3.1. Surface and Cross-Sectional Morphology
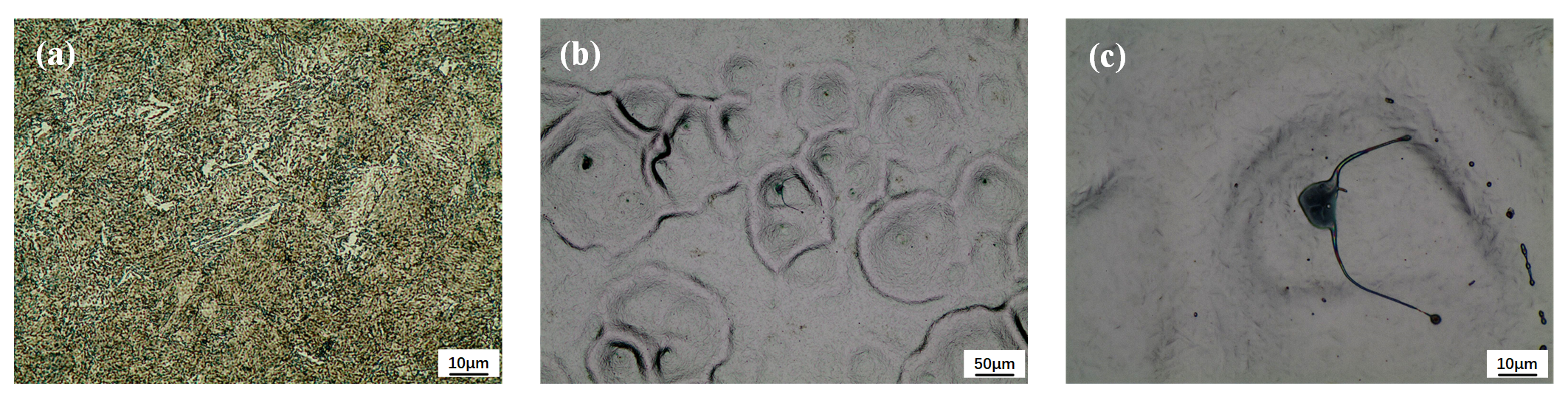
After quenching and tempering heat treatment, the original microstructure of 40CrNiMo7 steel becomes tempered sorbite, and the composite microstructure of carbide pellet is generated in the matrix ferrite [
7]. It can be seen from the figure that the high-temperature tempering martensite strips were generated, among which dispersedly distributed with cementite, carbide precipitate and a small amount of strip ferrite phase structure of different sizes. The original grain size is about 10 microns, as shown in
Figure 1a.
After once pulse HCPEB treatment, as shown in
Figure 1b, a large number of molten pits were formed on the surface of the sample, and the thermophysical difference between the original ferrite, cementite and carbide results in the modified surface melt and form molten droplets at the phase boundary firstly, which is consistent with the literature [
18,
19], the eruption source of the molten pit is closely related to the phase structure defects of HCPEB treated surface and the difference in thermophysical properties of the surface phase structure. In addition, it is commonly recognized that the electron beam irradiation penetrating heating mode causes the melting of treated samples to start in the subsurface layer. It can be observed from
Figure 1c that the morphology of splash of molten droplets is shown in the enlarged metallographic diagram of molten pits. The reason is that when the energy density increases to a certain value, the molten metal tends to gasify, along with the spill of molten metal droplets, and the morphology of transient plume distribution can be generated.
