MnNH4P2O7-Based Coating for High Temperature Assessment on the Surfaces of Cement Composites
Abstract
1. Introduction
2. Inorganic Color Changing Pigment
3. Experiments
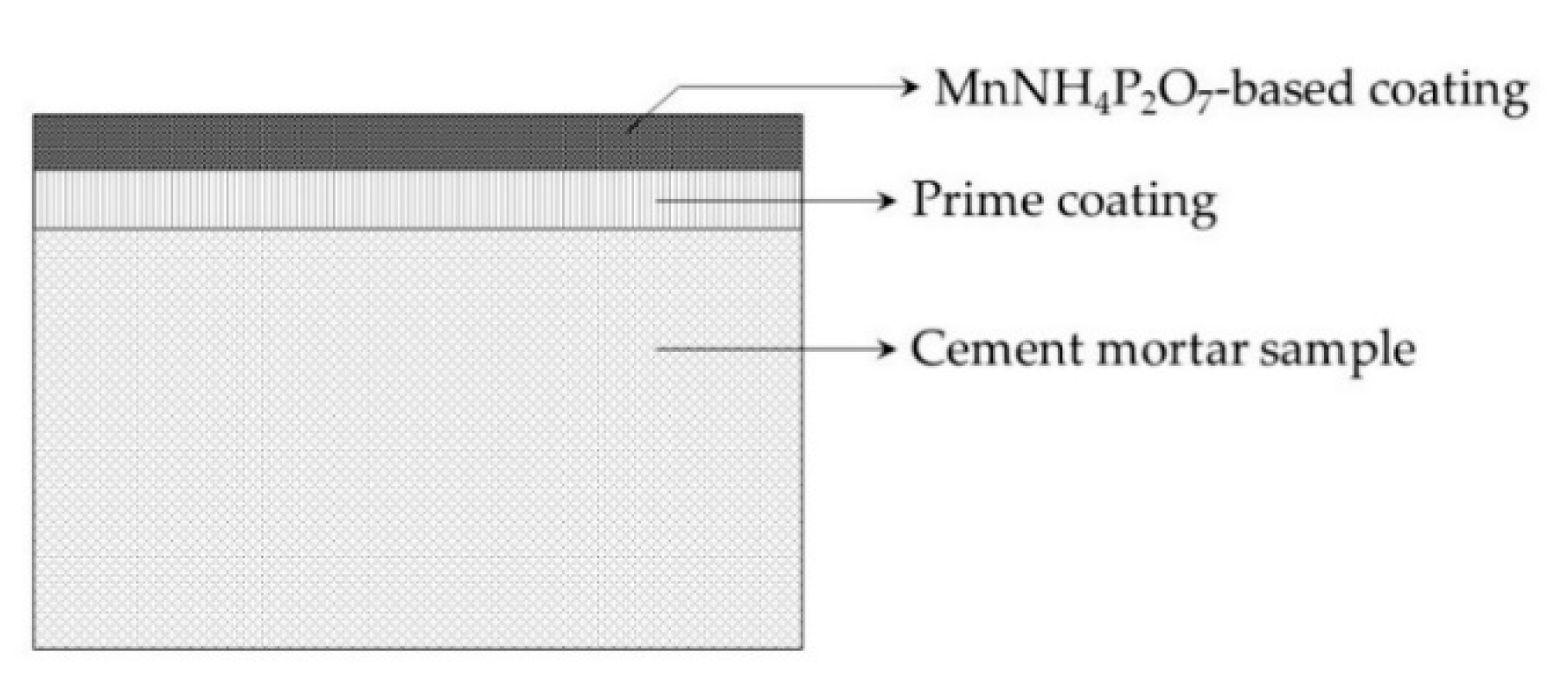
3.1. Preparation of Specimens and Test Variables
3.2. Image Acquisition and Post Processing
4. Results and Discussion
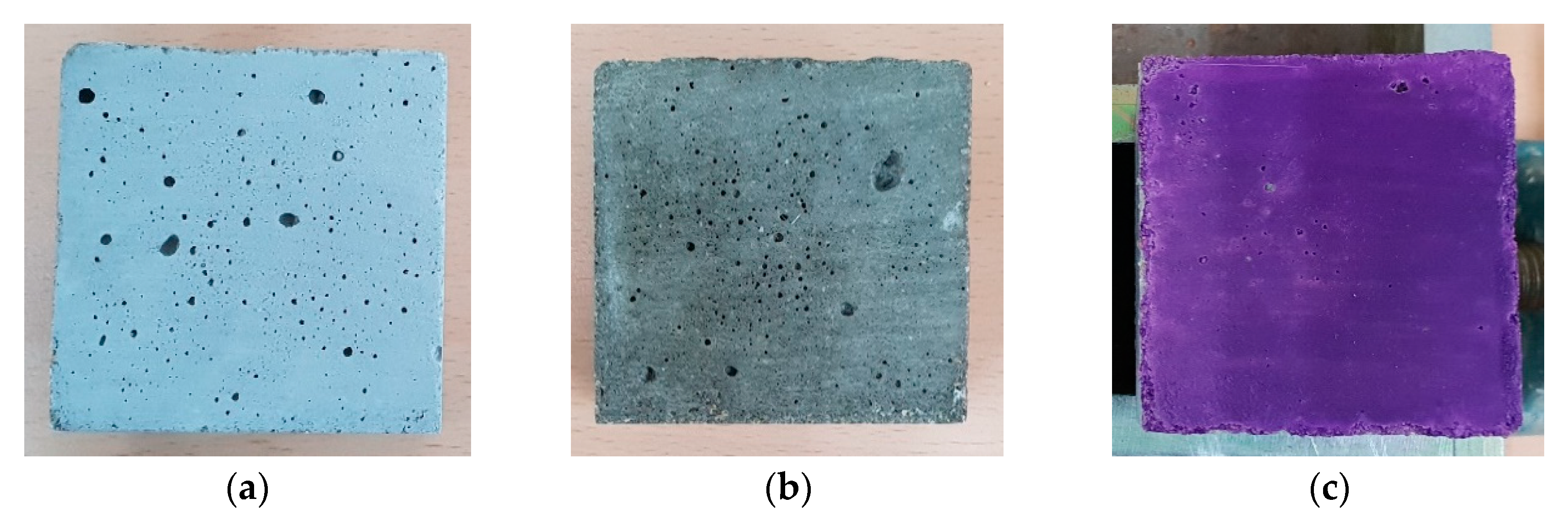
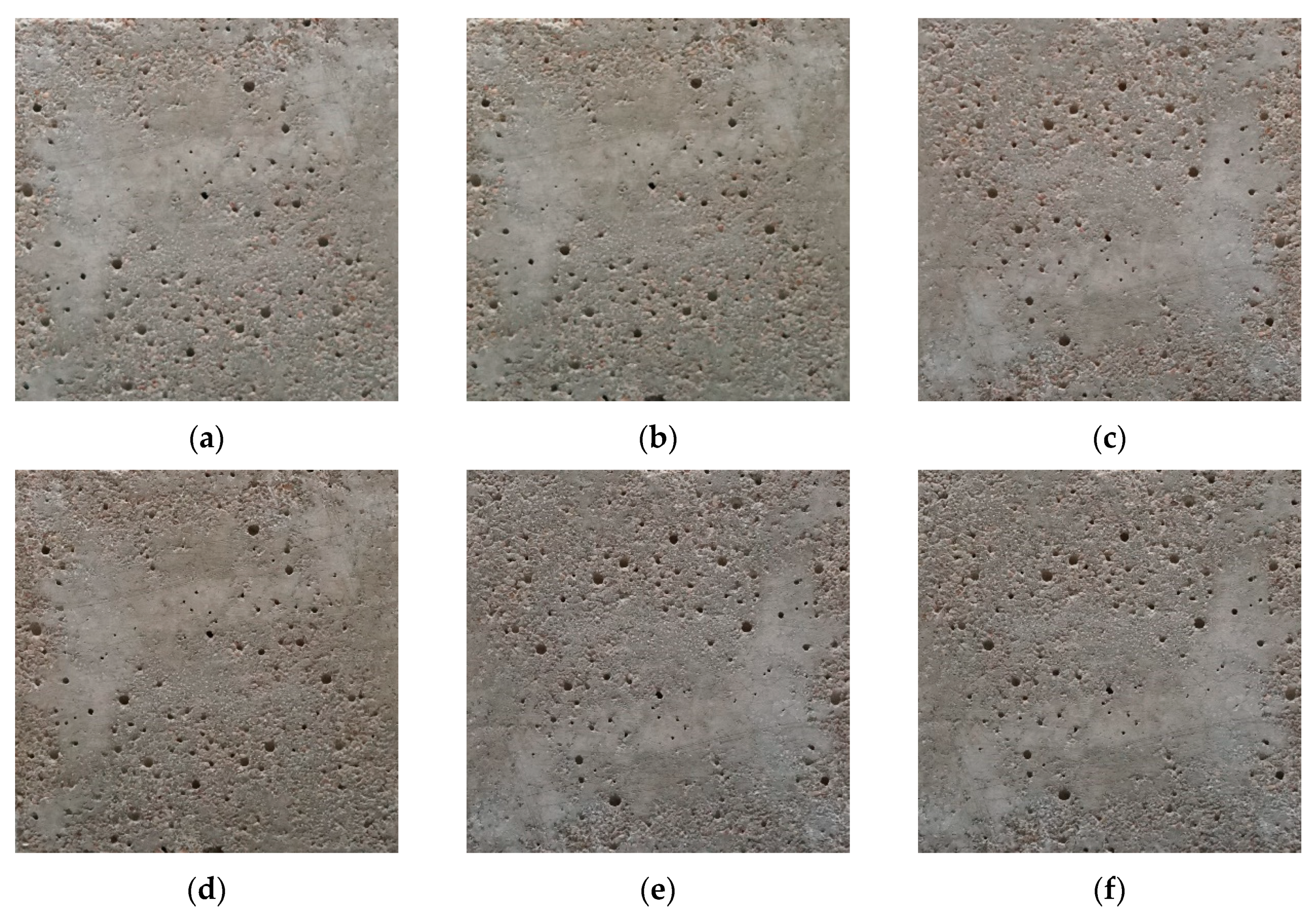
4.1. Thermal Response of Cement Composites and MnNH4P2O7-Based Coatings with Temperatures
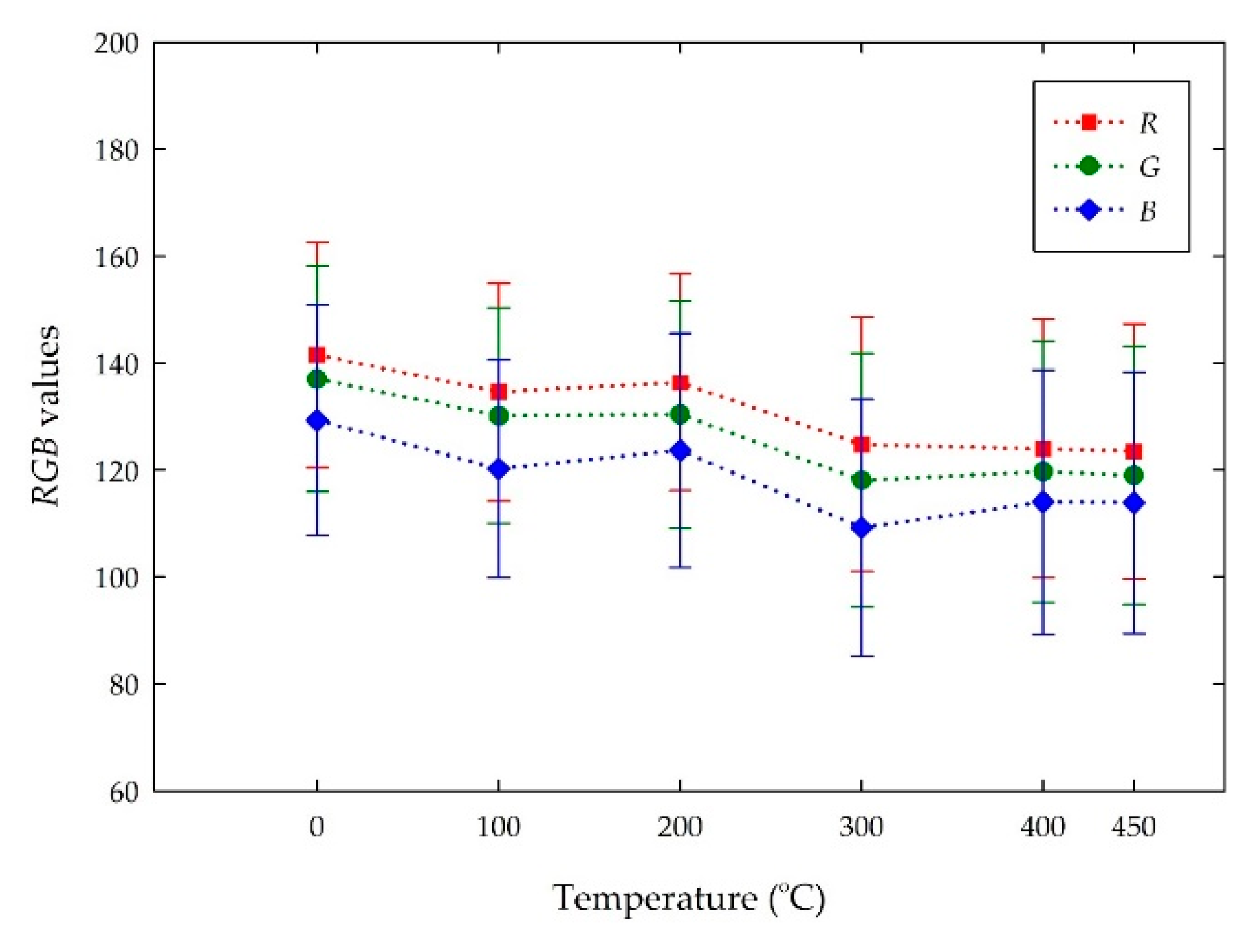
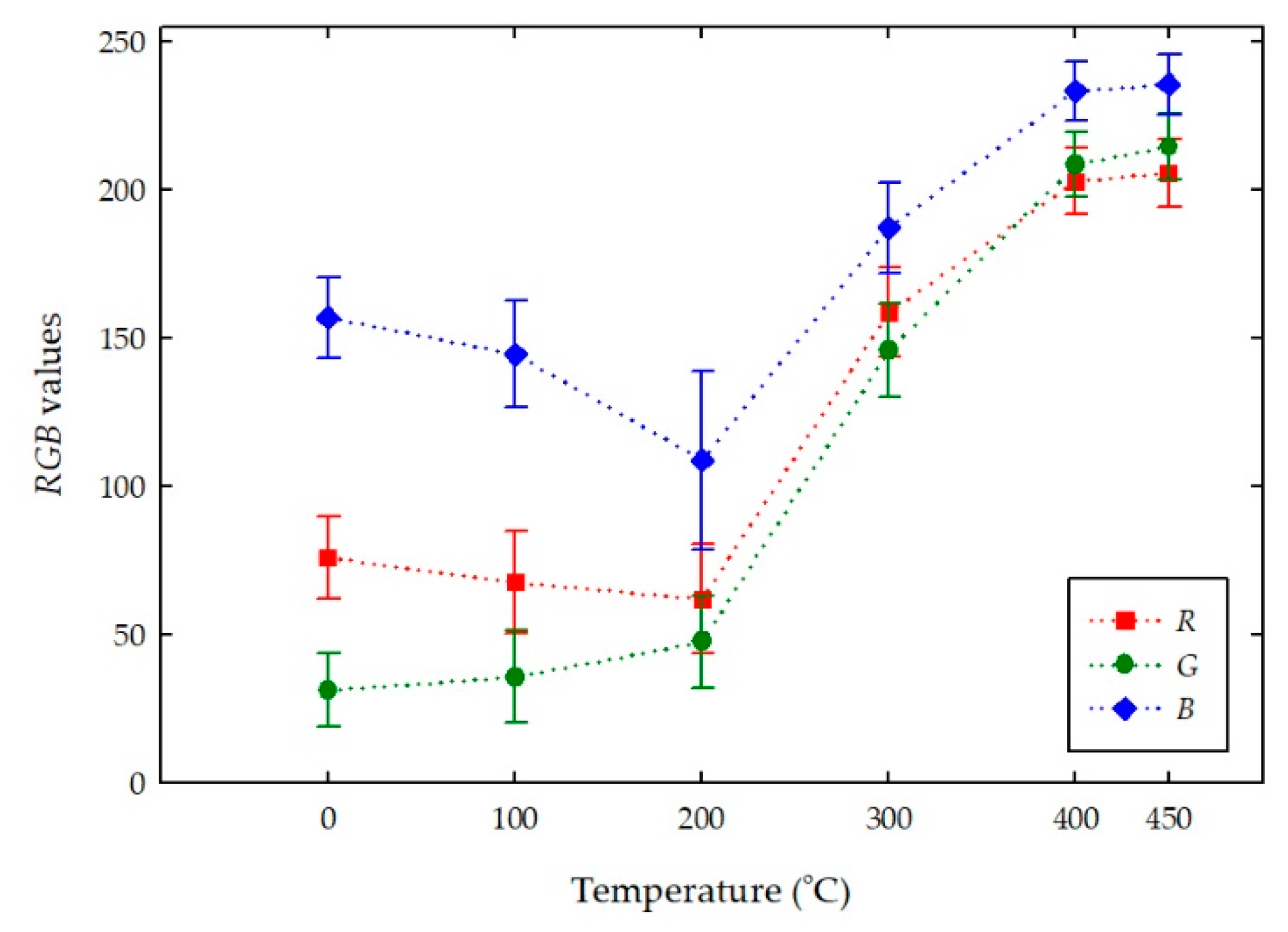
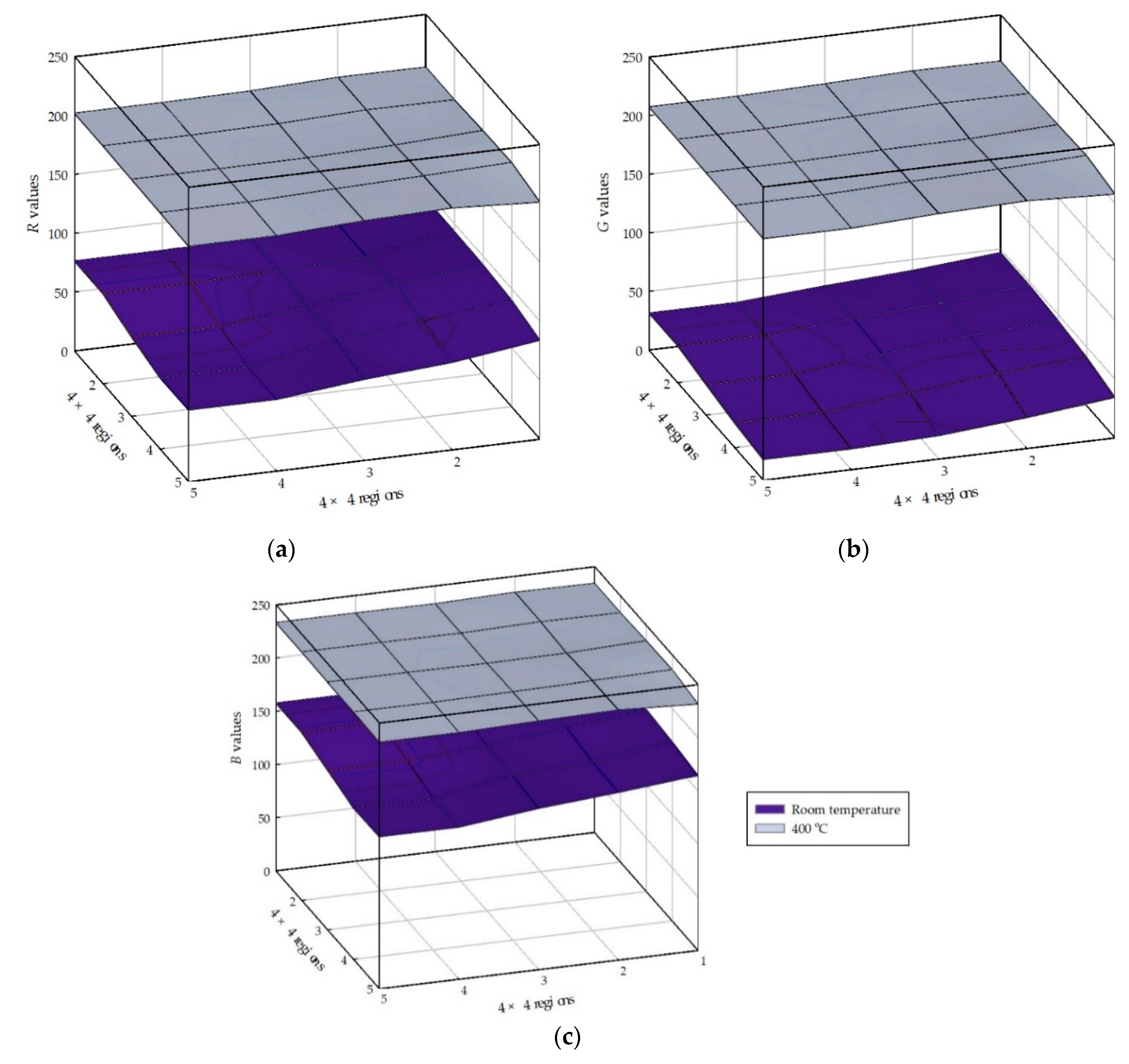
4.2. Color Changes Obtained in the RGB Color Space from Digital Images with Temperatures
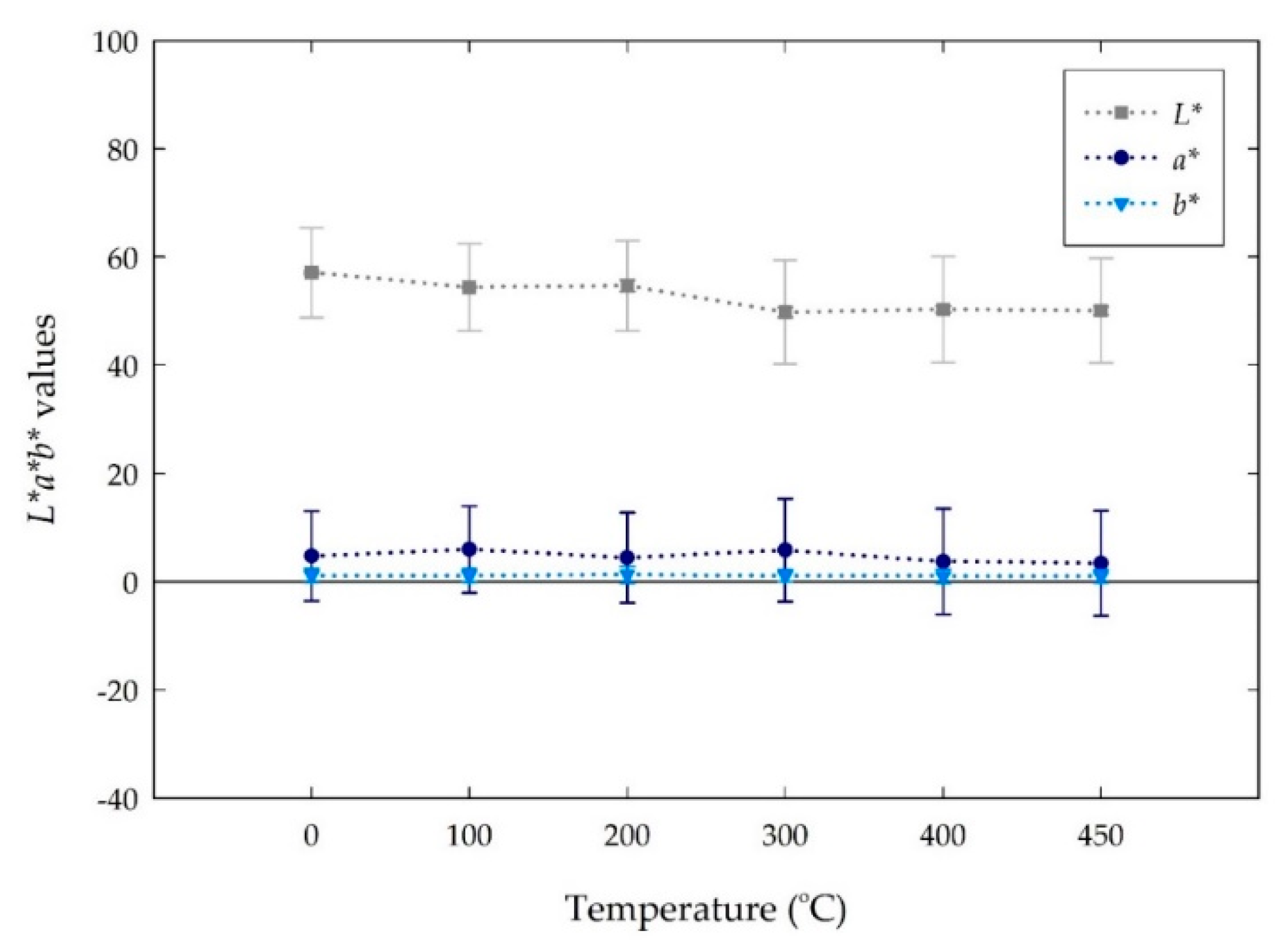
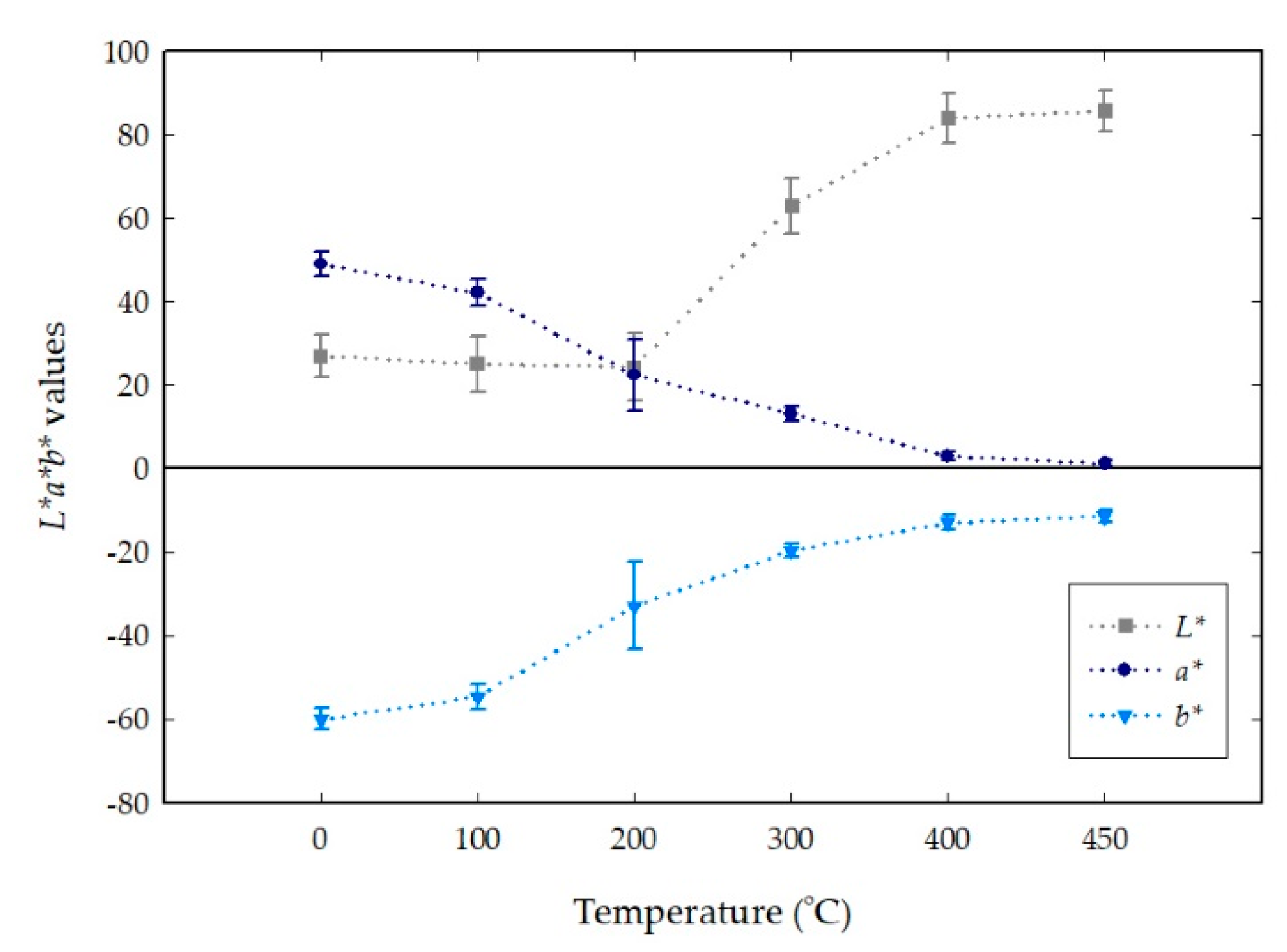
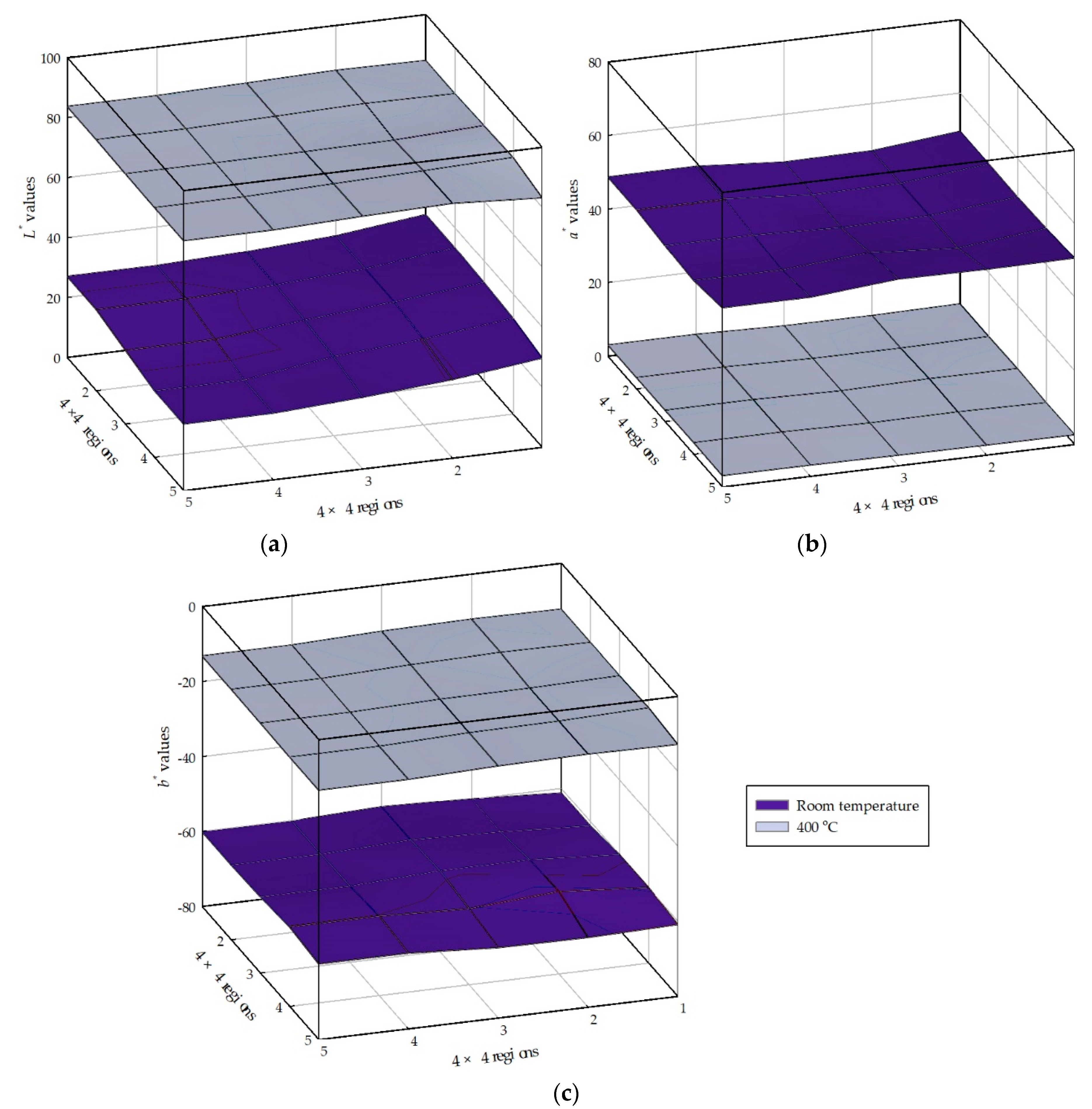
4.3. Color Changes Obtained in the L*a*b* Color Space from Digital Images with Temperatures
5. Summary
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferrara, M.; Bengisu, M. Materials That Change Color. In Springer Briefs in Applied Sciences and Technology; Springer Science and Business Media LLC: Heidelberg, Germany, 2013. [Google Scholar]
