Surface Modification of Bacterial Cellulose by Copper and Zinc Oxide Sputter Coating for UV-Resistance/Antistatic/Antibacterial Characteristics
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
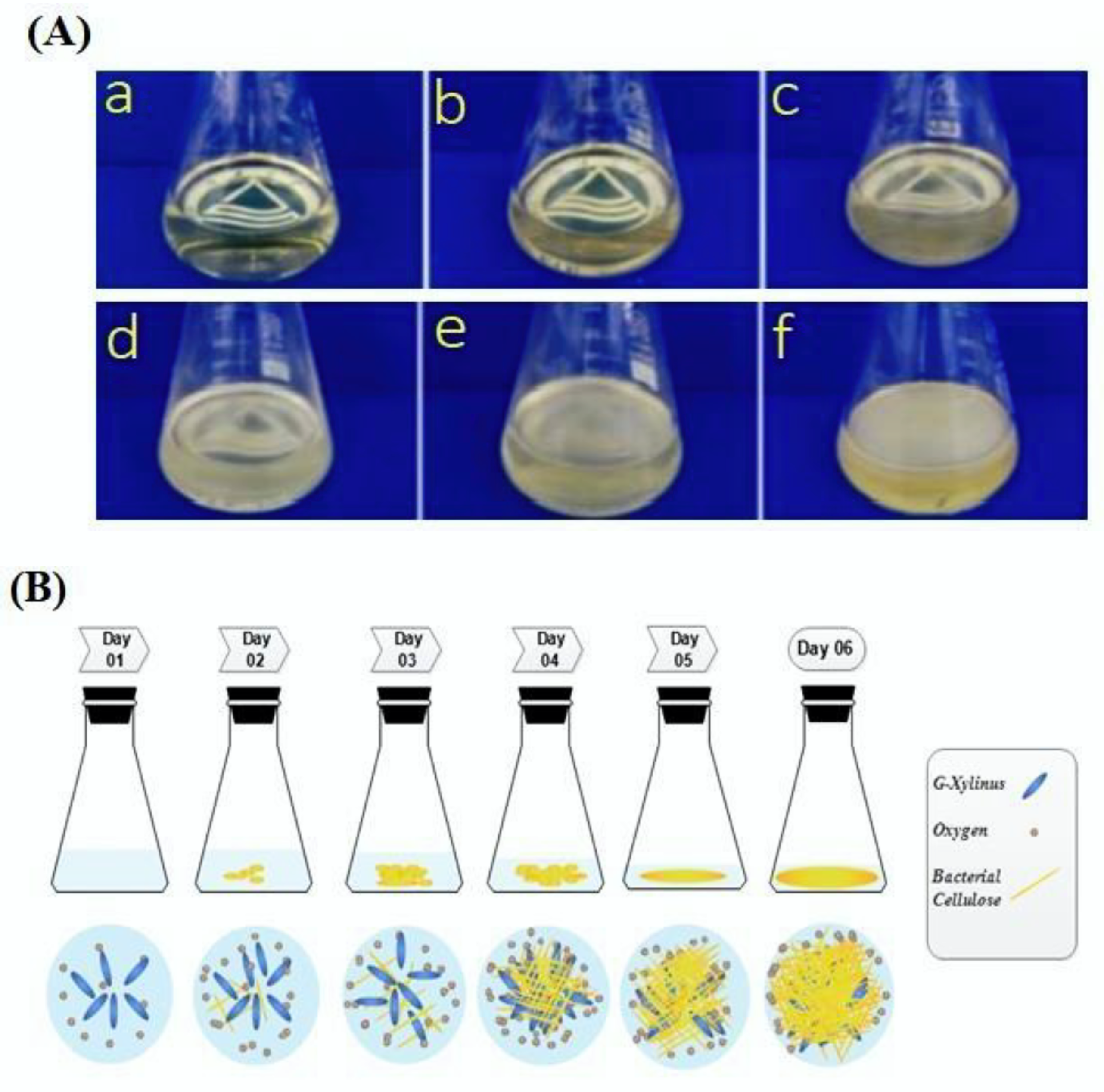
2.2. Synthesis of Bacterial Cellulose
2.3. Synthesis of Copper and Zinc Oxide Nanocomposite Films
2.4. Characterization
3. Results and Discussion
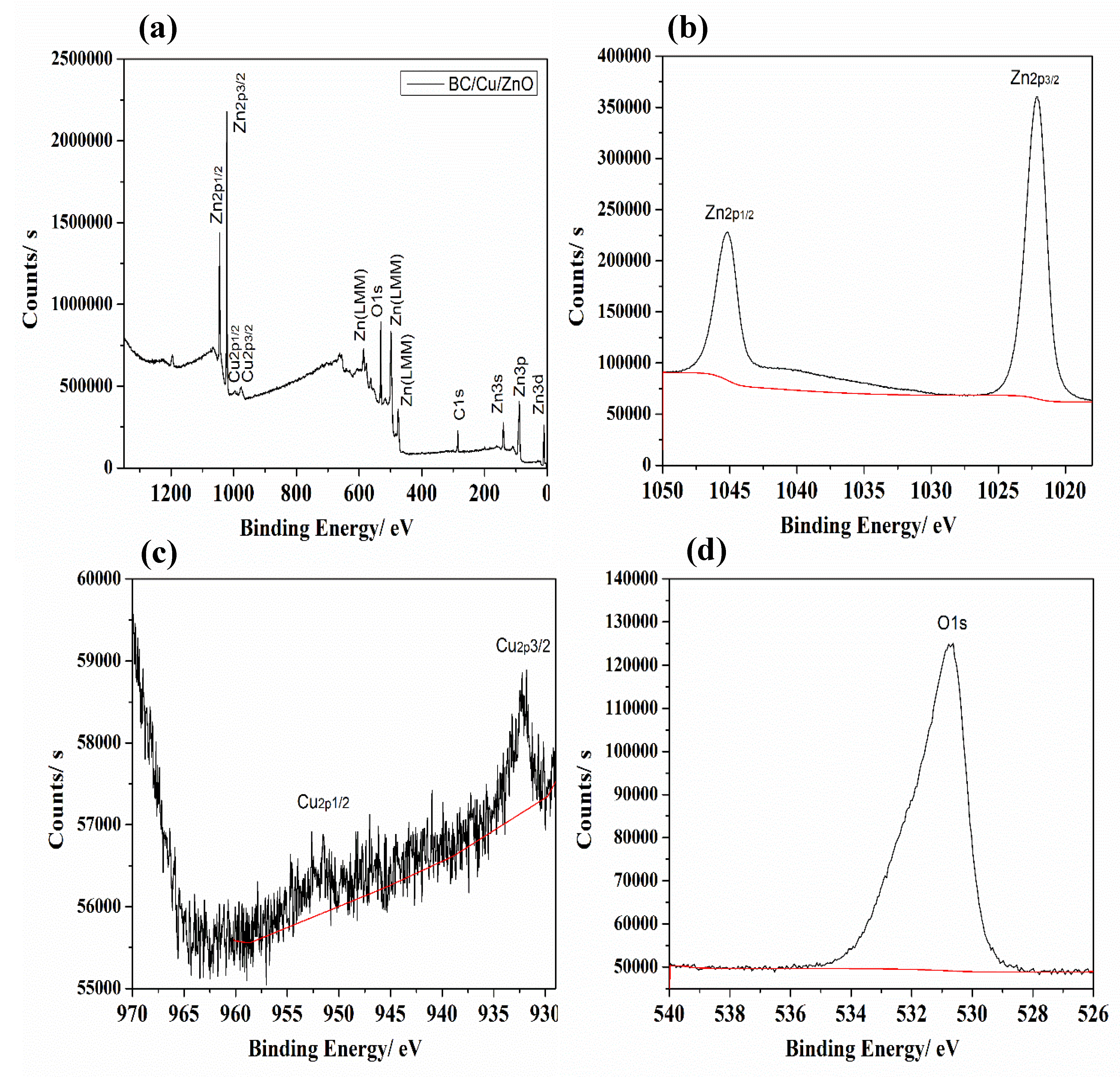
3.1. XPS Analysis
3.2. EDS Analysis
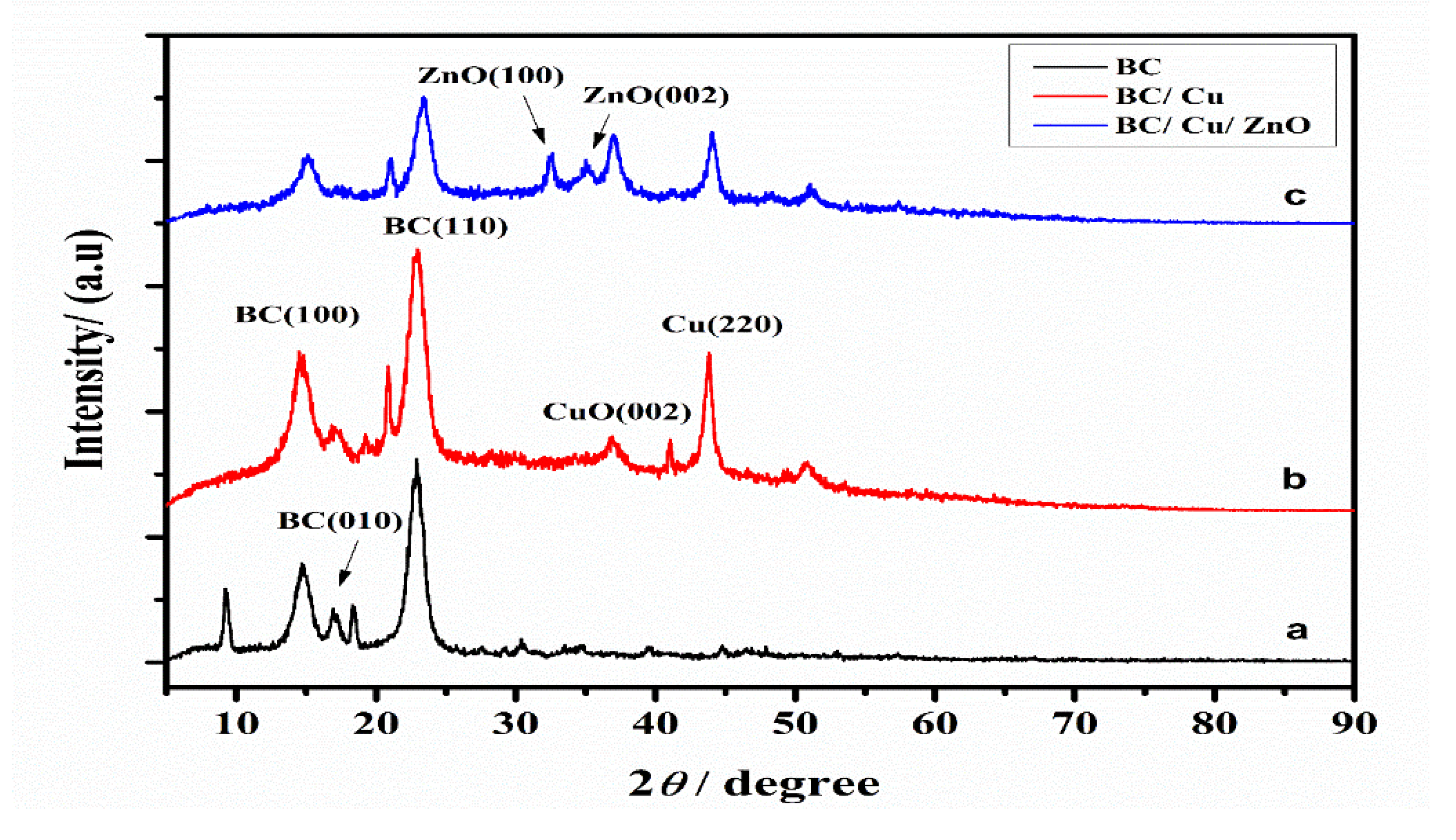
3.3. XRD Analysis
3.4. Antistatic Analysis
3.5. Ultraviolet Resistance Analysis
3.6. Antibacterial Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Chen, Q.; Ge, L.; Yin, L.; Fan, B.; Wang, H.; Lu, H.; Xu, H.; Zhang, R.; Shao, G. Synthesis and Ag-loading-density-dependent photocatalytic activity of Ag@TiO2 hybrid nanocrystals. Appl. Surf. Sci. 2013, 284, 921–929. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Mohapatra, S.; Singhal, R.; Avasthi, D.; Agarwal, D.C.; Ogale, S. Au–ZnO: A tunable localized surface plasmonic nanocomposite. Appl. Phys. Lett. 2008, 92, 043107. [Google Scholar] [CrossRef]
- Barakat, A.N.; Al-Deyab, S.; Kim, H.Y. Synthesis and study of the photoluminescence and optical characteristics of Cd/CdO nanorods prepared by the electrospinning process. Mater. Lett. 2012, 66, 225–228. [Google Scholar] [CrossRef]
- Geetha, D.; Kavitha, S.; Ramesh, P. A novel bio-degradable polymer stabilized Ag/TiO2 nanocomposites and their catalytic activity on reduction of methylene blue under natural sun light. Ecotoxicol. Environ. Saf. 2015, 121, 126–134. [Google Scholar] [CrossRef]
- Lee, D.-S.; Chen, Y.-W. Nano Ag/TiO2 catalyst prepared by chemical deposition and its photocatalytic activity. J. Taiwan Inst. Chem. Eng. 2014, 45, 705–712. [Google Scholar] [CrossRef]
- Bazant, P.; Kuritka, I.; Munster, L.; Kalina, L. Microwave solvothermal decoration of the cellulose surface by nanostructured hybrid Ag/ZnO particles: A joint XPS, XRD and SEM study. Cellulose 2015, 22, 1275–1293. [Google Scholar] [CrossRef]