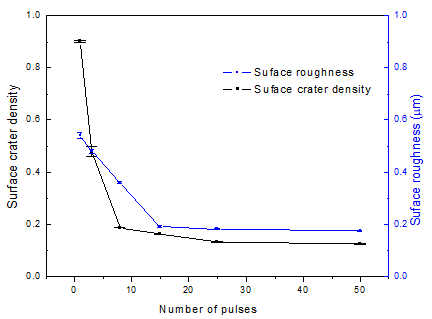
The surface roughness of samples before and after HCPEB treatment was measured by surface profilometer. The results showed that the average surface roughness (Ra) of the original polished samples was 0.13
m. After one pulse treatment, the roughness of the modified sample surface increase to 0.54
m. This is because a large number of craters formed on the sample surface after the first HCPEB treatment, hence the roughness of the modified surface increased correspondingly. The surface roughness was 0.48
m and 0.36
m after 3 and 8 pulses HCPEB treatments, respectively. After 15 pulses treatment, the modified surface became smoother with the roughness less than 0.2
m. With the increase of pulse numbers, the treated sample surface underwent repeated remelting and the crater density gradually decreased, then the modified surface became smoother and denser, and the microstructure was refined and uniform, in this way, the modified surface roughness gradually decreased as shown in
Figure 2. Wang et al. [
20] studied the influence of surface roughness on micro-dynamic friction and wear properties. They found that the wear rate decreases monotonically with the decrease of surface roughness in dry friction condition, and the worn surface with low roughness has a few furrows and slight plastic deformation.
The high magnification SEM morphology observation results of the modified surface after HCPEB treatments are shown in
Figure 3. After the initial pulse treatment, the modified surface of the sample showed obvious melting traces, and many embedded acicular structures were observed covering the modified surface (
Figure 3a). When the cooling rate is sufficient to avoid the diffusion phase transition, martensitic phase transition can occur in all metals and alloys at high temperature, while the appearance of embedded acicular structure on the modified surface indicates that martensitic phase transition may occur on the modified surface after HCPEB treatment, with the formation of martensitic phase transition. After 15 pulse treatments, the modified surface gradually flattens out, and the embedded needle structure becomes smaller in size and more evenly distributed (
Figure 3c). After 50 pulse treatments, it can be seen from
Figure 3d that the modified surface is more smooth and even, and the size of the embedded needle structure is more refined. The results showed that with the increase of HCPEB treatment times, the formation of embedded acicular structures related to martensitic transformation in the modified subsurface layer was gradually inhibited. In addition, the SEM morphology observation results of the modified surface at high power are shown in