- Jorgensen, G. Electrochromic and Thermochromic Materials for Solar Energy Applications with Emphasis on Niobium and Vanadium Oxides; University of California: Berkeley, CA, USA, 1984. [Google Scholar]
- Ma, Y.; Zhu, B.; Wu, K. Preparation and solar reflectance spectra of chameleon-type building coatings. Sol. Energy 2001, 70, 417–422. [Google Scholar]
- Zheng, S.; Xu, Y.; Shen, Q.; Yang, H. Preparation of thermochromic coatings and their energy saving analysis. Sol. Energy 2015, 112, 263–271. [Google Scholar] [CrossRef]
- Apostolakis, K.; Karlessi, T.; Santamouris, M.; Didaskalopoulos, P.; Synnefa, A.; Assimakopoulos, D. Development and testing of PCM doped cool colored coatings to mitigate urban heat island and cool buildings. Build. Environ. 2010, 46, 570–576. [Google Scholar]
- Apostolakis, K.; Karlessi, T.; Synnefa, A.; Livada, I.; Santamouris, M. Development and testing of thermochromic coatings for buildings and urban structures. Sol. Energy 2008, 83, 538–551. [Google Scholar]
- Saliari, M.; Papantoniou, S.; Santamouris, M.; Kolokotsa, D.; Vangeloglou, E.; Karlessi, T.; Maravelaki-Kalaitzaki, P. Development and analysis of mineral based coatings for buildings and urban structures. Sol. Energy 2012, 86, 1648–1659. [Google Scholar]