- Sun, F.; Qiao, X.; Tan, F.; Wang, W.; Qiu, X. One-step microwave synthesis of Ag/ZnO nanocomposites with enhanced photocatalytic performance. J. Mater. Sci. 2012, 47, 7262–7268. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Cheng, J.; Cheng, J.; Cheng, S. l-Arginine assisted preparation of Ag/ZnO nanocomposites with enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2015, 26, 2775–2781. [Google Scholar] [CrossRef]
- Gunasekar, V.; Ponnusami, V. Plasmonic photocatalysis and kinetics of reactive dye degradation in aqueous solution using enzymatically synthesized Ag/ZnO. J. Sol-Gel Sci. Technol. 2015, 74, 84–93. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Cady, C.N.; Behnke, J.L.; Strickland, A.D. Copper-Based Nanostructured Coatings on Natural Cellulose: Nanocomposites Exhibiting Rapid and Efficient Inhibition of a Multi-Drug Resistant Wound Pathogen, A. baumannii, and Mammalian Cell Biocompatibility In Vitro. Adv. Funct. Mater. 2011, 21, 2506–2514. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Payne, G.F.; Sun, S.; Wang, X. A highly conductive, pliable and foldable Cu/cellulose paper electrode enabled by controlled deposition of copper nanoparticles. Nanoscale 2019, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kishanji, M.; Mamatha, G.; Reddy, K.O.; Rajulu, A.V.; Madhukar, K. In situ generation of silver nanoparticles in cellulose matrix using Azadirachta indica leaf extract as a reducing agent. Int. J. Polym. Anal. Charact. 2017, 22, 734–740. [Google Scholar] [CrossRef]
- Hannon, J.C.; Kerry, J.P.; Cruz-Romero, M.; Azlin-Hasim, S.; Morris, M.A.; Cummins, E. Assessment of the migration potential of nanosilver from nanoparticle-coated low-density polyethylene food packaging into food simulants. Food Addit. Contam. Part A 2016, 33, 167–178. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Rajalu, A.V.; Kaliaraj, G.S.; Siengchin, S.; Parameswaranpillai, J.; Saraswathi, R. Preparation of cellulose/copper nanoparticles bionanocomposite films using a bioflocculant polymer as reducing agent for antibacterial and anticorrosion applications. Compos. Part B Eng. 2019, 175, 107177. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Foresti, L.M.; Vázquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef]