Figure 3b,d,f. The embedded needle-like structure of the modified surface is shown as a ridge of uplift and is covered by a layer of fine nanoscale grains. The following XRD and TEM analysis results show that these nanoscale grains can be identified as a complex phase structure, the majority of which is a nano-austenitic phase. Thus, the crystallization process occurred on the modified surface during rapid heating and rapid cooling after HCPEB treatment, resulting in the formation of nanoscale grains.
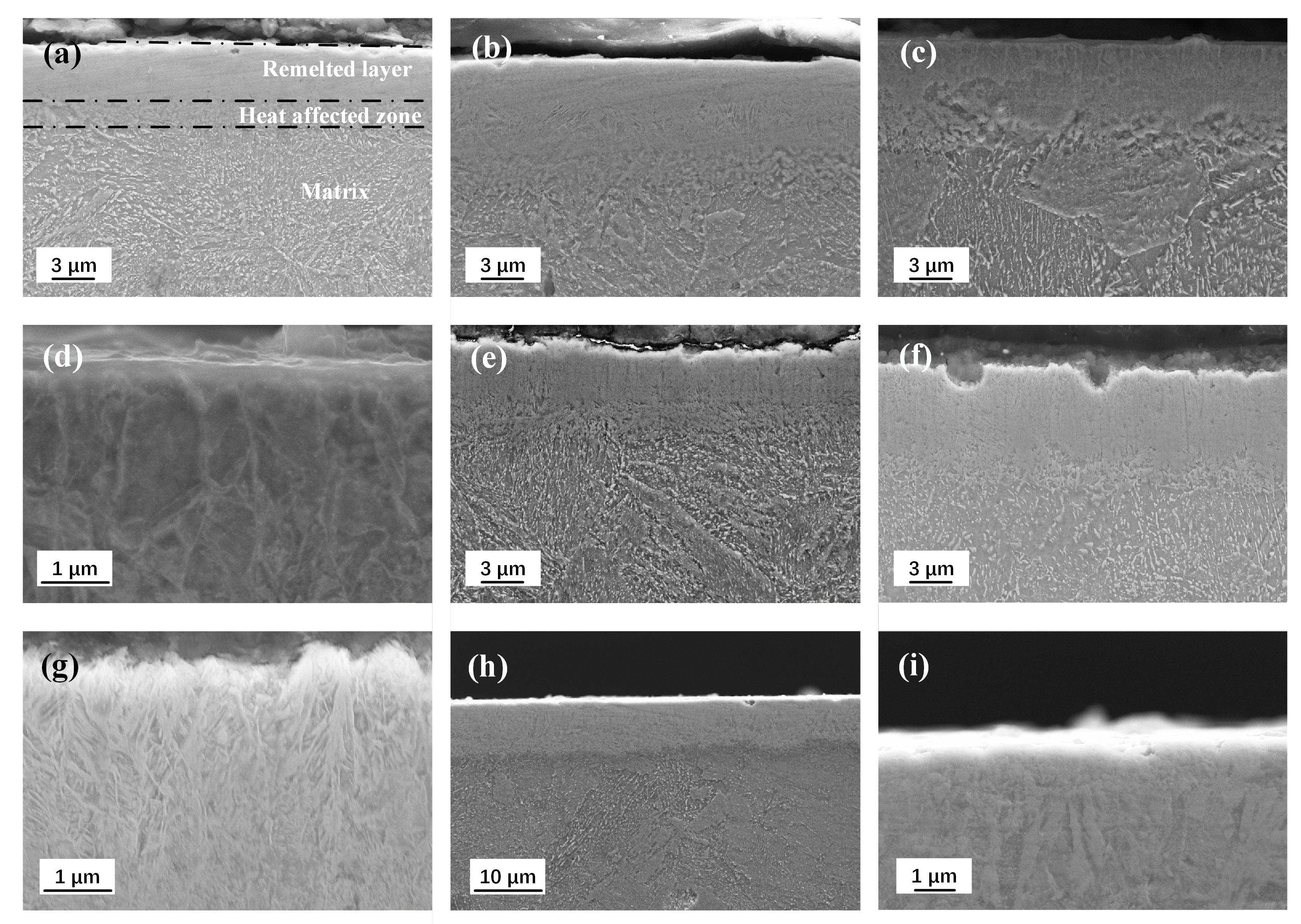
Figure 4 shows the cross-sectional SEM morphologies of the samples after HCPEB treatment with different pulse numbers. The modified surface shows obvious stratification, remelting layer, heat-affected zone and matrix can be seen from top to bottom. After one and three pulse treatments, the remelting layer of the modified surface shows uniform contrast, and the microstructure in the heat-affected zone is significantly more refined than that in the matrix (
Figure 4a,b). After 8 pulses HCPEB treatments, columnar crystals growing parallel to the incident direction of the electron beam in the remelting layer were observed in
Figure 4c,d, and the texture in the heat-affected zone was evenly refined. The cooling rate can reach up to
∼
ks
and the crystallization rate can reach up to 2∼5 m/s. The melt generated on the irradiation surface grew rapidly into the columnar crystal in the opposite direction from the heat dissipation direction.
After 15 pulse treatments, the typical layered structure of the modified surface can be observed, as shown in
Figure 4e. After 25 pulses HCPEB treatments, the dendritic structure can be clearly seen in high magnification SEM morphology (
Figure 4f,g). Such highly branched crystal with primary dendrites parallel to the electron beam heat flow direction are called columnar dendrites. When the liquid phase of the columnar crystals formed in the modified remelting layer was extremely subcooled before solidification, the latent heat released during solidification increased the temperature of the solid-liquid phase interface, and then the interface will bulge and form columnar crystals. A similar process could occur on the sides of the column crystals, which grows in a dendritic form and form dendrites. Temperature gradient and growth rate are two basic solidification parameters that determine the morphology and size of the microstructure. With the increase of pulse number, alloy and carbon elements in modified surface were fully diffused and can be spread to the clearance between two grains, or the front end of the crystallization, thus provide the premise of lateral crystal branching. In addition, the cooling rate is gradually reduced in the modified remelting layer, and the grain growth rate decreased gradually, which also provides enough growth time for the lateral branch, i.e., columnar dendrites can be generated.
