GLAD Magnetron Sputtered Ultra-Thin Copper Oxide Films for Gas-Sensing Application
Abstract
1. Introduction
2. Materials and Methods
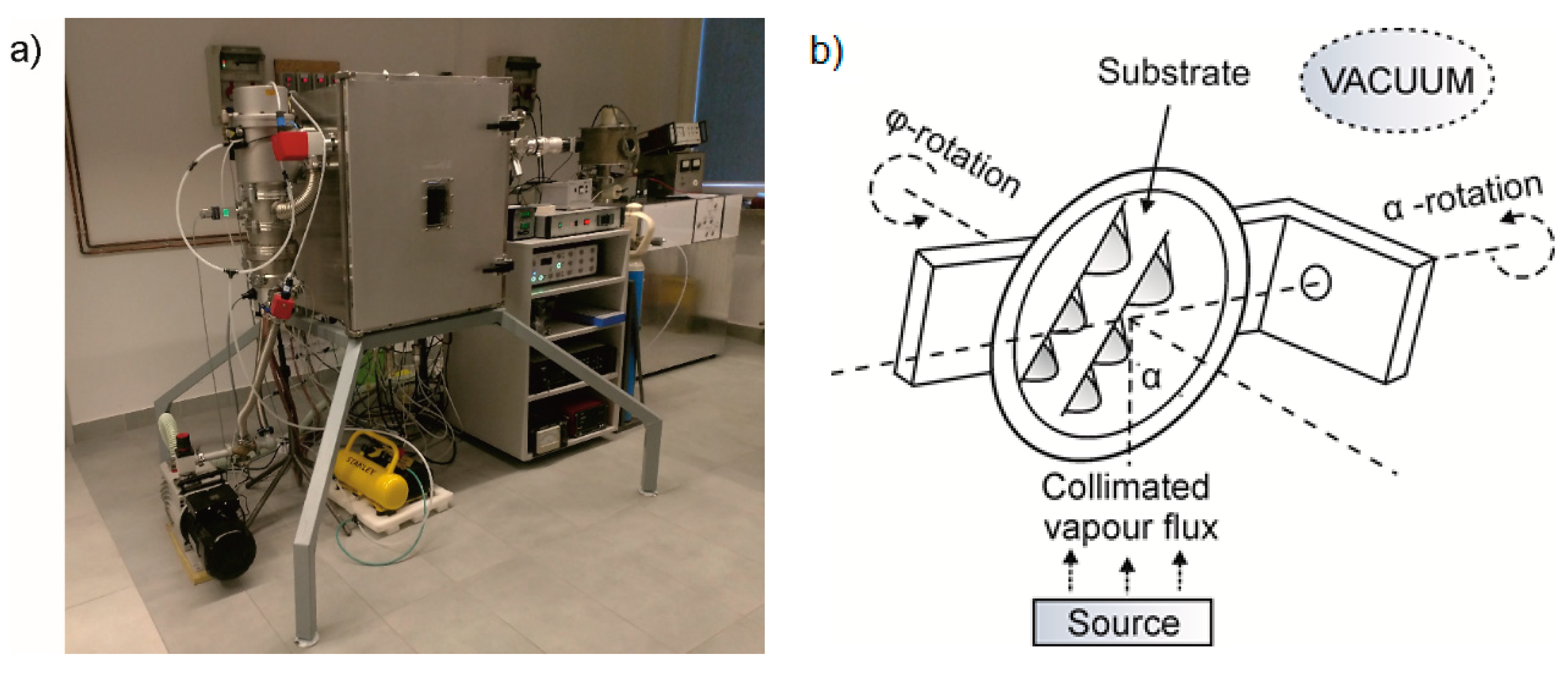
2.1. CuO Deposition
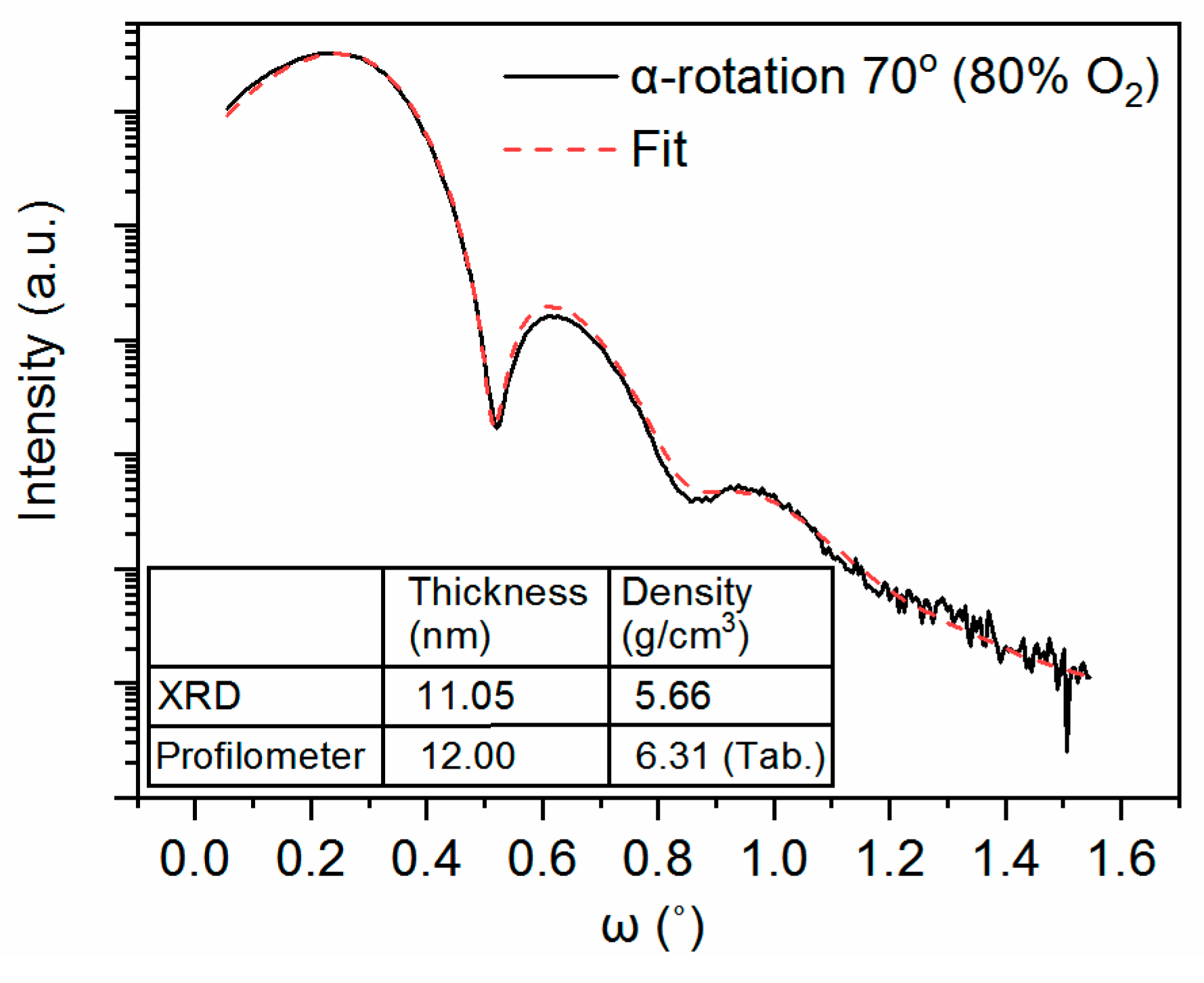
2.2. XRD/XRR Measurements
2.3. SEM/EDX Measurements
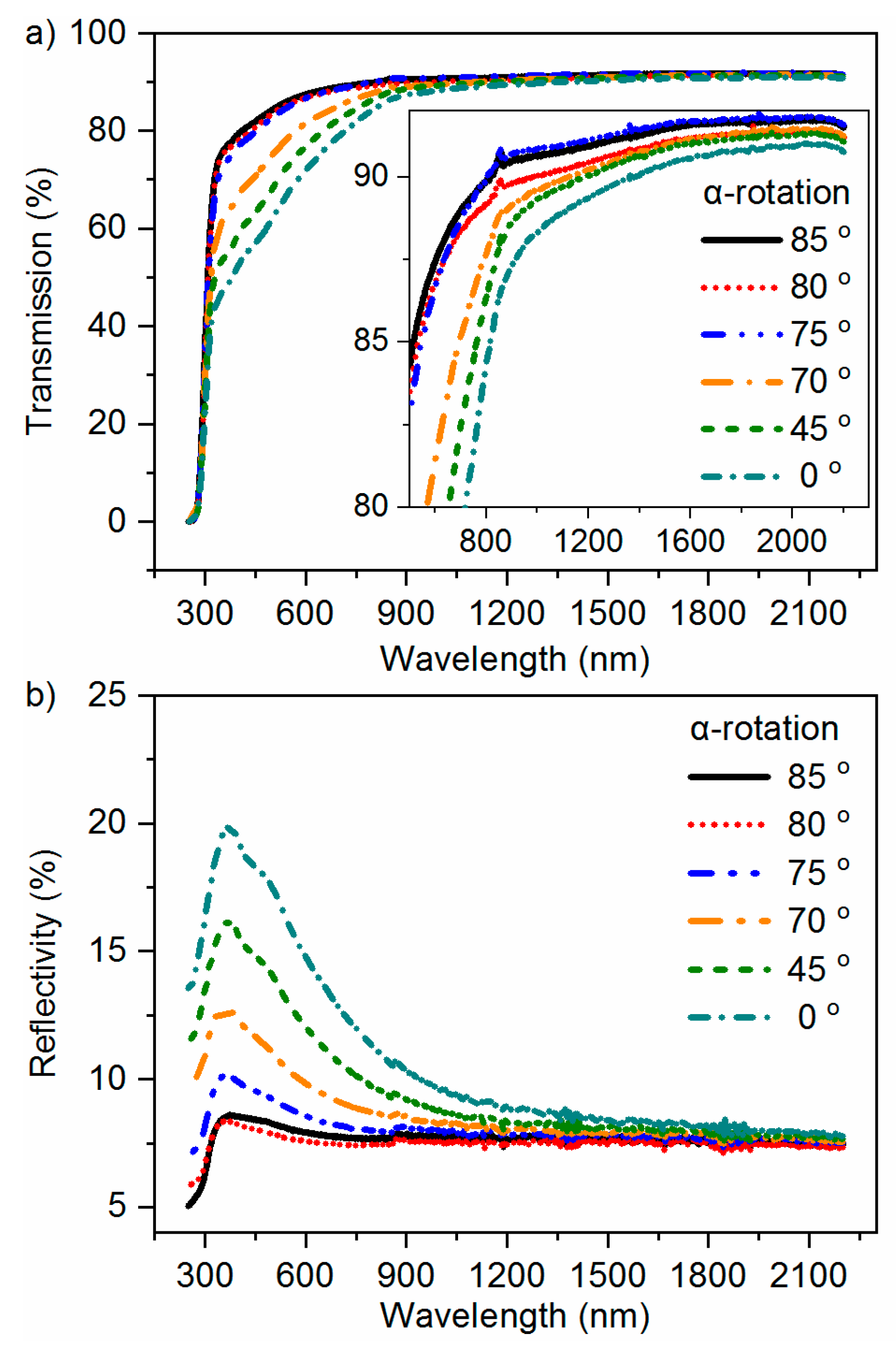
2.4. Optical Measurements
2.5. Thickness Measurements
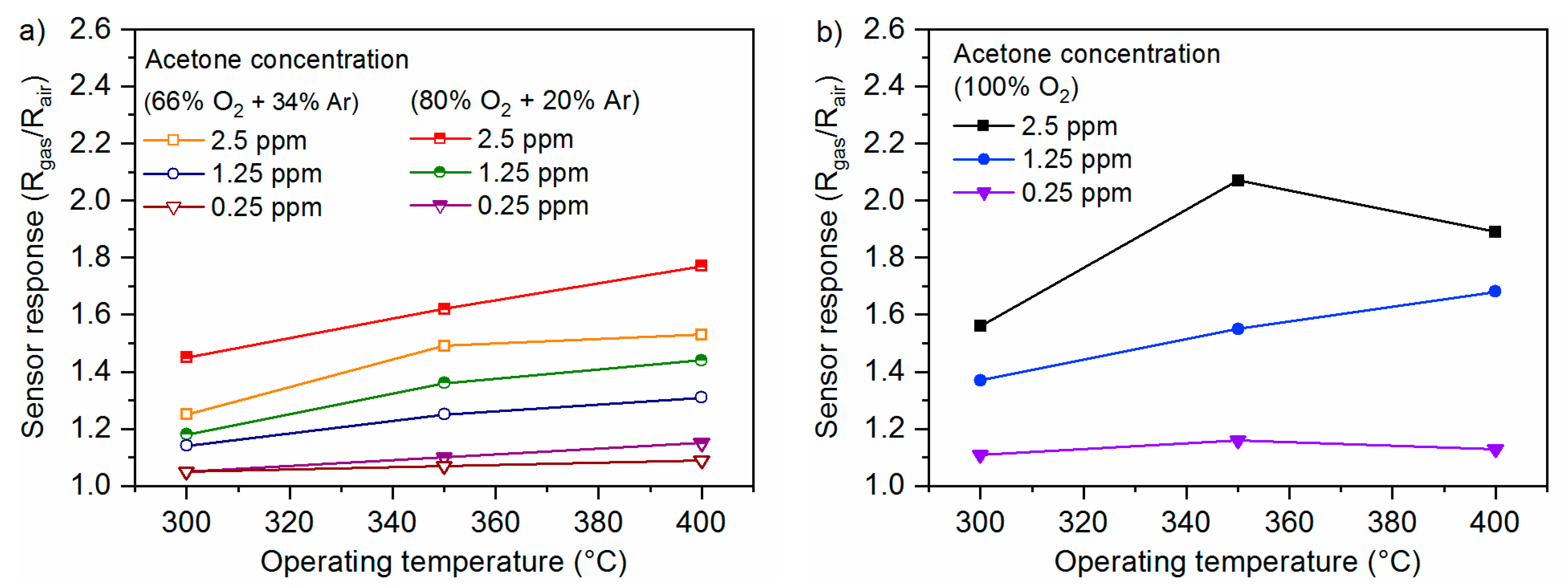
2.6. Gas-Sensing Setup
3. Results and Discussion
3.1. Material Characterization Results
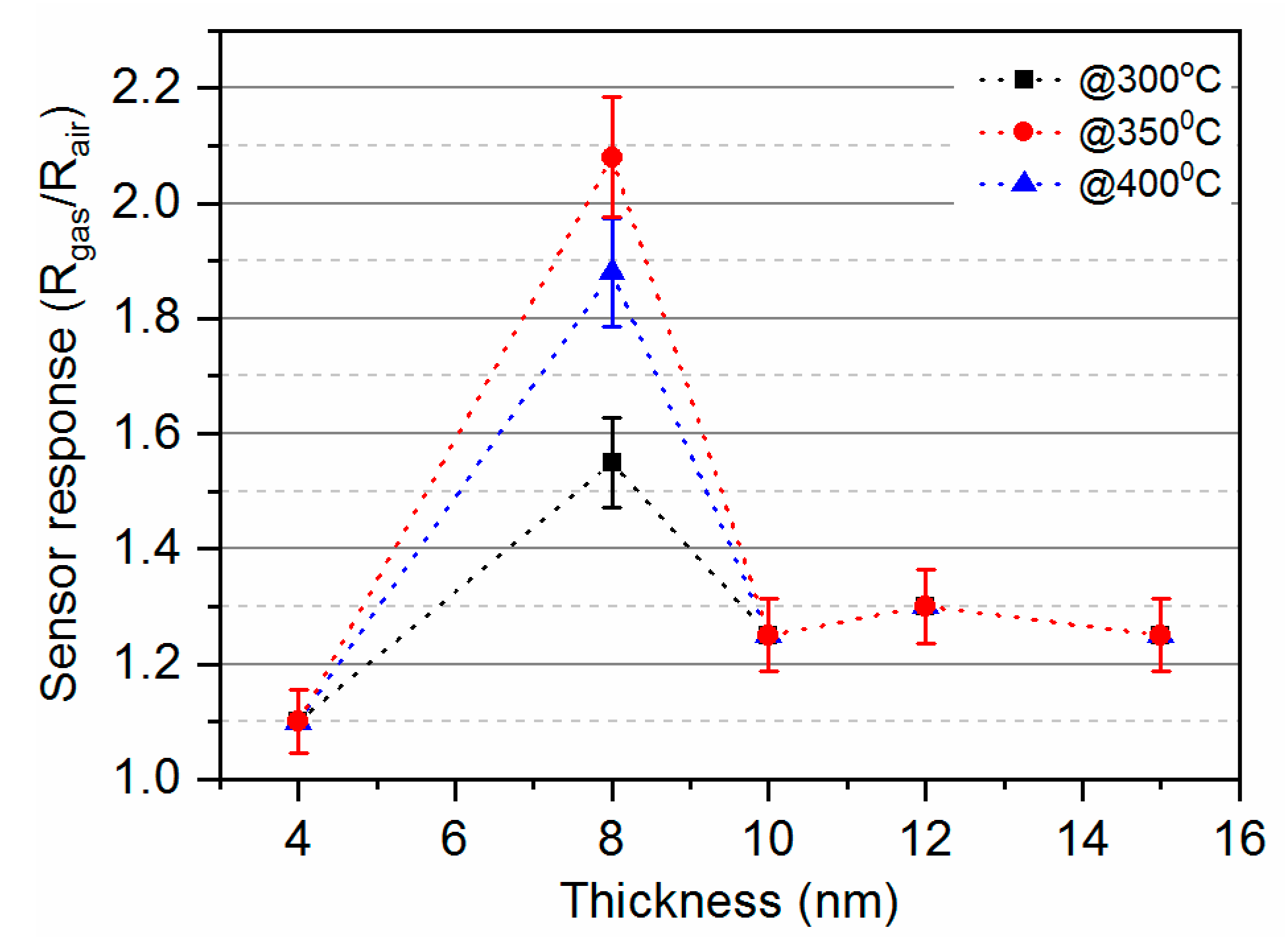
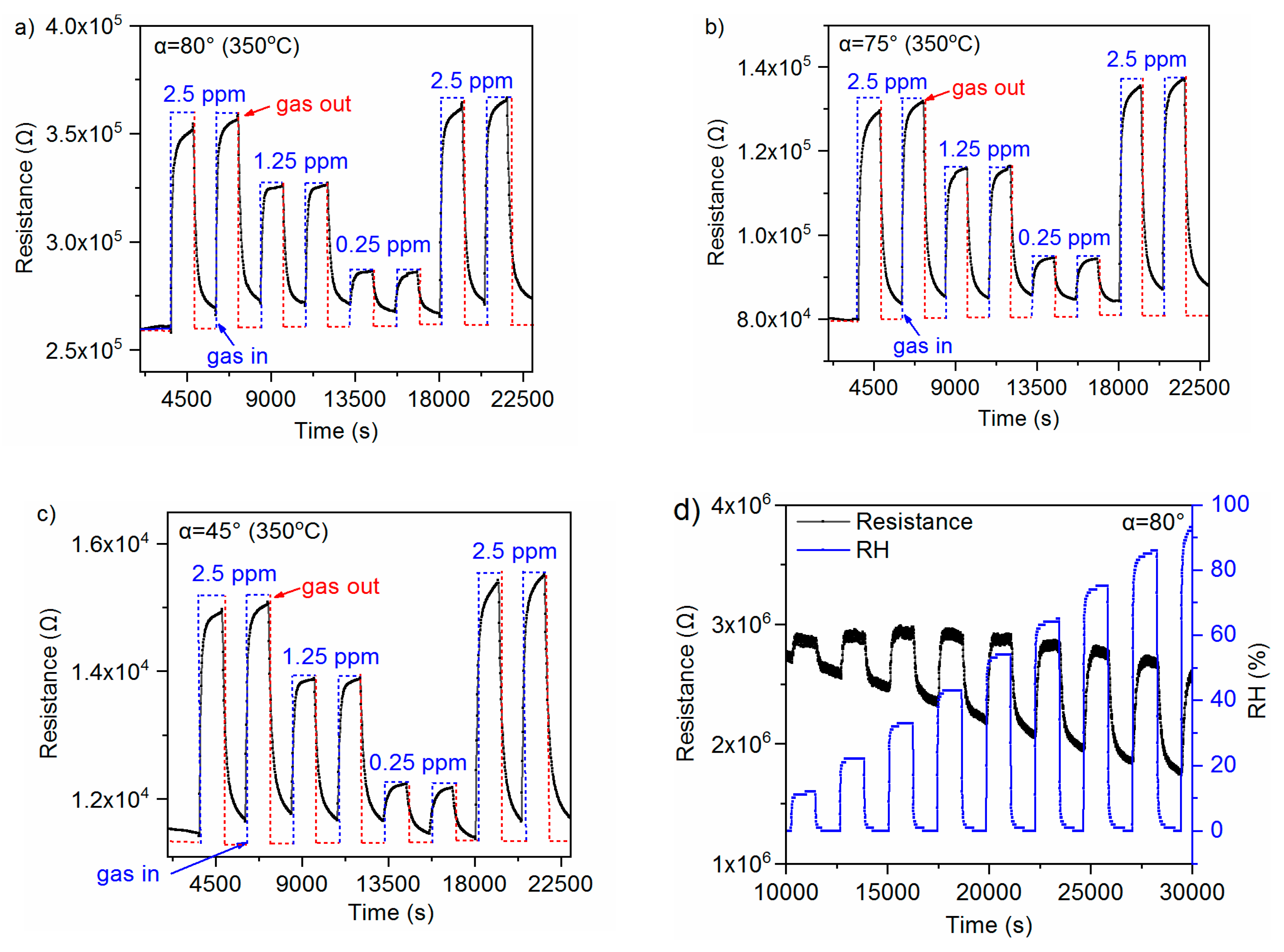
3.2. Gas-Sensing Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Markets and Markets. Gas Sensors Market by Gas Type (Oxygen, Carbon Monoxide, Carbon Dioxide, Ammonia, Chlorine, Hydrogen Sulfide, Nitrogen Oxide, Volatile Organic Compounds, Hydrocarbons), Technology, Application, Geography—Global Forecast 2024; MarketsandMarkets Research Private Ltd.: Pune, India, November, 2019. [Google Scholar]
- Staszek, K.; Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Six-port microwave system for volatile organic compounds detection. Sens. Actuators B Chem. 2017, 245, 882–894. [Google Scholar] [CrossRef]
- Jakubik, W.; Krzywiecki, M.; MacIak, E.; Urbańczyk, M. Bi-layer nanostructures of CuPc and Pd for resistance-type and SAW-type hydrogen gas sensors. Sens. Actuators B Chem. 2012, 175, 255–262. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A.; Szkudlarek, A.; Ziabka, M.; Domanski, K.; Maziarz, W. Performance of Si-doped WO3 thin films for acetone sensing prepared by glancing angle DC magnetron sputtering. IEEE Sens. J. 2015, 16, 1004–1012. [Google Scholar] [CrossRef]