- Booshehri, A.Y.; Wang, R.; Xu, R. Simple method of deposition of CuO nanoparticles on a cellulose paper and its antibacterial activity. Chem. Eng. J. 2015, 262, 999–1008. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High Photocatalytic Activity of ZnO−Carbon Nanofiber Heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef]
- Wu, J.; Xue, D. Progress of science and technology of ZnO as advanced material. Sci. Adv. Mater. 2011, 3, 127–149. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Pandimurugan, R.; Thambidurai, S. UV protection and antibacterial properties of seaweed capped ZnO nanoparticles coated cotton fabrics. Int. J. Biol. Macromol. 2017, 105, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, D. UV protection textile materials. AUTEX Res. J. 2007, 7, 53–62. [Google Scholar]
- Ranjan Das, B. UV radiation protective clothing. Open Text. J. 2010, 3, 14–21. [Google Scholar]
- Parejo, P.G.; Zayat, M.; Levy, D. Highly efficient UV-absorbing thin-film coatings for protection of organic materials against photodegradation. J. Mater. Chem. 2006, 16, 2165–2169. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Alamdari, S.; Han, W.; Park, H.-H. Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomed. 2017, 12, 207. [Google Scholar] [CrossRef]
- Calvo, M.E.; Smirnov, J.R.C.; Míguez, H. Novel approaches to flexible visible transparent hybrid films for ultraviolet protection. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 945–956. [Google Scholar] [CrossRef]
- Rusdi, R.; Rahman, A.A.; Mohamed, N.S.; Kamarudin, N.; Kamarulzaman, N. Preparation and band gap energies of ZnO nanotubes, nanorods and spherical nanostructures. Powder Technol. 2011, 210, 18–22. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Ruby, D.S.; Peters, D.W.; McKenzie, B.B.; Hsu, J.W.P. ZnO Nanostructures as Efficient Antireflection Layers in Solar Cells. Nano Lett. 2008, 8, 1501–1505. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Wang, X.; Ali, N.; El-Mohamedy, R. Nanoparticle Coatings for UV Protective Textiles. Res. J. Text. Appar. 2010, 14, 9–20. [Google Scholar] [CrossRef]