After 50 pulses HCPEB treatment, the dendrite growth marks and the formation of near-surface equiaxed crystals (nanoscale) in the modified remelting layer can be observed from SEM morphologies, as shown in
Figure 4h,i. In the process of HCPEB treatment, when the solidification reaches to a certain extent, the melt near the surface in front of the dendrite growth in the remelting layer becomes supercooled due to rapid cooling. In the liquid phase, such a supercooled melt may nucleate independently and grow until it meets other crystal nuclei, i.e., it becomes nanoscale equiaxed crystal on the modified near-surface.
3.2. XRD Analysis and TEM Observation
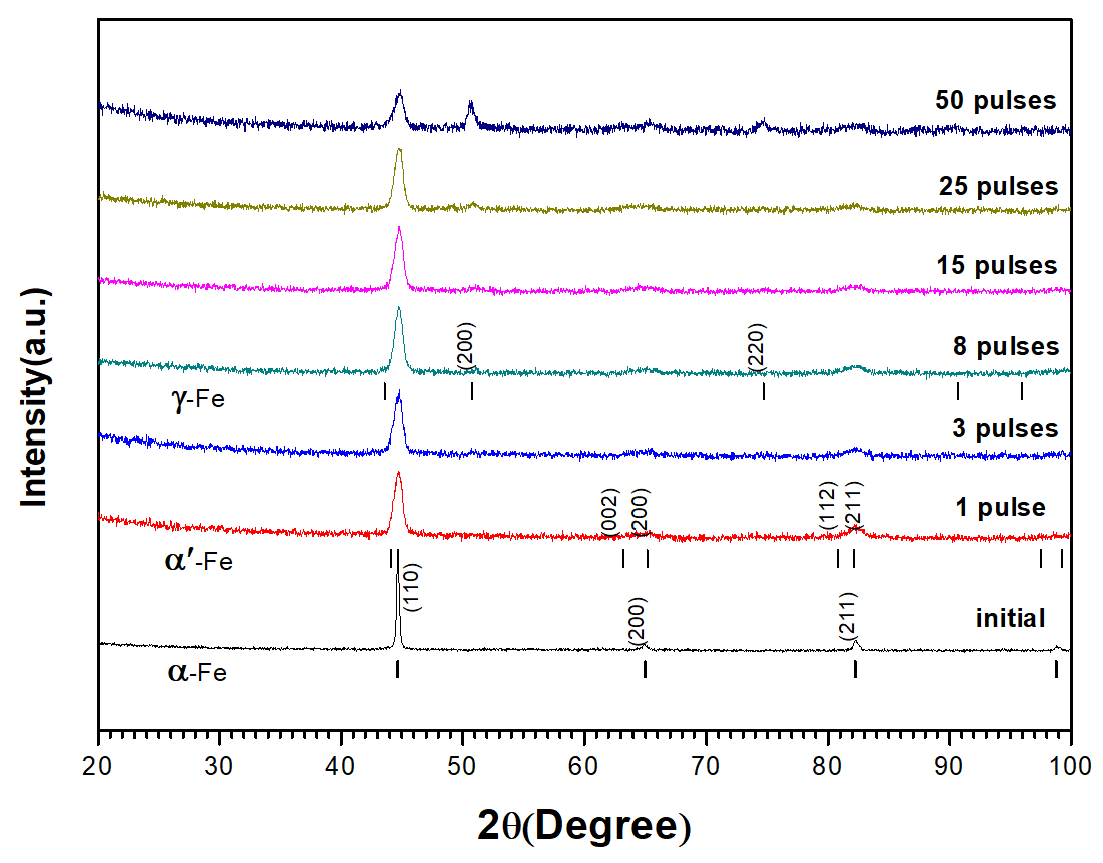
Figure 5 shows the XRD diffraction pattern of grazing incident on the sample surface before and after HCPEB treatment. The results prove that the modified surface is composed of austenitic and martensitic phases. The diffraction peak intensity of the newly formed
-fe phase increased with the increasing pulse number, and
-fe phase became the dominating phase of the modified surface layer.