- Hariharan, V.; Gnanavel, B.; Sathiyapriya, R.; Aroulmoji, V. A Review on Tungsten Oxide (WO3) and their Derivatives for Sensor Applications. Int. J. Adv. Sci. Eng. 2019, 5, 1163–1168. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G. Study on TiO2-SnO2 core-shell heterostructure nanofibers with different work function and its application in gas sensor. Sens. Actuators B Chem. 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Li, Z. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrog. Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Tan, W.; Yu, Q.; Ruan, X.; Huang, X. Design of SnO2-based highly sensitive ethanol gas sensor based on quasi molecular-cluster imprinting mechanism. Sens. Actuators B Chem. 2015, 212, 47–54. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Chang, H.; Liu, A.; Xia, B. Characterization of a hybrid composite of SnO2 nanocrystal-decorated reduced graphene oxide for ppm-level ethanol gas sensing application. RSC Adv. 2015, 5, 18666–18672. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Yan, H.; Yang, Z.; Wang, Q. A simple large-scale synthesis of mesoporous In2O3 for gas sensing applications. Appl. Surf. Sci. 2016, 378, 443–450. [Google Scholar] [CrossRef]
- Kalantarian, H.; Alshurafa, N.; Sarrafzadeh, M. Detection of Gestures Associated with Medication Adherence Using Smartwatch-Based Inertial Sensors. IEEE Sens. J. 2016, 16, 1054–1061. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, X.; Yang, A.; Zong, X. Hierarchical assembly of urchin-like alpha-iron oxide hollow microspheres and molybdenum disulphide nanosheets for ethanol gas sensing. J. Colloid Interface Sci. 2018, 523, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chen, J.; Liu, K.; Huang, X. Synthesis of porous α-Fe2O3 microrods via in situ decomposition of FeC2O4 precursor for ultra-fast responding and recovering ethanol gas sensor. Sens. Actuators B Chem. 2016, 230, 46–53. [Google Scholar] [CrossRef]
