Synthesis of Polypyrrole/V2O5 Composite Film on the Surface of Magnesium Using a Mild Vapor Phase Polymerization (VPP) Method for Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
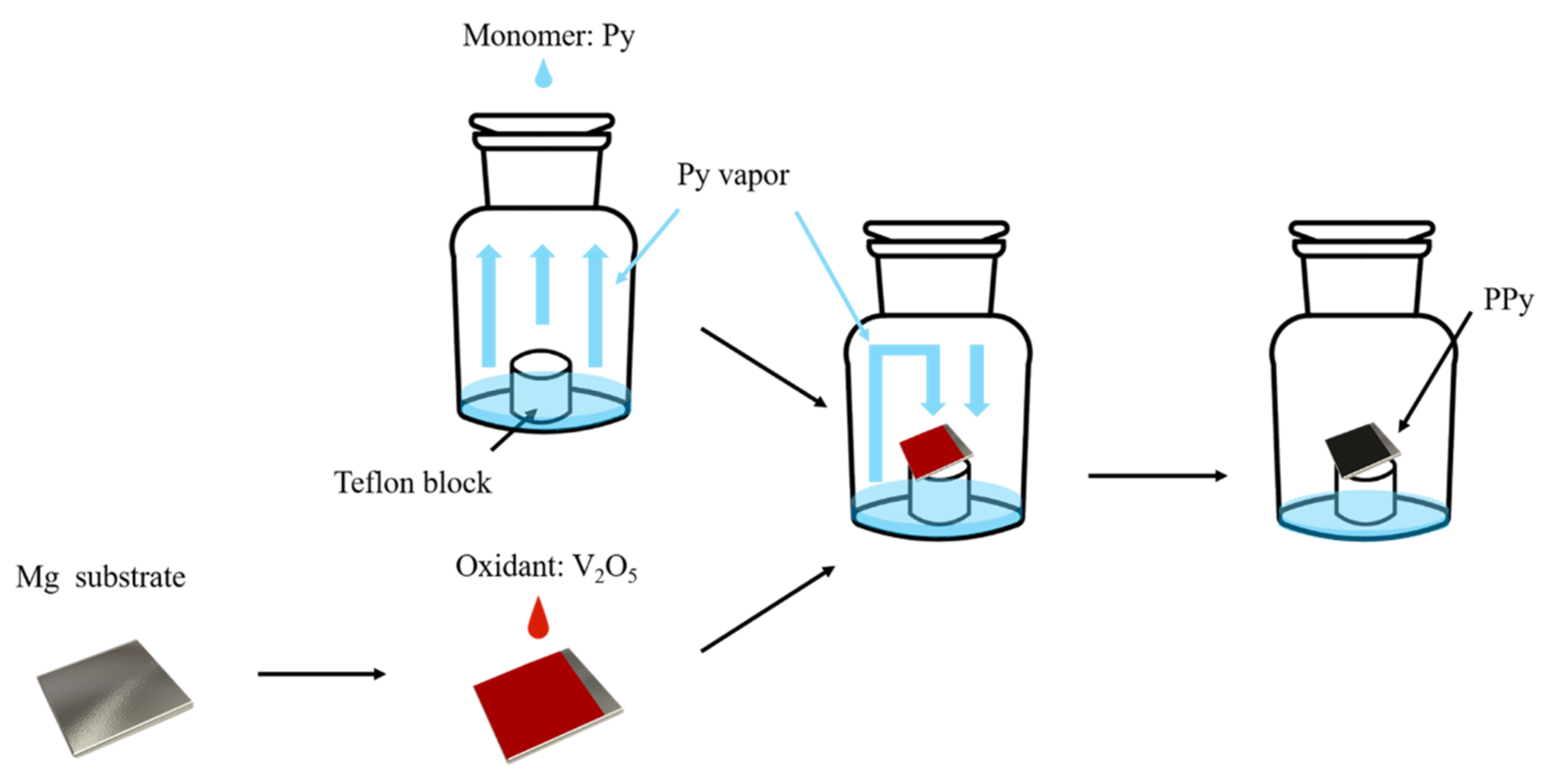
2.2. Synthesis of PPy/V2O5 Film on the Surface of Mg
2.3. Performance Test
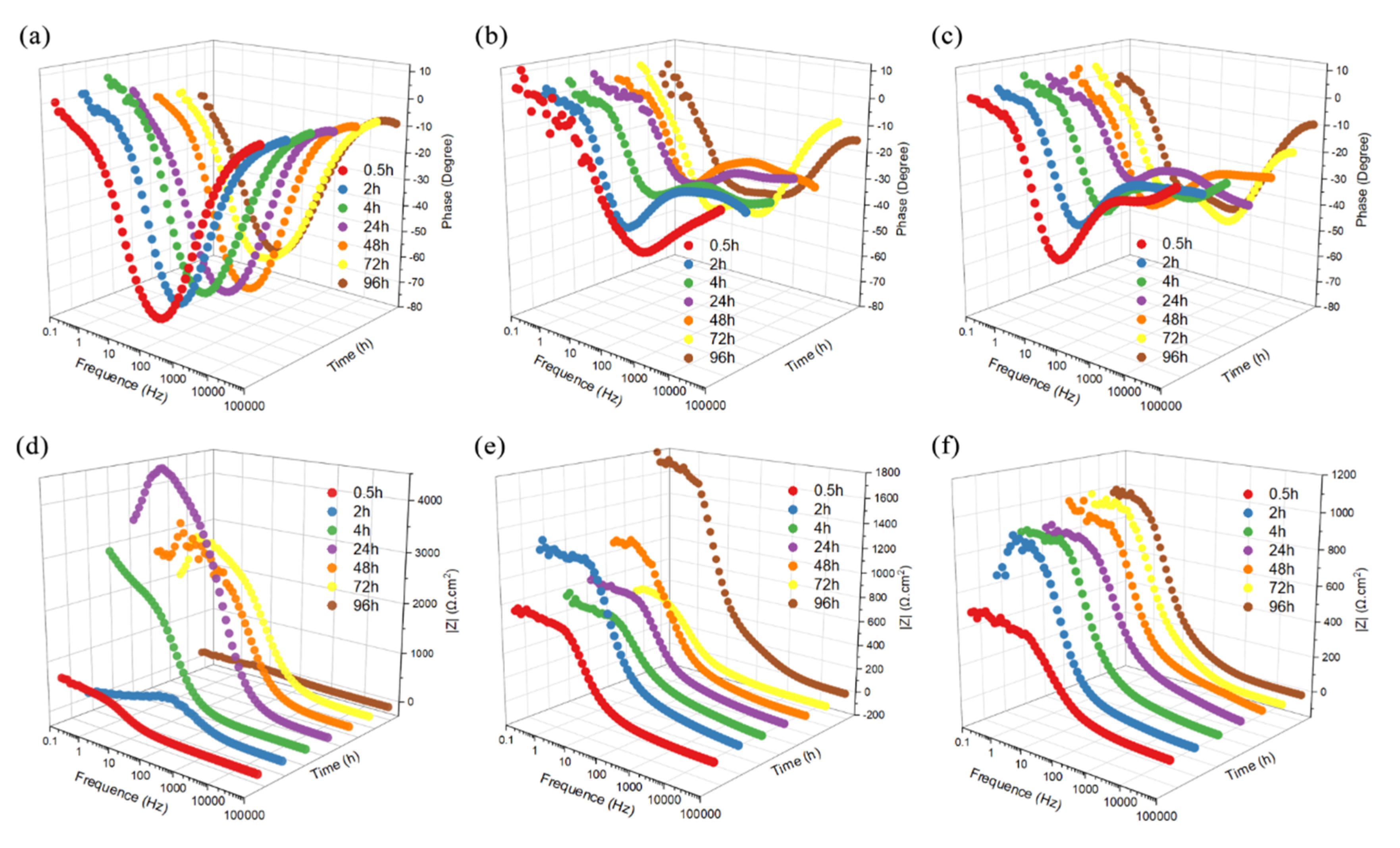
3. Results and Discussion
3.1. Reaction Process
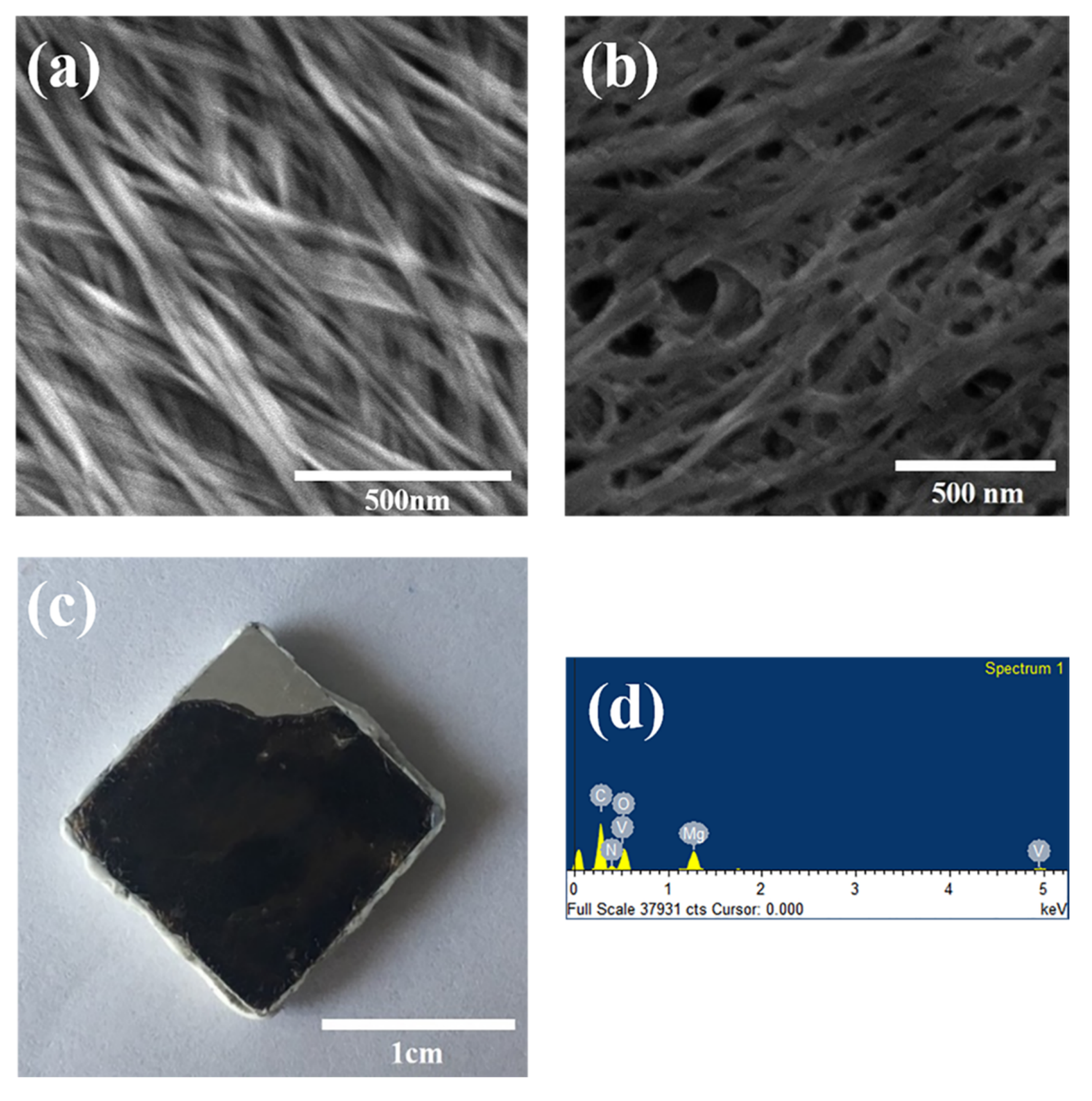
3.2. Morphologies of the PPy/V2O5 Film on the Mg
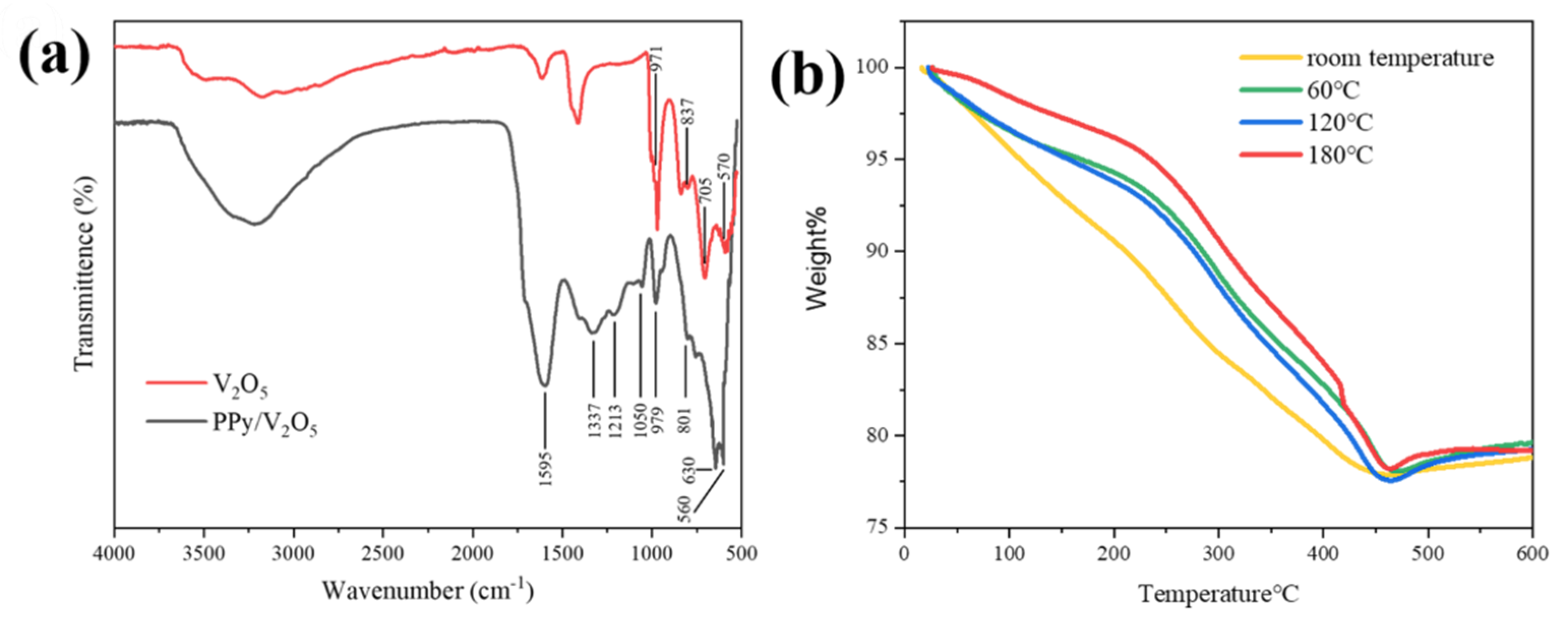
3.3. FTIR Spectroscopic Analysis
3.4. Thermogravimetric Analysis
3.5. Conductivity
3.6. Characterization of the Corrosion Resistance
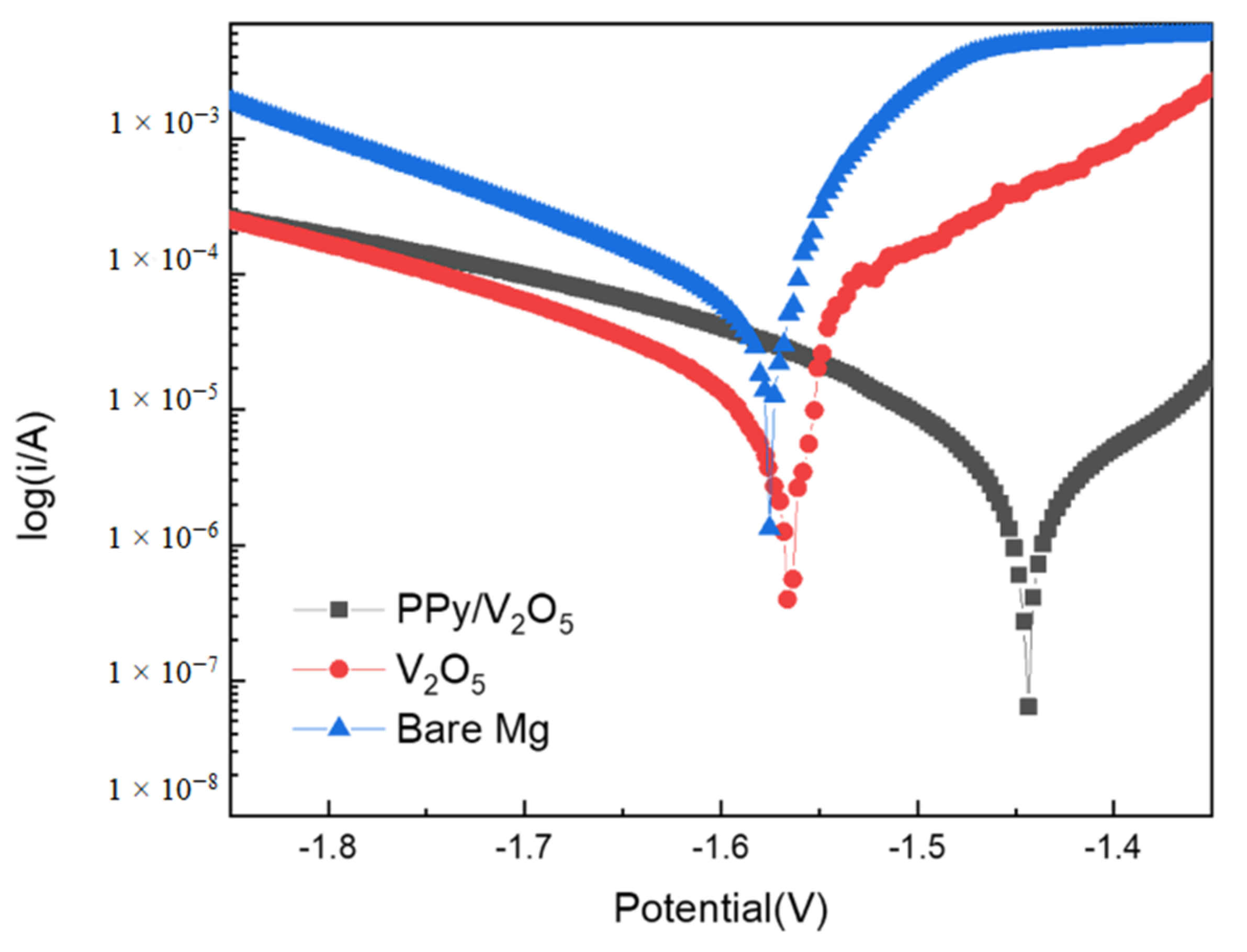
3.6.1. Tracing Quasistationary Polarization
3.6.2. Electrochemical Impedance Spectroscopy
3.7. Mechanism of the Corrosion Resistance
3.8. The Role of V2O5
3.9. The Limitations of Our VPP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Turhan, M.C.; Weiser, M.; Jha, H.; Virtanen, S. Optimization of electrochemical polymerization parameters of polypyrrole on Mg-Al alloy (AZ91D) electrodes and corrosion performance. Electrochim. Acta 2011, 56, 5347–5354. [Google Scholar] [CrossRef]