The XRD peaks of the original samples correspond to the diffraction peaks of a single pattern
-Fe phase, and the diffraction peaks are sharp. After HCPEB treatment, XRD diffraction peaks become dull and wider. After three pulses HCPEB treatment, it can be found that the diffraction peaks corresponding to (200) and (211) crystal planes show obvious peak cleavage by observing the diffraction spectra. In general, such diffraction peak cleavage can be seen in the XRD spectra of newly formed martensite. Refer to standard PDF CARDS of martensite (
-Fe) diffraction spectrum and SEM appearance (
Figure 5) of electron beam modification quenched and tempered 40CrNiMo7 steel microstructure, it can be deduced that after HCPEB treatment, with the rapid melting, cooling and solidification process on the sample surface, the surface modification layer of quenched and tempered 40CrNiMo7 steel sample will appear large-scale grain refining and martensite phase transformation. As the number of pulses increased to eight, significant new phase diffraction peaks appeared in the diffraction pattern, corresponding to the diffraction peaks of austenitic phase (
-fe) in the standard PDF card. In addition, with the increase of pulse number, the diffraction peak intensity of
-fe increases gradually, which is proved by the XRD diffraction peak intensity of the modified surface after 50 pulses HCPEB treatment as shown in the figure.
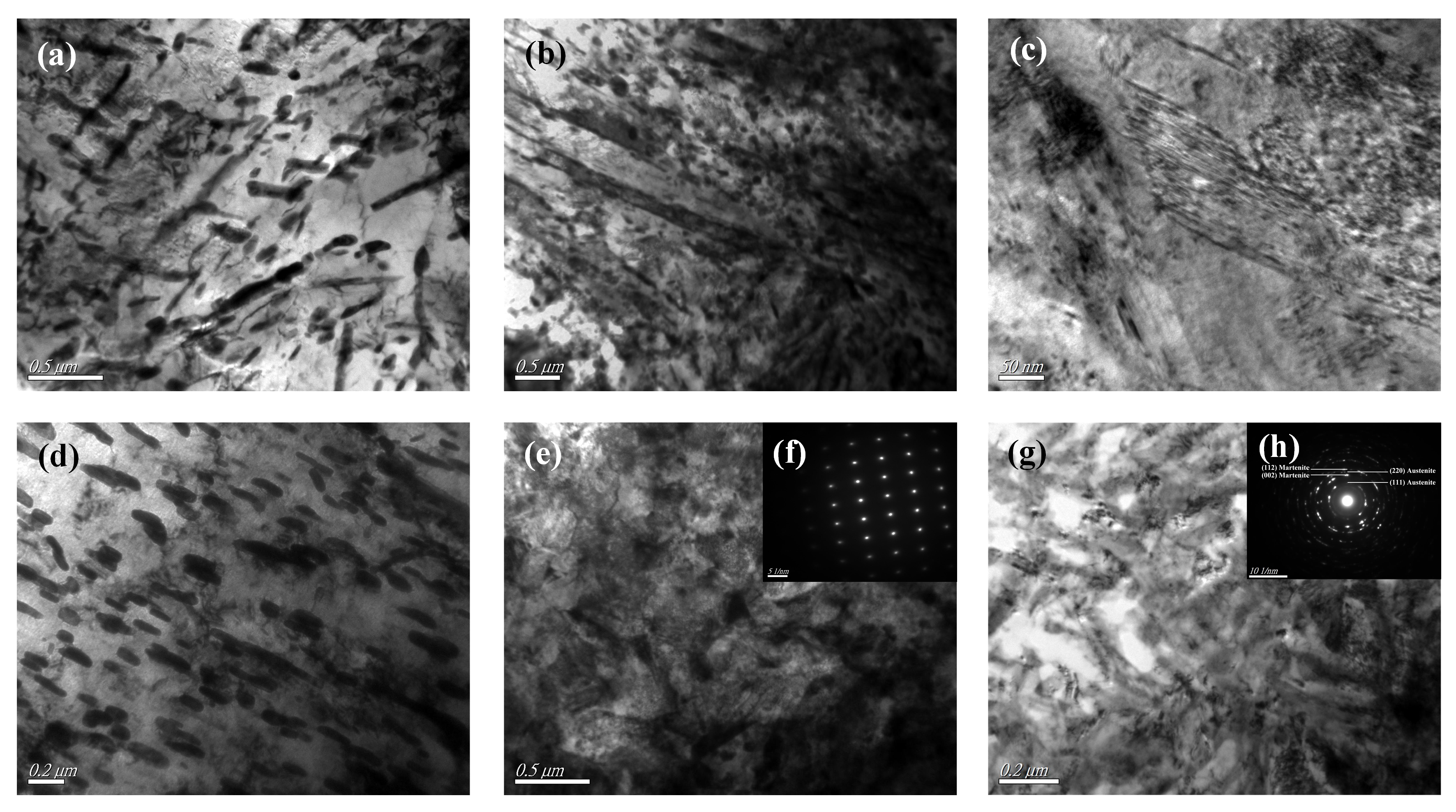
TEM micrograph (
Figure 6a) shows that these carbides appear as long shapes and with black contrast due to high carbon content, and with sizes ranging from 1.10 to 0.25
m embedded in
-Fe. After one pulse HCPEB treatment, as shown in
Figure 6b,c, the newly formed lath martensite and acicular martensite in the modified surface layer can be clearly observed. In addition, the size of carbide particles in the original structure was significantly reduced, as shown in