- Kamalisarvestani, M.; Saidur, R.; Mekhilef, S.; Javadi, F.S. Performance, materials and coating technologies of thermochromic thin films on smart windows. Renew. Sustain. Energy Rev. 2013, 26, 353–364. [Google Scholar] [CrossRef]
- Chen, X.; Yoon, J. A thermally reversible temperature sensor based on polydiacetylene: Synthesis and thermochromic properties. Dye. Pigment. 2011, 89, 194–198. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.M. The research of temperature indicating paints and its application in aero-engine temperature measurement. Procedia Eng. 2015, 99, 1152–1157. [Google Scholar] [CrossRef]
- Lempereur, C.; Andral, R.; Prudhomme, J.Y. Surface temperature measurement on engine components by means of irreversible thermal coatings. Meas. Sci. Technol. 2008, 19, 105501. [Google Scholar] [CrossRef]
- Popescu, M.; Serban, L.; Popescu, M. Thermo-indicating paint for damage warning. J. Therm. Anal. Calorim. 1996, 46, 317–321. [Google Scholar] [CrossRef]
- Rabhiou, A.; Feist, J.; Kempf, A.; Skinner, S.; Heyes, A. Phosphorent thermal history sensors. Sens. Actuators A Phys. 2011, 169, 18–26. [Google Scholar] [CrossRef]