- Srinivasan, A.; Ranjani, P.; Rajendran, N. Electrochemical polymerization of pyrrole over AZ31 Mg alloy for biomedical applications. Electrochim. Acta 2013, 88, 310–321. [Google Scholar] [CrossRef]
- Truong, V.T.; Lai, P.K.; Moore, B.T.; Muscat, R.F.; Russo, M.S. Corrosion protection of magnesium by electroactive polypyrrole/paint coatings. Synthetic Met. 2000, 110, 7–15. [Google Scholar] [CrossRef]
- Bazzaoui, M.; Martins, J.I.; Bazzaoui, E.A.; Albourine, A. Environmentally friendly process for nickel electroplating of ABS. Appl. Surf. Sci. 2012, 258, 7968–7975. [Google Scholar] [CrossRef]
- Ishizaki, T.; Hieda, J.; Saito, N.; Saito, N.; Takai, O. Corrosion resistance and chemical stability of super-hydrophobic film deposited on magnesium alloy AZ31 by microwave plasma-enhanced chemical vapor deposition. Electrochim. Acta 2010, 55, 7094–7101. [Google Scholar] [CrossRef]
- Li, G.L.; Schenderlein, M.; Men, Y.J.; Mohwald, H.; Shchukin, D.G. Monodisperse Polymeric Core-Shell Nanocontainers for Organic Self-Healing Anticorrosion Coatings. Adv. Mater. Interfaces 2014, 1, 130019. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarswat, P.K.; Free, M.L. Metal oxides and novel metallates coated stable engineered steel for corrosion resistance applications. Appl. Surf. Sci. 2018, 456, 328–341. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Cui, X.K.; Zhang, Y.; Chen, S.G.; Dong, M.Y.; Liu, H.; Shao, Q.; Ding, T.; Wu, S.D.; Guo, Z.H. Poly (vinyl butyral)/Graphene oxide/poly (methylhydrosiloxane) nanocomposite coating for improved aluminum alloy anticorrosion. Polymer 2019, 172, 415–422. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.J.; Yu, G.H. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, Q.M.; Zhang, S.D.; Yin, R.; Liu, X.H.; He, Y.X.; Dai, K.; Shan, C.X.; Guo, J.; Liu, C.T.; et al. Electrically conductive polymer composites for smart flexible strain sensors: A critical review. J. Mater. Chem. C 2018, 6, 12121–12141. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Gleason, K.K. Device Fabrication Based on Oxidative Chemical Vapor Deposition (oCVD) Synthesis of Conducting Polymers and Related Conjugated Organic Materials. Adv. Mater. Interfaces 2019, 6, 1801564. [Google Scholar] [CrossRef] [Green Version]
- Cacialli, F.; Bruschi, P. Site-selective chemical-vapor-deposition of submicron-wide conducting polypyrrole films: Morphological investigations with the scanning electron and the atomic force microscope. J. Appl. Phys. 1996, 80, 70–75. [Google Scholar] [CrossRef]