Figure 6d. The results show that the rapid heating of the quenched 40CrNiMo7 steel by initial HCPEB treatment results in surface melting. When the liquid metal became cool and crystallized, the atomic diffusion occurred at first, and the melting locations were where the carbides dissolved. Subsequently, the rapid cooling rate induced the martensitic transformation in the surface-modified layer.
After eight pulse HCPEB treatments, as shown in
Figure 6e, the modified area of the sample became more flat and dense, and the microstructure characteristics corresponding to martensitic transformation are even less. The analysis results of the electron diffraction SAED (
Figure 6f) shows that the refined grains are austenitic phase and located near the modified surface, which matched the X-ray diffraction analysis results. With more HCPEB treatments, the carbides were fully melted and dissolved.
After 50 times HCPEB treatments, The carbide particles basically disappeared in the modified surface as shown in
Figure 6g, and the modified surface composition was evenly distributed, SAED analysis results show that (
Figure 6h) the microstructure of the modified surface was compound phase structure consists of austenitic phase and a small amount of nano martensite phase composition, this result is consistent with grazing incidence XRD analysis results (
Figure 5). Researches show that even though the cooling rate during the electron beam process can reach up to
∼
ks
, the ultrafine grain size in the austenitic phase and the large amount of dissolved alloying elements still inhibit the normal martensitic phase transition. F. Meyers et al. [
21] found that nanostructures appeared in the shear zone of 304 stainless steel in the study of high-speed deformation with strain rate up to
s
, and F. Meyers believed that rapid heating and cooling in the shear zone was conducive to the formation of non-equilibrium structures.
3.3. Microstructure Evolution of Modified Layer
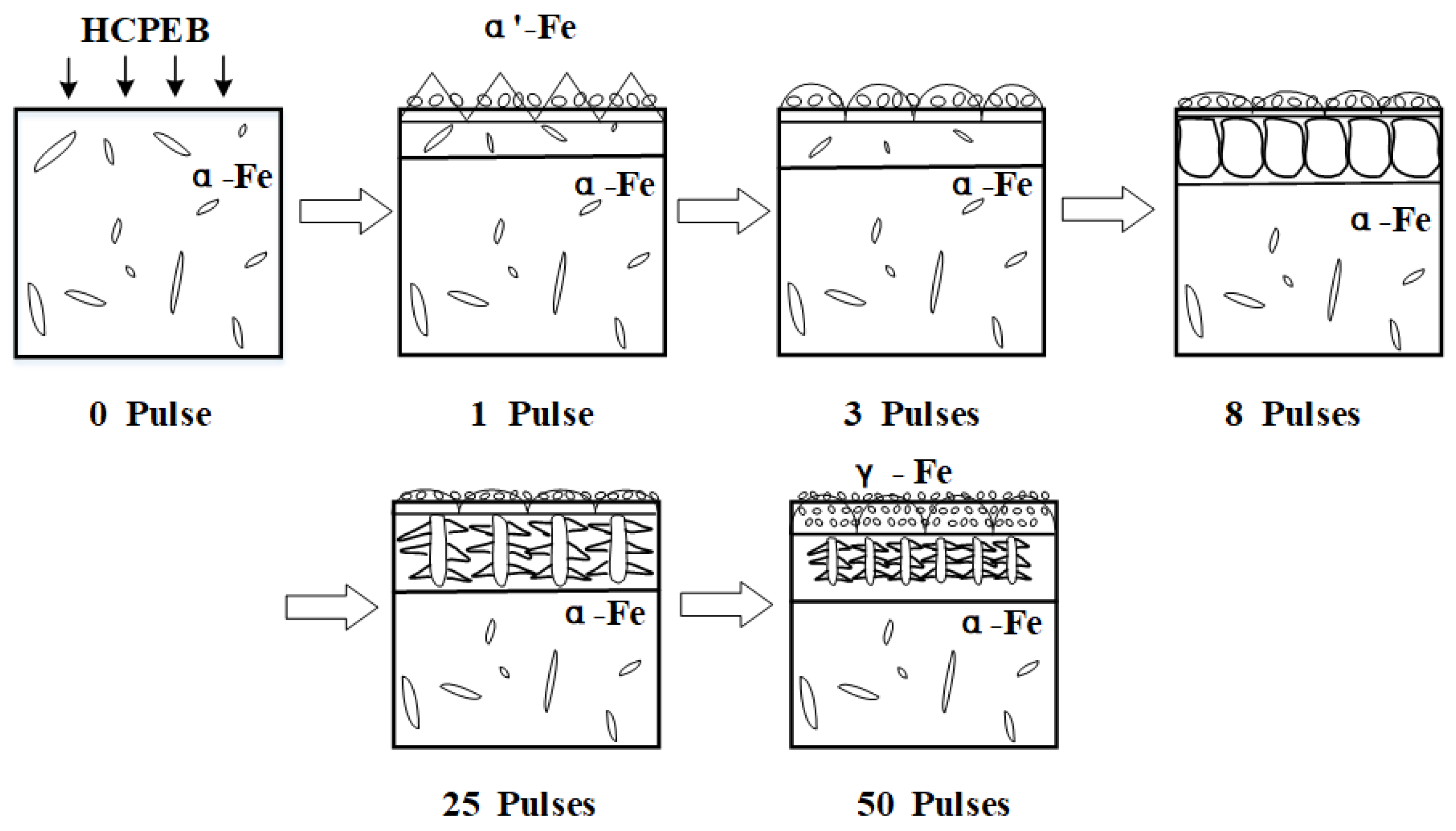