- Pospíšil, J.; Nešpůrek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Çakır, B.A.; Budama, L.; Topel, Ö.; Hoda, N. Synthesis of ZnO nanoparticles using PS-b-PAA reverse micelle cores for UV protective, self-cleaning and antibacterial textile applications. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 132–139. [Google Scholar] [CrossRef]
- Han, Y.B.; Han, J.B.; Ding, S.; Chen, D.J.; Wang, Q.Q. Optical nonlinearity of ZnO microcrystallite enhanced by interfacial state. Opt. Express 2005, 13, 9211–9216. [Google Scholar] [CrossRef]
- Niu, Q.-X.; Liu, Y.-J.; Song, D.-J.; Gao, Y.-J.; Dai, C.-L.; Yang, H.-W. Research of anti-ultraviolet nano-film structure based on the FDTD method. Optik 2016, 127, 539–543. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Jia, G.; Lei, H. Optical Prosperities of Ag-ZnO Composition Nanofilm Synthesized by Chemical Bath Deposition. Acta Phys. Pol. A 2011, 119, 451–454. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Top. Curr. Chem. 2020, 378, 7. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.; Zhou, Z.; Tian, K. Preparation of Ag nanoparticles coated tetrapod-like ZnO whisker photocatalysts using photoreduction. Mater. Sci. Eng. B 2011, 176, 978–983. [Google Scholar] [CrossRef]
- Lu, W.; Gao, S.; Wang, J. One-Pot Synthesis of Ag/ZnO Self-Assembled 3D Hollow Microspheres with Enhanced Photocatalytic Performance. J. Phys. Chem. C 2008, 112, 16792–16800. [Google Scholar] [CrossRef]
- Kim, K.; Lee, D.-H.; Lee, S.Y.; Jang, G.-E.; Kim, J.-S. Effect of Ag/Al co-doping method on optically p-type ZnO nanowires synthesized by hot-walled pulsed laser deposition. Nanoscale Res. Lett. 2012, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, Q.; Cai, Y.; Gao, W.; Bo, C. Characterization of polymer nanofibers coated by reactive sputtering of zinc. J. Mater. Process. Technol. 2009, 209, 2028–2032. [Google Scholar] [CrossRef]
- Waykar, R.G.; Pawbake, A.; Kulkarni, R.R.; Jadhavar, A.A.; Funde, A.; Waman, V.S.; Pathan, H.M.; Jadkar, S. Influence of RF power on structural, morphology, electrical, composition and optical properties of Al-doped ZnO films deposited by RF magnetron sputtering. J. Mater. Sci. Mater. Electron. 2015, 27, 1134–1143. [Google Scholar] [CrossRef]