- Arulprakasajothi, M.; Rupesh, P.L. Surface temperature measurement of gar turbine combustor using temperature-indicating paint. Int. J. Amb. Energy 2020. [Google Scholar] [CrossRef]
- Rupesh, P.L.; Arulprakasajothi, M. Thermal history analysis on a hot surface using temperature indicating paints. Int. J. Amb. Energy 2020. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, B. Research on the preparation of reversibly thermochromic cement based materials at normal temperature. Cem. Concr. Res. 2009, 39, 90–94. [Google Scholar] [CrossRef]
- Lee, J. The effect of high temperature on color and residual compressive strength of concrete. In Proceedings of the 7th international Conference on Fracture Mechanics of Concrete and Concrete Structures. High Performance, Fiber Reinforced Concrete, Special Loadings and Structural Applications, Jeju, Korea, 23–28 May 2010; pp. 1772–1775. [Google Scholar]
- Hager, I. Behaviour of cement concrete at high temperature. Bull. Polish Acad. Sci. Tech. Sci. 2013, 61, 1–10. [Google Scholar] [CrossRef]
- Hajiloo, H.; Green, M.F. Post-fire residual properties of GFRP reinforced concrete slabs: A holistic investigation. Compos. Struct. 2018, 201, 398–413. [Google Scholar] [CrossRef]
- Yu, B.; Kodur, V.K.R. Effect of high temperature on bond strength of near-surface mounted FRP reinforcement. Compos. Struct. 2014, 110, 88–97. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Wang, Z.; Zhao, X.L. Fatigue behavior of concrete beams reinforced with glass- and carbon-fiber reinforced polymer (GFRP/CFRP) bars after exposure to elevated temperatures. Compos. Struct. 2019, 229, 111427. [Google Scholar] [CrossRef]
- Annerel, E.; Taerwe, L. Revealing the temperature history in concrete after fire exposure by microscopic analysis. Cem. Concr. Res. 2009, 39, 1239–1249. [Google Scholar] [CrossRef]
- Hager, I. Colour change in heated concrete. Fire Technol. 2014, 50, 945–958. [Google Scholar] [CrossRef]
- Yuzer, N.; Akoz, F.; Ozturk, D.L. Compressive strength–color change relation in mortars at high temperature. Cem. Concr. Res. 2004, 34, 1803–1807. [Google Scholar] [CrossRef]
- Begum, Y.; Wright, A.J. Relating highly distorted Jahn-Teller MnO6 to colouration in manganese violet pigments. J. Mater. Chem. 2012, 22, 21110–21116. [Google Scholar] [CrossRef]