- Cho, J.; Shin, K.H.; Jang, J. Polyaniline micropattern onto flexible substrate by vapor deposition polymerization-mediated inkjet printing. Thin Solid Films 2010, 518, 5066–5070. [Google Scholar] [CrossRef]
- Dall’Acqua, L.; Tonin, C.; Varesano, A.; Canetti, M.; Porzio, W.; Catellani, M. Vapour phase polymerisation of pyrrole on cellulose-based textile substrates. Synthetic Met. 2006, 156, 379–386. [Google Scholar] [CrossRef]
- Shin, K.H.; Cho, J.; Jang, J.; Jang, H.S.; Park, E.S.; Song, K.; Kim, S.H. Polypyrrole top-contact electrodes patterned by inkjet printing assisted vapor deposition polymerization in flexible organic thin-film transistors. Org. Electron. 2012, 13, 715–720. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Howden, R.M.; Borrelli, D.C.; Gleason, K.K. Vapor phase oxidative synthesis of conjugated polymers and applications. J. Polym. Sci. 2012, 50, 1329–1351. [Google Scholar] [CrossRef]
- Robinson, M.T.; Simons, C.E.; Cliffel, D.E.; Jennings, G.K. Photocatalytic photosystem I/PEDOT composite films prepared by vapor-phase polymerization. Nanoscale 2017, 9, 6158–6166. [Google Scholar] [CrossRef]
- Yasin, M.N.; Brooke, R.K.; Rudd, S.; Chan, A.; Chen, W.T.; Waterhouse, G.I.N.; Evans, D.; Rupenthal, I.D.; Svirskis, D. 3-Dimensionally ordered macroporous PEDOT ion-exchange resins prepared by vapor phase polymerization for triggered drug delivery: Fabrication and characterization. Electrochim. Acta 2018, 269, 560–570. [Google Scholar] [CrossRef]