- Ramazanov, S.M.; Kurbanov, M.K.; Safaraliev, G.K.; Bilalov, B.A.; Kargin, N.I.; Gusev, A.S. Structural properties of the epitaxial (SiC) 1− x (AlN) x solid solution films fabricated by magnetron sputtering of SiC-Al composite targets. Tech. Phys. Lett. 2014, 40, 300–302. [Google Scholar] [CrossRef]
- Li, H.T.; Wang, T.; Wang, Q. Composition and residual stress analysis of Ti-Si/(Ti-Si)xN film on Mg–Al alloy substrate prepared by magnetron sputtering. Mater. Res. Innov. 2015, 19, 1268–1271. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, B.; Hu, W.; Zhang, W.; Yin, N.; Wang, H. Polyol mediated synthesis of ZnO nanoparticles templated by bacterial cellulose. Carbohydr. Polym. 2013, 92, 1953–1959. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, S.; Wang, H.; Wang, B.; Jiang, J. Biosynthesis of bacterial cellulose/multi-walled carbon nanotubes in agitated culture. Carbohydr. Polym. 2008, 74, 659–665. [Google Scholar] [CrossRef]
- Wen, L.; Ma, Y.; Dai, B.; Zhou, Y.; Liu, J.; Pei, C. Preparation and dielectric properties of SiC nanowires self-sacrificially templated by carbonated bacterial cellulose. Mater. Res. Bull. 2013, 48, 687–690. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Chen, G.-Y.; Wang, Y.-B.; Yao, J. A facile one-pot route for preparing cellulose nanocrystal/zinc oxide nanohybrids with high antibacterial and photocatalytic activity. Cellulose 2014, 22, 261–273. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef]
- Lv, P.; Wei, A.; Li, D.; Lucia, L.; Wei, Q.; Wang, Y.; Zhang, J. Copper nanoparticles-sputtered bacterial cellulose nanocomposites displaying enhanced electromagnetic shielding, thermal, conduction, and mechanical properties. Cellulose 2016, 23, 3117–3127. [Google Scholar] [CrossRef]
- Long, C.; Qi, D.; Wei, T.; Yan, J.; Jiang, L.; Fan, Z. Nitrogen-Doped Carbon Networks for High Energy Density Supercapacitors Derived from Polyaniline Coated Bacterial Cellulose. Adv. Funct. Mater. 2014, 24, 3953–3961. [Google Scholar] [CrossRef]