The surface modification mechanism of HCPEB of quenched and tempered 40CrNiMo7 steel is summarized as follows: the surface of the sample melted after irradiation of electron beam. Due to the self-cooling effect of the matrix, solid–liquid interface exists, and liquid metal firstly nucleated at the solid-liquid interface junction. After initial pulse treatment, the modified surface carbide partially melted, and the molten liquid metal in the remelting layer presented greater condensate depression, the molten layer was shallow, and the cooling rate was very high. In this case, the nucleation rate is extremely high, accompanied by the martensite phase transformation and the formation of ridge topography, which were shown in the ridge shape and fine grain in
Figure 7 (1 pulses). With the increase of pulse number, the remelting layer deepening continuously and the melt carbides dissolve more fully, meanwhile, carbon atoms were fully dissolved and spread, the nucleation rate increase gradually. The generated nanometer austenitic organization became slighter, and the nanoscale grain inhibited the occurrence of martensite phase transformation. The modified ridge shape surface morphology became more round, this is because extreme shear stress was needed during the martensite phase transformation, whereas it is not possible to overcome the stress disorder in the process of HCPEB treatments. The cooling rate in the remelting layer was gradually reduced, thus the grains could grow along the opposite direction of heat dissipation, and then extended into the liquid solution and eventually formed columnar crystals, which can be proved by the parallel-aligned columnar crystal in the remelting layer as shown in
Figure 7 (8 pulses). When the cooling rate continues to reduce, columnar dendrites might generate in the remelting layer, as shown in
Figure 7 (25 pulses). After 50 times HCPEB treatments, almost all surface carbides melted, when the cooling rate decreased to a certain extent, the melt which located in front of the columnar dendrites became subcooled and may independently nuclear and growth in the liquid phase, ultimately turned into nanometer isometric when contacting other crystal nuclei, as shown in
Figure 7 (50 pulses).