- Zuber, K.; Shere, H.; Rehmen, J.; Wheaton, V.; Fabretto, M.; Murphy, P.J.; Evans, D.R. Influence of Postsynthesis Heat Treatment on Vapor-Phase-Polymerized Conductive Polymers. Acs Omega 2018, 3, 12679–12687. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.R.; Aliev, A.E.; Gnade, B.; Balkus, K.J. Fabrication of silver vanadium oxide and V2O5 nanowires for electrochromics. Acs Nano 2008, 2, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Malook, K.; Khan, H.; Shah, M. Synthesis, characterization and electrical properties of polypyrrole/V2O5 composites. Korean J. Chem. Eng. 2018, 35, 12–19. [Google Scholar] [CrossRef]
- Perera, S.D.; Patel, B.; Nijem, N.; Roodenko, K.; Seitz, O.; Ferraris, J.P.; Chabal, Y.J.; Balkus, K.J. Vanadium Oxide Nanowire-Carbon Nanotube Binder-Free Flexible Electrodes for Supercapacitors. Adv. Energy Mater. 2011, 1, 936–945. [Google Scholar] [CrossRef]
- Zia, J.; Kashyap, J.; Riaz, U. Facile synthesis of polypyrrole encapsulated V2O5 nanohybrids for visible light driven green sonophotocatalytic degradation of antibiotics. J. Mol. Liq. 2018, 272, 834–850. [Google Scholar] [CrossRef]