- Zhou, T. Electrically conductive bacterial cellulose composite membranes produced by the incorporation of graphite nanoplatelets in pristine bacterial cellulose membranes. Express Polym. Lett. 2013, 7, 756–766. [Google Scholar] [CrossRef]
- Czaja, W.; Romanovicz, D.; Brown, R.M. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Mohamed, A.; El-Naggar, M.E.; Shaheen, T.I.; Hassabo, A. Laminating of chemically modified silan based nanosols for advanced functionalization of cotton textiles. Int. J. Boil. Macromol. 2017, 95, 429–437. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Yang, Y.; Zhang, Y.; Song, W.; Yang, X.; Chen, J.; Chen, Q.; Ji, X. Antimony nanoparticles anchored on interconnected carbon nanofibers networks as advanced anode material for sodium-ion batteries. J. Power Sour. 2015, 284, 227–235. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, W.; Huang, F.; Chen, N.; Wei, Q. Polyester fabric coated with Ag/ZnO composite film by magnetron sputtering. Appl. Surf. Sci. 2016, 390, 863–869. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Jiang, M.; Zhu, X. Oxidation of copper during physical sputtering deposition: Mechanism, avoidance and utilization. arXiv 2014, arXiv:1412.2031. [Google Scholar]
- Kim, D.-W.; Rhee, K.-Y.; Park, S.-J. Synthesis of activated carbon nanotube/copper oxide composites and their electrochemical performance. J. Alloys Compd. 2012, 530, 6–10. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Song, X.-P.; Liang, S.; Wang, L.; Yang, Z. Bottom-up assembly of hierarchical Cu2O nanospheres: Controllable synthesis, formation mechanism and enhanced photochemical activities. CrystEngComm 2012, 14, 3545. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.S.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Hassabo, A.; El-Naggar, M.E.; Mohamed, A.L.; Hebeish, A. Development of multifunctional modified cotton fabric with tri-component nanoparticles of silver, copper and zinc oxide. Carbohydr. Polym. 2019, 210, 144–156. [Google Scholar] [CrossRef]