- Rajagopalan, S.R.; Lee, J.H. High temperature sensing and detection for cementitious materials using manganese violet pigment. Materials 2020, 13, 993. [Google Scholar]
- Lee, J.D.; Browne, L.S. The nature and properties of manganese violet. J. Chem. Soc. A 1968, 559–561. [Google Scholar] [CrossRef]
- Liu, T.; Sullivan, J.P. Pressure and Temperature Sensitive Paints. In Technology and Engineering; Springer: Berlin, Germany, 2019; Volume 53. [Google Scholar]
- Annerel, E.; Taerwe, L. Methods to quantify the colour development of concrete exposed to fire. Constr. Build. Mater. 2011, 25, 3989–3997. [Google Scholar] [CrossRef]
- Felicetti, R. Digital camera colorimetry for the assessment of fire damaged concrete. In Proceedings of the International Conference on Fire deign of Concrete Structures: What now? What next? Milan, Italy, 2–4 December 2004. [Google Scholar]
- Wei, Y.; Kong, W.K.; Wan, C.; Wang, Y.Q. The colorimetry method in assessing fire-damaged concrete. J. Adv. Concr. Technol. 2019, 17, 282–294. [Google Scholar] [CrossRef]
- Short, N.R.; Guise, S.E.; Purkiss, J.A. Assessment of fire damaged concrete using colour image analysis. Constr. Build. Mater. 2001, 15, 9–15. [Google Scholar] [CrossRef]