- Bai, M.H.; Bian, L.J.; Song, Y.; Liu, X.X. Electrochemical Codeposition of Vanadium Oxide and Polypyrrole for High-Performance Supercapacitor with High Working Voltage. Acs Appl. Mater. Interfaces 2014, 6, 12656–12664. [Google Scholar] [CrossRef]
- Jadhav, N.; Jensen, M.B.; Gelling, V. Tungstate and vanadate-doped polypyrrole/aluminum flake composite coatings for the corrosion protection of aluminum 2024-T3. J. Coat. Technol. Res. 2015, 12, 259–276. [Google Scholar] [CrossRef]
- Ren, X.Z.; Shi, C.; Zhang, P.X.; Jiang, Y.K.; Liu, J.H.; Zhang, Q.L. An investigation of V2O5/polypyrrole composite cathode materials for lithium-ion batteries synthesized by sol-gel. Mater. Sci. Eng. B Adv. 2012, 177, 929–934. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, W.S.; Rhee, H.W.; Song, M.K. Iron corrosion protection by ultra-thin conductive films based on polypyrrole/poly(methyl methacrylate) composite. Mol. Cryst. Liq. Cryst. 2006, 445, 193–200. [Google Scholar] [CrossRef]
- Li, F.; Li, G.X.; Zeng, J.; Gao, G.H. Molybdate-Doped Copolymer Coatings for Corrosion Prevention of Stainless Steel. J. Appl. Polym. Sci. 2014, 131, 40602. [Google Scholar] [CrossRef]