- Shen, J. Synthesis of Ni-doped A-MoO3 nanolamella and their improved gas sensing properties. Sens. Actuators B Chem. 2017, 252, 757–763. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2019, 6, 46–62. [Google Scholar] [CrossRef]
- Pisarkiewicz, T.; Kenig, T.; Rydosz, A.; Maziarz, W. Solution growth of ZnO sub-micro rods enhanced by electric field. Bull. Polish Acad. Sci. Tech. Sci. 2011, 59, 425–428. [Google Scholar] [CrossRef][Green Version]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Aboott, D. High-Sensitivity Metamaterial-Inspired Sensor. IEEE Sens. J. 2014, 14, 1345–1351. [Google Scholar] [CrossRef]
- Rydosz, A. Amorphous and nanocrystalline magnetron sputtered CuO thin films deposited on low temperature cofired ceramics substrates for gas sensor applications. IEEE Sens. J. 2014, 14, 1600–1607. [Google Scholar] [CrossRef]
- Rydosz, A. The use of copper oxide thin films in gas-sensing applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef]
- Mikami, K.; Kido, Y.; Akaishi, Y.; Quitain, A.; Kida, T. Synthesis of Cu2O/CuO nanocrystals and their application to H 2 S sensing. Sensors 2019, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Schwind, B.; Weinberger, C.; Tiemann, M.; Wagner, T. Gas Responsive Nanoswitch: Copper Oxide Composite for Highly Selective H2S Detection. Adv. Funct. Mater. 2019, 29, 1904505. [Google Scholar] [CrossRef]
- Ayesh, A.I. Production of sensitive gas sensors using CuO/SnO2 nanoparticles. Appl. Phys. A 2019, 125, 550. [Google Scholar] [CrossRef]
- Cerqui, C.; Ponzoni, A.; Zappa, D.; Comini, E.; Sberveglieri, G. Copper oxide nanowires for surface ionization based gas sensor. Procedia Eng. 2014, 87, 1023–1026. [Google Scholar] [CrossRef]
- Krcmar, P. Fully inkjet-printed cuo sensor on flexible polymer substrate for alcohol vapours and humidity sensing at room temperature. Sensors 2019, 19, 3068. [Google Scholar] [CrossRef] [PubMed]