- Guo, S.-P.; Li, C.-X.; Chi, Y.; Ma, Z.; Xue, H.-G. Novel 3-D network SeS/NCPAN composites prepared by one-pot in-situ solid-state method and its electrochemical performance as cathode material for lithium-ion battery. J. Alloys Compd. 2016, 664, 92–98. [Google Scholar] [CrossRef]
- Chow, L.; Lupan, O.; Chai, G.; Khallaf, H.; Ono, L.; Cuenya, B.R.; Tiginyanu, I.; Ursaki, V.; Sontea, V.; Schulte, A. Synthesis and characterization of Cu-doped ZnO one-dimensional structures for miniaturized sensor applications with faster response. Sens. Actuators A Phys. 2013, 189, 399–408. [Google Scholar] [CrossRef]
- Xiang, Z.; Gao, W.; Chen, L.; Lan, W.; Zhu, J.; Runge, T.M. A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose 2015, 23, 493–503. [Google Scholar] [CrossRef]
- Chen, M.; Kang, H.; Gong, Y.; Guo, J.; Zhang, H.; Liu, R. Bacterial Cellulose Supported Gold Nanoparticles with Excellent Catalytic Properties. ACS Appl. Mater. Interfaces 2015, 7, 21717–21726. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hang, T.; Hu, A.; Chen, Z.; Lu, Q.; Li, M. Solid state diffusion between Sn and Cu microcones on Cu microcones. J. Alloys Compd. 2014, 582, 408–413. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumar, N.; Khare, S. Enhancement in UV emission and band gap by Fe doping in ZnO thin films. Opto-Electr. Rev. 2014, 22, 68–76. [Google Scholar] [CrossRef]


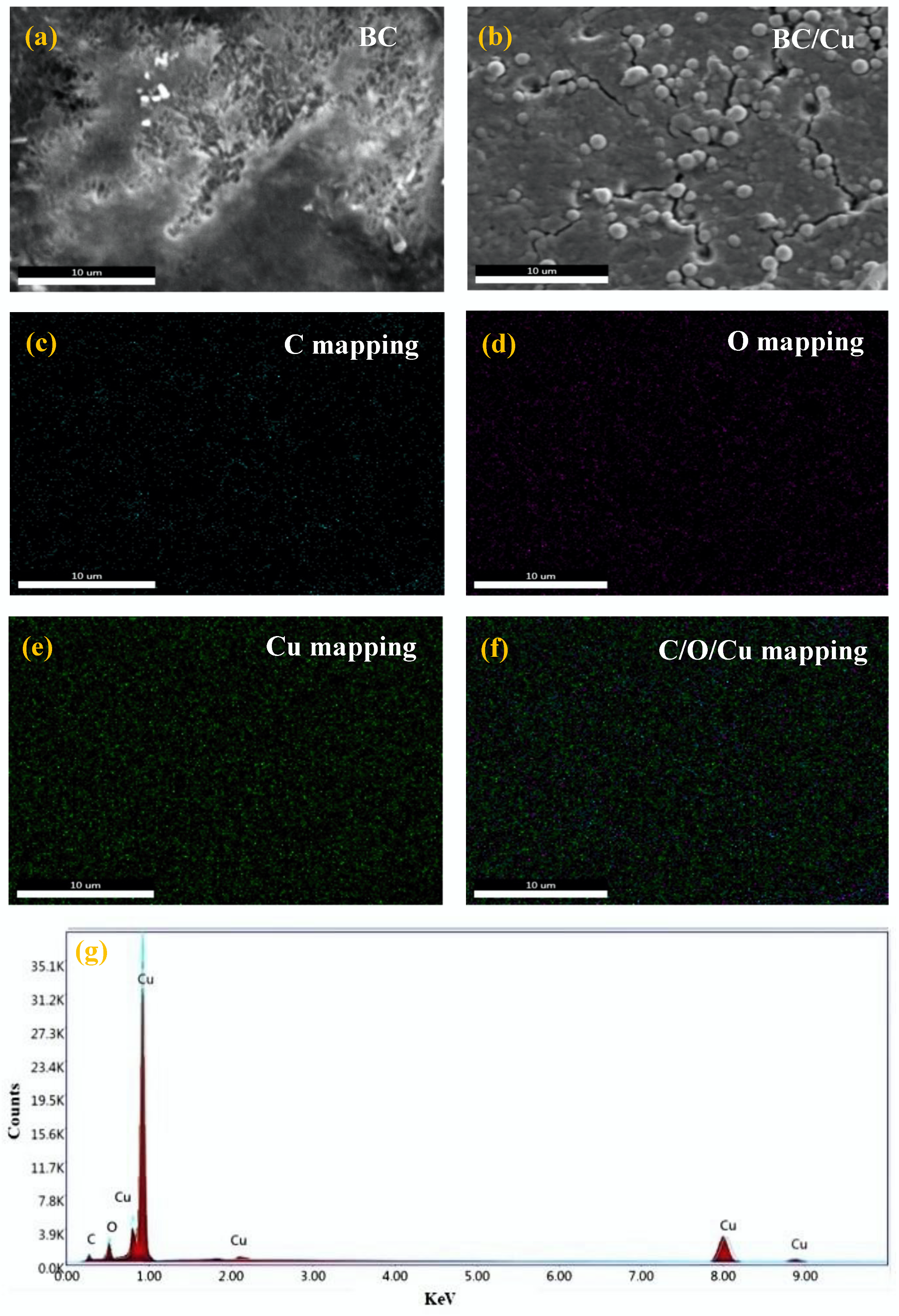

| Samples | Atomic Content % of Elements | |||
|---|---|---|---|---|
| Carbon | Oxygen | Copper | Zinc | |
| Bacterial Cellulose/Copper | 17.64 | 14.08 | 68.28 | |
| Bacterial Cellulose/Copper/Zinc Oxide | 16.44 | 38.87 | 1.22 | 43.47 |
| Nanocomposite Materials | Static Half Period/s | Instantaneous Electrostatic Voltage/V | Reference |
|---|---|---|---|
| Polyester fabric/Ag | 0.51 ± 0.23 | 188 ± 9.83 | [56] |
| Polyester fabric/Ag2O | 4.658 ± 0.39 | 380 ± 8.94 | |
| Polyester fabric/Ag/ZnO | 70.34 ± 10.42 | 565 ± 5.48 | |
| Polyester fabric/Ag2O/ZnO | 25.83 ± 3.58 | 315 ± 5.48 | |
| Bacterial Cellulose/Copper | 3.386 ± 0.56 | 259.66 ± 5.50 | This work |
| Bacterial Cellulose/Copper/Zinc Oxide | 51.50 ± 4.10 | 349.3 ± 6.02 |
| Nanocomposite Materials | T(UVA)/% | T(UVB)/% | UPF | Reference |
|---|---|---|---|---|
