg, P. Ingrowth of bone into pores in titanium chambers implanted in rabbits: Effect of pore cross-sectional shape in the presence of dynamic shear. J. Biomed. Mater. Res. 1993, 27, 247–253. [Google Scholar] [CrossRef]
- Pal, S.; Gubeljak, N.; Hudak, R.; Lojen, G.; Rajtukova, V.; Predan, J.; Kokol, V.; Drstvensek, I. Tensile properties of selective laser melting products affected by building orientation and energy density. Mater. Sci. Eng. A 2018, 743, 637–647. [Google Scholar] [CrossRef]
- Rulison, C. Two-component surface energy characterization as a predictor of wettability and dispersability. Krus. Appl. note AN213 2000, 1–22. [Google Scholar]
- Zandi, M. Studies on the Gelation of Gelatin Solutions and on the Use of Resulting Gels for Medical Scaffolds. Ph.D. Thesis, University of Duisburg-Essen, Duisburg, Germany, February 2008. [Google Scholar]
- Gornall, J.L.; Terentjev, E.M. Universal kinetics of helix-coil transition in gelatin. Phys. Rev. E: Stat. Nonlinear Soft Matter Phys. 2008, 77, 031908. [Google Scholar] [CrossRef] [PubMed]
- Lan Levengood, S.K.; Polak, S.J.; Poellmann, M.J.; Hoelzle, D.J.; Maki, A.J.; Clark, S.G.; Wheeler, M.B.; Wagoner Johnson, A.J. The effect of BMP-2 on micro- and macroscale osteointegration of biphasic calcium phosphate scaffolds with multiscale porosity. Acta Biomater. 2010, 6, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- Mantila Roosa, S.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. - Part A 2010, 92, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Itala, A.I.; Ylanen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Joint Surg. Am. 2001, 83, S105–S115. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Terpilowski, K. Comparison of apparent surface free energy of some solids determined by different approaches. In Contact Angle, Wettability and Adhesion, Volume 6, 1st ed.; Mittal, K.L., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 281–310. [Google Scholar]
- Cicciu, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive titanium surfaces: Interactions of eukaryotic and prokaryotic cells of nano devices applied to dental practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef]
- Knorr, S.D.; Combe, E.C.; Wolff, L.F.; Hodges, J.S. The surface free energy of dental gold-based materials. Dent. Mater. 2005, 21, 272–277. [Google Scholar] [CrossRef]
- Sun, C.C.; Lee, S.C.; Hwang, W.C.; Hwang, J.S.; Tang, I.T.; Fu, Y.S. Surface free energy of alloy nitride coatings deposited using closed field unbalanced magnetron sputter ion plating. Mater. Trans. 2006, 47, 2533–2539. [Google Scholar] [CrossRef]
- Peršin, Z.; Stenius, P.; Stana-Kleinschek, K. Estimation of the surface energy of chemically and oxygen plasma-treated regenerated cellulosic fabrics using various calculation models. Text. Res. J. 2011, 81, 1673–1685. [Google Scholar] [CrossRef]
- Schuster, J.M.; Schvezov, C.E.; Rosenberger, M.R. Analysis of the results of surface free energy measurement of Ti6Al4V by different methods. Procedia Mater. Sci. 2015, 8, 732–741. [Google Scholar] [CrossRef]
- Fowkes, F.M. Donor-acceptor interactions at interfaces. J. Adhes. 1972, 4, 155–159. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Moses, R.L.; Jacobs, J.J. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001, 7, 55–71. [Google Scholar] [CrossRef]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Clark, R.; Moses, R.L. Cell adhesion to biomaterials: Correlations between surface charge, surface roughness, adsorbed protein, and cell morphology. J. Long-Term Eff. Med. Implant. 1995, 5, 209–231. [Google Scholar]