- Annerel, E.V.; Taerwe, L.R. Assessment techniques for the evaluation of concrete structures after fire. J. Struct. Fire Eng. 2013, 4, 123–130. [Google Scholar]
- Felicetti, R. Combined while-drilling techniques for the assessment of deteriorated concrete cover. In Proceedings of the 7th International Symposium on Nondestructive Testing in Civil Engineering, Nantes, France, 30 June–3 July 2009. [Google Scholar]
- Tkaleie, M.; Tasie, J.F. Color Spaces—Perceptual, Historical and Applicational Background; IEEE: Ljubljana, Slovenia 2003; pp. 304–308. [Google Scholar]
- Kheng, L.W. Color spaces and color-difference equations. Color Res. Appl. 2002, 24, 186–198. [Google Scholar]
- MnNH4P2O7. Available online: https://www.kremer-pigmente.com (accessed on 1 January 2018).
- Pascale, D. RGB Coordinates of the Macbeth Color Checker; Babel Color Co.: Montreal, Canada, 2006; pp. 1–16. [Google Scholar]
- RGB Tables. Available online: https://www.rapidtables.com/web/color/RGB_Color.html (accessed on 1 November 2019).
- ColorHexa. Available online: https://www.color-hex.com/ (accessed on 1 November 2019).