3.4. Corrosion Property
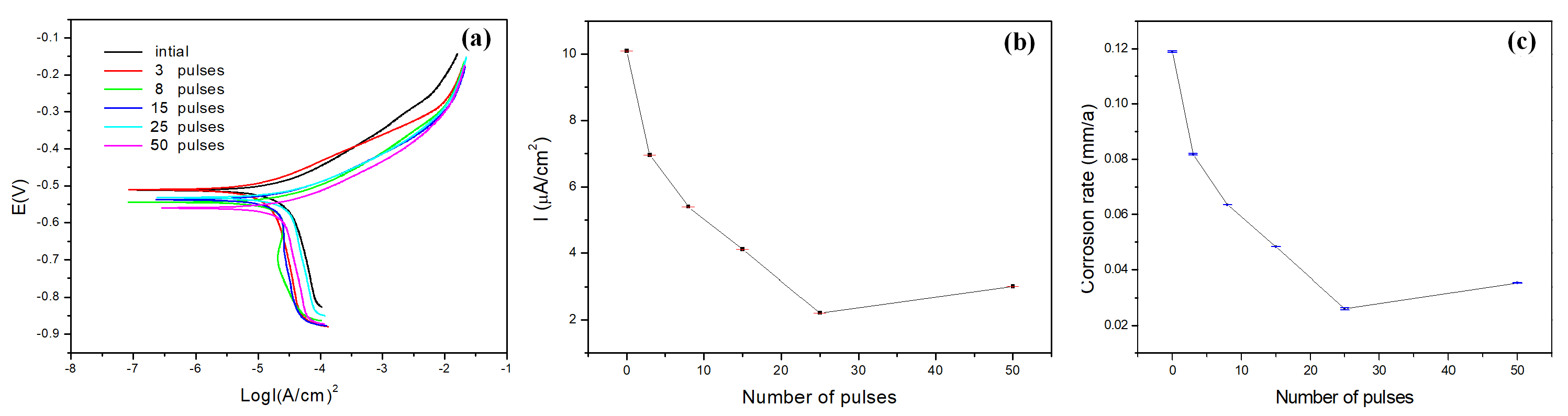
Figure 8a shows typical potentiodynamic polarization cures of 40CrNiMo7 steel recorded in the NaCl (3.5%) solution before and after HCPEB treatment. The corrosion potentials
of HCPEB treated samples shift indifferently as compared with the initial sample. While the self- corrosion current density (
) and corrosion rate decreases significantly. After 25 pulses of HCPEB treatment. The result indicated the significant improvement on corrosion resistance of the alloying samples the
value was reduced greatly from the initial
to 2.2099
A/cm
as shown in
Figure 8b, according to the inverse relationship between the corrosion resistance (
) and the Icorr, the
of treated samples would be improved. Correspondingly, the corrosion rate was decreased drastically from the initial 0.12 to 0.02 mm/a given in
Figure 8c. However, the surface corrosion resistance of the samples decreased slightly after 50 pulses of HCPEB treatment.
The unbalanced electrochemical distribution on the material surface leads to the potential difference and forms corrosion microbattery. The surface roughness and the microstructure inhomogeneity of the material surface will lead to unbalanced electrochemical distribution. Li [
22] illustrated that surface roughness accelerated the corrosion process of material surface. Three possible mechanisms that increased corrosion resistance can be concluded as follows:
The selective purification of the sample surface and the homogenization of the modified surface components by HCPEB effectively reduced the generation probability of the microbattery on material surface;
Refined grain structure in modified layer and compact and smooth of surface effectively reduce the direct contact between the material and the corrosive medium, meanwhile, intergranular corrosion is also reduced;
The reduction of surface roughness effectively reduces the corrosion rate of the material surface.
Optimal performance of the material surface corrosion resistance was obtained after 25 pulses of HCPEB treatment, which mainly attributed to the generation of the austenitic phase with high alloy content.