- Ceotto, D. Empirical relation between surface tension and density for some pure liquid metals. Russ. J. Phys. Chem. A 2015, 89, 2327–2330. [Google Scholar] [CrossRef]
- Eng, C.; Osgouei, A.; Abbas, G. Density and temperature dependencies of liquid surface tension. Iran J. Chem. Chem. Eng. (IJCCE) 2011, 30, 79–90. [Google Scholar]
- Osorio, F.A.; Bilbao, E.; Bustos, R.; Alvarez, F. Effects of concentration, bloom degree, and pH on gelatin melting and gelling temperatures using small amplitude oscillatory rheology. Int. J. Food Prop. 2007, 10, 841–851. [Google Scholar] [CrossRef]
- Cox, R.A. Acids and Bases. Solvent effects on acid-base strength. By Brian G. Cox. Angew. Chem. Int. Ed. 2013, 52, 7638. [Google Scholar] [CrossRef]
- Wang, J.; De Boer, J.; De Groot, K. Preparation and characterization of electrodeposited calcium phosphate/chitosan coating on Ti6Al4V plates. J. Dent. Res. 2004, 83, 296–310. [Google Scholar] [CrossRef]
- Ramay, H.R.R.; Zhang, M. Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials 2004, 25, 5171–5180. [Google Scholar] [CrossRef] [PubMed]
| Substrate | Place of Coating | Range of Pore Size (µm) | ||
|---|---|---|---|---|
| 1% GEL | 5% GEL | 10% GEL | ||
| Sub-1 | A | 20–45 | 25–40 | 10–20 |
| B | 100–140 | 75–100 | 75–100 | |
| Sub-2 | A | 10–20 | 20–30 | 5–10 |
| B | 75–125 | 75–100 | 50–60 | |
| Sub-3 | A | 25–50 | 30–40 | 20–50 |
| B | 110–160 | 70–90 | 75–100 | |
| Sub-4 | A | 15–30 | 20–30 | 20–30 |
| B | 130–180 | 60–80 | 50–60 | |
| Sample (% Polarity) | |||||||
|---|---|---|---|---|---|---|---|
| Test Liquids (% Polarity) | GEL Solutions (% Polarity) | ||||||
| W (63.73%) | EG (44.65%) | DI (0%) | F (50.68%) | G1 (35.47%) | G5 (32.98%) | G10 (35.39%) | |
| Sub-1 (4.31%) | 7.80 | −12.91 | −20.47 | −6.24 | −17.87 | −19.37 | −19.05 |
| Sub-2 (8.82%) | −8.01 | −25.50 | −28.57 | −20.27 | −29.38 | −30.38 | −29.94 |
| Sub-3 (6.25%) | −2.75 | −21.64 | −27.28 | −15.85 | −26.0 | −27.18 | −16.66 |
| Sub-4 (14.29%) | −21.37 | −35.70 | −33.53 | −31.80 | −38.51 | −39.05 | −38.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, S.; Peršin, Z.; Vuherer, T.; Drstvenšek, I.; Kokol, V. The Effect of Ti-6Al-4V Alloy Surface Structure on the Adhesion and Morphology of Unidirectional Freeze-Coated Gelatin. Coatings 2020, 10, 434. https://doi.org/10.3390/coatings10050434
Pal S, Peršin Z, Vuherer T, Drstvenšek I, Kokol V. The Effect of Ti-6Al-4V Alloy Surface Structure on the Adhesion and Morphology of Unidirectional Freeze-Coated Gelatin. Coatings. 2020; 10(5):434. https://doi.org/10.3390/coatings10050434
Chicago/Turabian StylePal, Snehashis, Zdenka Peršin, Tomaž Vuherer, Igor Drstvenšek, and Vanja Kokol. 2020. "The Effect of Ti-6Al-4V Alloy Surface Structure on the Adhesion and Morphology of Unidirectional Freeze-Coated Gelatin" Coatings 10, no. 5: 434. https://doi.org/10.3390/coatings10050434
APA StylePal, S., Peršin, Z., Vuherer, T., Drstvenšek, I., & Kokol, V. (2020). The Effect of Ti-6Al-4V Alloy Surface Structure on the Adhesion and Morphology of Unidirectional Freeze-Coated Gelatin. Coatings, 10(5), 434. https://doi.org/10.3390/coatings10050434