- Song, S.; Song, G.L.; Shen, W.; Liu, M. Corrosion and Electrochemical Evaluation of Coated Magnesium Alloys. Corrosion 2012, 68. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; He, Y.; Sun, Y.; Zhang, X.; Shi, W.; Ge, D. Synthesis of Polypyrrole/V2O5 Composite Film on the Surface of Magnesium Using a Mild Vapor Phase Polymerization (VPP) Method for Corrosion Resistance. Coatings 2020, 10, 402. https://doi.org/10.3390/coatings10040402
Li J, He Y, Sun Y, Zhang X, Shi W, Ge D. Synthesis of Polypyrrole/V2O5 Composite Film on the Surface of Magnesium Using a Mild Vapor Phase Polymerization (VPP) Method for Corrosion Resistance. Coatings. 2020; 10(4):402. https://doi.org/10.3390/coatings10040402
Chicago/Turabian StyleLi, Jiawei, Yuan He, Yanan Sun, Xiuming Zhang, Wei Shi, and Dongtao Ge. 2020. "Synthesis of Polypyrrole/V2O5 Composite Film on the Surface of Magnesium Using a Mild Vapor Phase Polymerization (VPP) Method for Corrosion Resistance" Coatings 10, no. 4: 402. https://doi.org/10.3390/coatings10040402
APA StyleLi, J., He, Y., Sun, Y., Zhang, X., Shi, W., & Ge, D. (2020). Synthesis of Polypyrrole/V2O5 Composite Film on the Surface of Magnesium Using a Mild Vapor Phase Polymerization (VPP) Method for Corrosion Resistance. Coatings, 10(4), 402. https://doi.org/10.3390/coatings10040402