- Sihar, N. Ultraviolet Light-Assisted Copper Oxide Nanowires Hydrogen Gas Sensor. Nanoscale Res. Lett. 2018, 13, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O. Influence of CuO nanostructures morphology on hydrogen gas sensing performances. Microelectron. Eng. 2016, 164, 63–70. [Google Scholar] [CrossRef]
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal oxide thin films prepared by magnetron sputtering technology for volatile organic compound detection in the microwave frequency Range. Materials 2019, 12, 877. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, Q.; Shao, Z. Magnetron sputtering for ZnO: Ga scintillation film production and its application research status in nuclear detection. Crystals 2019, 9, 263. [Google Scholar] [CrossRef]
- Sharmin, A.; Tabassum, S.; Bashar, M.S.; Mahmood, Z.H. Depositions and characterization of sol–gel processed Al-doped ZnO (AZO) as transparent conducting oxide (TCO) for solar cell application. J. Theor. Appl. Phys. 2019, 13, 123–132. [Google Scholar] [CrossRef]
- Au, B.W.C.; Chan, K.Y.; Pang, W.L.; Lee, C.L.; Mustafa, A.H. Tungsten oxide (WO3) films prepared by sol-gel spin-coating technique. Solid State Phenom. 2018, 280, 71–75. [Google Scholar] [CrossRef]
- Djebian, R.; Boudjema, B.; Kabir, A.; Sedrati, C. Physical characterization of CuO thin films obtained by thermal oxidation of vacuum evaporated Cu. Solid State Sci. 2020, 101, 106147. [Google Scholar] [CrossRef]
- Guo, B. Single-crystalline metal oxide nanostructures synthesized by plasma-enhanced thermal oxidation. Nanomaterials 2019, 9, 1405. [Google Scholar] [CrossRef] [PubMed]
- Outokesh, M. Hydrothermal synthesis of CuO nanoparticles: Study on effects of operational conditions on yield, purity, and size of the nanoparticles. Ind. Eng. Chem. Res. 2011, 50, 3540–3554. [Google Scholar] [CrossRef]