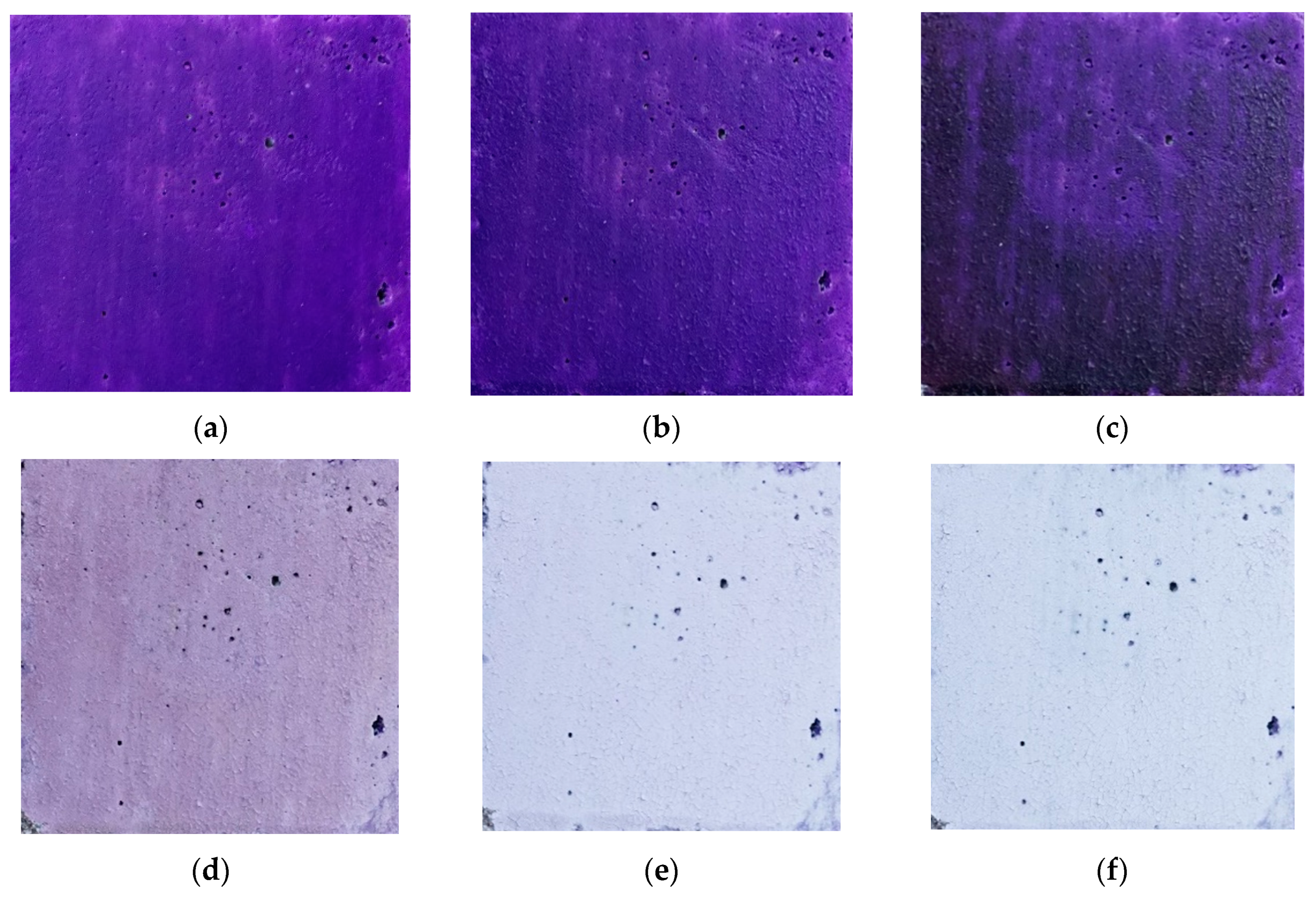
| Parameter | Characteristics |
|---|---|
| Color | Dark violet |
| Chemical characterization | Ammonium manganese III pyrophosphate |
| Density | 2.7–2.9 kg/m3 |
| Bulk density | 0.60 g/cm3 |
| Average particle size | 2.30 µm |
| pH value | 2.5–4.7 |
| Thermal decomposition | >400 °C |
| Temperature (°C) | Color Values | Hex Code | Color Description | ||
|---|---|---|---|---|---|
| Red (R) | Green (G) | Blue (B) | |||
| Room Temperature | 76 ± 13 | 31 ± 12 | 157 ± 13 | #4b1f9c | Dark violet |
| 100 | 67 ± 17 | 35 ± 15 | 144 ± 18 | #432390 | Dark violet |
| 200 | 62 ± 18 | 47 ± 15 | 109 ± 30 | #3d2f6c | Dark desaturated blue |
| 300 | 159 ± 15 | 146 ± 15 | 187 ± 15 | #9E91BB | Grayish violet |
| 400 | 202 ± 11 | 209 ± 11 | 233 ± 10 | #cad0e9 | Light grayish blue |
| 450 | 205 ± 11 | 214 ± 11 | 235 ± 10 | #cdd6eb | Light grayish blue |
| Temperature (°C) | Color Values | Hex Code | Color Description | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| Room Temperature | 26 ± 5 | 48 ± 3 | –60 ± 2 | #4b1f9c | Dark violet |
| 100 | 25 ± 6 | 42 ± 3 | –54 ± 3 | #432390 | Dark violet |
| 200 | 24 ± 8 | 22 ± 8 | –32 ± 10 | #3d2f6c | Dark desaturated blue |
| 300 | 62 ± 6 | 13 ± 1 | –19 ± 1 | #9E91BB | Grayish violet |
| 400 | 83 ± 5 | 2 ± 1 | –12 ± 1 | #cad0e9 | Light grayish blue |
| 450 | 85 ± 4 | 1 ± 1 | –11 ± 1 | #cdd6eb | Light grayish blue |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajadurai, R.S.; Lee, J.-H.; Choi, E.; Kang, J.-W. MnNH4P2O7-Based Coating for High Temperature Assessment on the Surfaces of Cement Composites. Coatings 2020, 10, 396. https://doi.org/10.3390/coatings10040396
Rajadurai RS, Lee J-H, Choi E, Kang J-W. MnNH4P2O7-Based Coating for High Temperature Assessment on the Surfaces of Cement Composites. Coatings. 2020; 10(4):396. https://doi.org/10.3390/coatings10040396
Chicago/Turabian StyleRajadurai, Rajagopalan Sam, Jong-Han Lee, Eunsoo Choi, and Joo-Won Kang. 2020. "MnNH4P2O7-Based Coating for High Temperature Assessment on the Surfaces of Cement Composites" Coatings 10, no. 4: 396. https://doi.org/10.3390/coatings10040396
APA StyleRajadurai, R. S., Lee, J.-H., Choi, E., & Kang, J.-W. (2020). MnNH4P2O7-Based Coating for High Temperature Assessment on the Surfaces of Cement Composites. Coatings, 10(4), 396. https://doi.org/10.3390/coatings10040396