| Polyester fabric/Ag | 3.52 ± 0.02 | 2.69 ± 0.05 | 35.26 ± 0.61 | [56] |
| Polyester fabric/Ag2O | 4.36 ± 0.43 | 3.67 ± 0.18 | 26.70 ± 1.64 | |
| Polyester fabric/Ag/ZnO | 3.94 ± 0.07 | 3.55 ± 0.14 | 27.68 ± 1.21 | |
| Polyester fabric/Ag2O/ZnO | 3.86 ± 0.24 | 3.21 ± 0.01 | 30.37 ± 0.20 | |
| Bacterial Cellulose/Copper | 0.22 ± 0.01 | 0.12 ± 0.02 | 918.27 ± 1.90 | This work |
| Bacterial Cellulose/Copper/Zinc Oxide | 0.16 ± 0.02 | 0.07 ± 0.01 | 1850.33 ± 2.12 |
| Samples | Bacterial Growth (%) | ||
|---|---|---|---|
| E. Coli (ATCC 22125) | S. aureus (ATCC 6538) | Reference | |
| FPEI-A | 5.99 | 7.89 | [61] |
| FPEI-AZ | 5.45 | 6.86 | |
| FPEI-AZC | 4.9 | 5.83 | |
| PMC-A | 2.49 | 1.85 | |
| PMC-AZ | 2.22 | 1.84 | |
| PMC-AZC | 1.95 | 1.83 | |
| Pure Bacterial Cellulose | 100 | 100 | This work |
| Bacterial Cellulose/Copper | 2.63 | 3.15 | |
| Bacterial Cellulose/Copper/Zinc Oxide | 1.55 | 1.89 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasim, M.; Khan, M.R.; Mushtaq, M.; Naeem, A.; Han, M.; Wei, Q. Surface Modification of Bacterial Cellulose by Copper and Zinc Oxide Sputter Coating for UV-Resistance/Antistatic/Antibacterial Characteristics. Coatings 2020, 10, 364. https://doi.org/10.3390/coatings10040364
Wasim M, Khan MR, Mushtaq M, Naeem A, Han M, Wei Q. Surface Modification of Bacterial Cellulose by Copper and Zinc Oxide Sputter Coating for UV-Resistance/Antistatic/Antibacterial Characteristics. Coatings. 2020; 10(4):364. https://doi.org/10.3390/coatings10040364
Chicago/Turabian StyleWasim, Muhammad, Muhammad Rafique Khan, Muhammad Mushtaq, Awais Naeem, Mengchen Han, and Qufu Wei. 2020. "Surface Modification of Bacterial Cellulose by Copper and Zinc Oxide Sputter Coating for UV-Resistance/Antistatic/Antibacterial Characteristics" Coatings 10, no. 4: 364. https://doi.org/10.3390/coatings10040364
APA StyleWasim, M., Khan, M. R., Mushtaq, M., Naeem, A., Han, M., & Wei, Q. (2020). Surface Modification of Bacterial Cellulose by Copper and Zinc Oxide Sputter Coating for UV-Resistance/Antistatic/Antibacterial Characteristics. Coatings, 10(4), 364. https://doi.org/10.3390/coatings10040364