- Duoc, V.T. New design of ZnO nanorod- And nanowire-based NO2 room-temperature sensors prepared by hydrothermal method. J. Nanomater. 2019, 2019, 9. [Google Scholar] [CrossRef]
- Ukoba, K.O.; Eloka-Eboka, A.C.; Inambao, F.L. Review of nanostructured NiO thin film deposition using the spray pyrolysis technique. Renew. Sustain. Energy Rev. 2018, 82, 2900–2915. [Google Scholar] [CrossRef]
- Romero, R.; Martin, F.; Ramos-Barrado, J.R.; Leinen, D. Synthesis and characterization of nanostructured nickel oxide thin films prepared with chemical spray pyrolysis. Thin Solid Films 2010, 518, 4499–4502. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Pillai, S.K.; Sinha Ray, S.; Jalama, K.; Krause, R.W.M. Recent trends in the microwave-assisted synthesis of metal oxide nanoparticles supported on carbon nanotubes and their applications. J. Nanomater. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Hawkeye, M.M.; Taschuk, M.T.; Brett, M.J. Glancing Angle Deposition of Thin Films. Engineering the Nanoscale; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Limnonthakul, P. Influence of oxygen flow rate on electrochromic property of WO3 nanorods prepared by glancing reactive magnetron sputtering. Mater. Today Proc. 2017, 4, 6218–6223. [Google Scholar] [CrossRef]
- Ollitrault, J.; Martin, N.; Rauch, J.-Y.; Sanchez, J.-B.; Berger, F. Improvement of ozone detection with GLAD WO3 films. Mater. Lett. 2015, 155, 1–3. [Google Scholar] [CrossRef]
- Song, Y.G.; Shim, Y.; Han, S.D.; Lee, H.R. Metal Oxide Nanocolumns for Extremely Sensitive Gas Sensors. J. Sens. Sci. Technol. 2016, 25, 184–188. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Tomar, M.; Gupta, V. Tunable nanostructured columnar growth of SnO2 for ef fi cient detection of CO gas. Nanotechnology 2018, 29, 065502. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.S.; Han, S.D.; Moon, H.G.; Yoon, S.; Kang, C. Highly Sensitive H2S Sensor Based on the Metal-Catalyzed SnO2 Nanocolumns Fabricated by Glancing Angle Deposition. Sensors 2015, 15, 15468–15477. [Google Scholar] [CrossRef] [PubMed]
- Beckers, N.A.; Taschuk, M.T.; Brett, M.J. Selective room temperature nanostructured thin film alcohol sensor as a virtual sensor array. Sens. Actuators B. Chem. 2013, 176, 1096–1102. [Google Scholar] [CrossRef]
- Han, S.D. Downsizing Gas Sensors based on Semiconducting Metal Oxide: Effects of electrodes on gas sensing properties. Sens. Actuators B. Chem. 2017, 248, 949–956. [Google Scholar]
- Tounsi, N. Structural and optical characterization of copper oxide composite thin films elaborated by GLAD technique. Vacuum 2015, 121, 9–17. [Google Scholar] [CrossRef]
- Chaffar Akkari, F.; Ben Jbara, H.; Abdelkader, D.; Gallas, B.; Kanzari, M. Effect of angle deposition γ on the structural, optical and electrical properties of copper oxide zigzag (+γ, −γ) nanostructures elaborated by glancing angle deposition. Thin Solid Films 2018, 657, 61–69. [Google Scholar] [CrossRef]
- Sorocki, J.; Rydosz, A. A prototype of a portable gas analyzer for exhaled acetone detection. Appl. Sci. 2019, 13, 2605. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 7, 2298. [Google Scholar] [CrossRef]
- Rydosz, A. Nanosensors for exhaled breath monitoring as a possible tool for noninvasive diabetes detection. In Nanosensors for Smart Cities; Elsevier: Amsterdam, The Netherlands, 2020; pp. 467–481. [Google Scholar]
- Staszek, K.; Szkudlarek, A.; Kawa, M.; Rydosz, A. Microwave system with sensor utilizing GO-based gas-sensitive layer and its application to acetone detection. Sens. Actuators B 2019, 297, 126699. [Google Scholar] [CrossRef]
- Amann, A.; Smith, D. Volatile Biomarkers. In Non-Invasive Diagnosis in Physiology and Medicine; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Andrysiewicz, W.; Krzeminski, J.; Marszalek, K.; Rydosz, A. Flexible Gas Sensor Printed on a Polymer Substrate for Sub-ppm Acetone Detection. Electron. Mater. Lett. 2020, 16, 146–155. [Google Scholar] [CrossRef]
- Weinstein, A.M.; D’Antonio, C.; Mukherjee, K. Density of Copper Thin Films measured by X-ray Absorption. Nature 1968, 220, 777–778. [Google Scholar] [CrossRef]
- Sahdan, M.Z. Fabrication and Characterization of Crystalline Cupric Oxide (CuO) Films by Simple Immersion Method. Procedia Manuf. 2015, 2, 379–384. [Google Scholar] [CrossRef]
- Shehayeb, S. Thin polymeric CuO film from EPD designed for low temperature photothermal absorbers. Electrochim. Acta 2019, 305, 295–303. [Google Scholar] [CrossRef]
- Chaugan, D.; Satsangi, V.R.; Dass, S. Preparation and characterization of nanostructured CuO thin films for photoelectrochemical splitting of water. Bull. Mater. Sci. 2006, 29, 709–716. [Google Scholar]
- Dahrul, M.; Alatas, H. Preparation and Optical Properties Study of CuO thin Film as Applied Solar Cell on LAPAN-IPB Satellite. Procedia Environ. Sci. 2016, 33, 661–667. [Google Scholar] [CrossRef]
- Rydosz, A.; Pisarkiewicz, T.; Maziarz, W. Formation a uniform temperature distribution in semiconductors resistance gas sensors in LTCC technology. Prz. Elektrotechniczny 2011, 4, 249–252. [Google Scholar]
- Bartsch, H.; Mueller, J.; Stöpel, D.; Rydosz, A. Printed heater elements for smart sensor packages in LTCC. In Proceedings of the Conference: EMPC 2017—21th European Microelectronics and Packaging Conference, Warsaw, Poland, 10–13 September 2017. [Google Scholar]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef] [PubMed]




| Deposition Conditions | Thickness [nm] | ||
|---|---|---|---|
| α [°] | O2/Ar [%] | Power [W] | |
| 85 | 100/0 | 50 | 4 |
| 80 | 100/0 | 8 | |
| 75 | 80/20 | 10 | |
| 70 | 80/20 | 12 | |
| 45 | 66/34 | 12 | |
| 0 | 66/34 | 15 | |
| Characterization Method Name | Instrumentation | Measurements Parameters |
|---|---|---|
| X-ray diffractometry (XRD) | X’Pert PRO MPD PANalytical system with a grazing angle of 3° using monochromatic Cu Kα1 radiation | structure |
| X-ray reflectometry (XRR) | structure thickness density | |
| Scanning electron microscope (SEM) with an attachment for the detection of characteristic X-ray radiation (EDS, EDAX Genesis) | Nova NANOSEM 200, FEI, USA | structure chemical composition |
| Optical measurements | Perkin-Elmer double-beam spectrophotometer Lambda 19 wavelength range from 300 to 2200 nm with a step of 1 nm | transmission coefficient reflective coefficient |
| Mechanical profilometry | TalyStep profilometer, Taylor Hobson, Leicester, UK | thickness |
| Gas-sensing system | Electrometer (34401A HP) | electrical resistance changes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydosz, A.; Dyndał, K.; Andrysiewicz, W.; Grochala, D.; Marszałek, K. GLAD Magnetron Sputtered Ultra-Thin Copper Oxide Films for Gas-Sensing Application. Coatings 2020, 10, 378. https://doi.org/10.3390/coatings10040378
Rydosz A, Dyndał K, Andrysiewicz W, Grochala D, Marszałek K. GLAD Magnetron Sputtered Ultra-Thin Copper Oxide Films for Gas-Sensing Application. Coatings. 2020; 10(4):378. https://doi.org/10.3390/coatings10040378
Chicago/Turabian StyleRydosz, Artur, Katarzyna Dyndał, Wojciech Andrysiewicz, Dominik Grochala, and Konstanty Marszałek. 2020. "GLAD Magnetron Sputtered Ultra-Thin Copper Oxide Films for Gas-Sensing Application" Coatings 10, no. 4: 378. https://doi.org/10.3390/coatings10040378
APA StyleRydosz, A., Dyndał, K., Andrysiewicz, W., Grochala, D., & Marszałek, K. (2020). GLAD Magnetron Sputtered Ultra-Thin Copper Oxide Films for Gas-Sensing Application. Coatings, 10(4), 378. https://doi.org/10.3390/coatings10040378







