Trap State and Charge Recombination in Nanocrystalline Passivized Conductive and Photoelectrode Interface of Dye-Sensitized Solar Cell
Abstract
1. Introduction
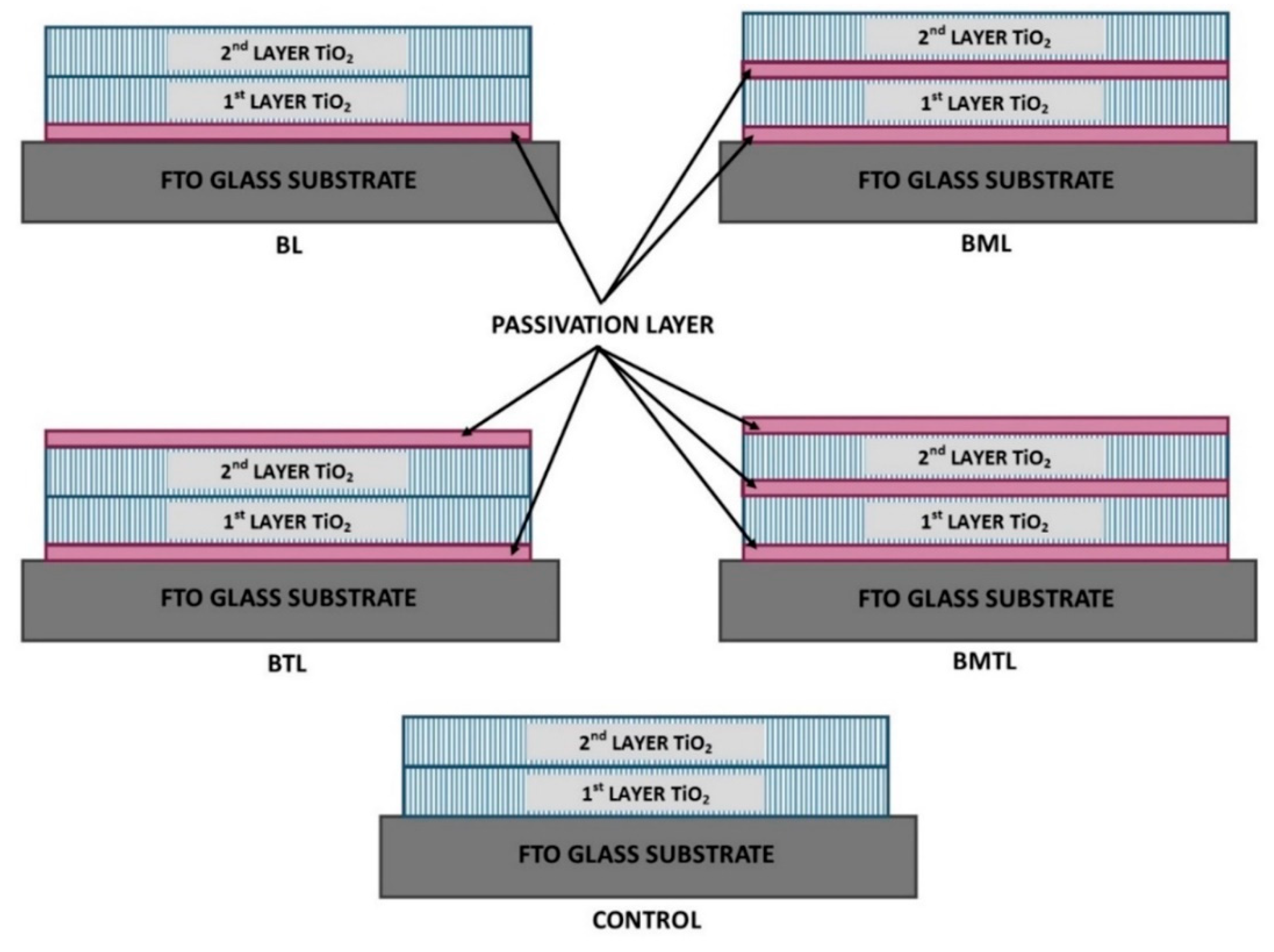
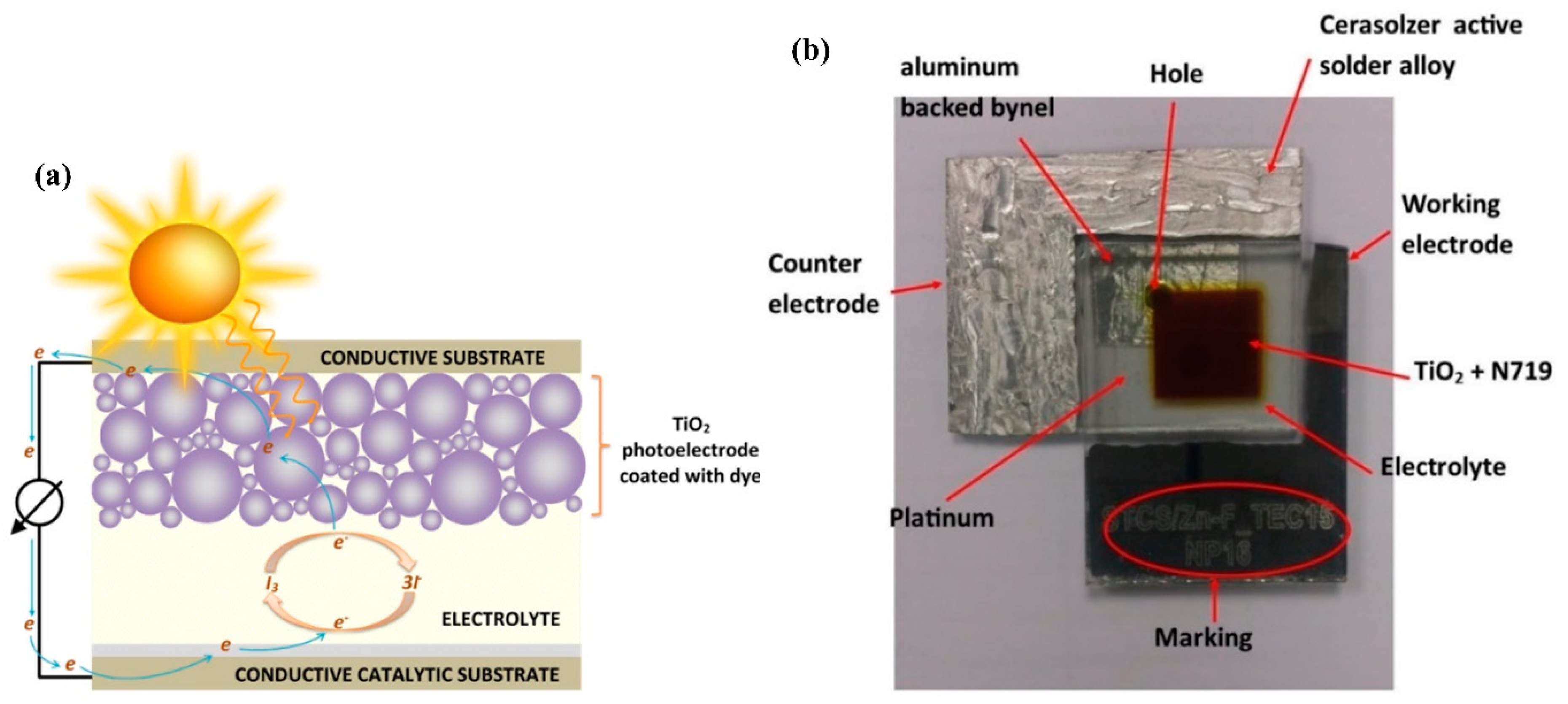
2. Materials and Methods
3. Results
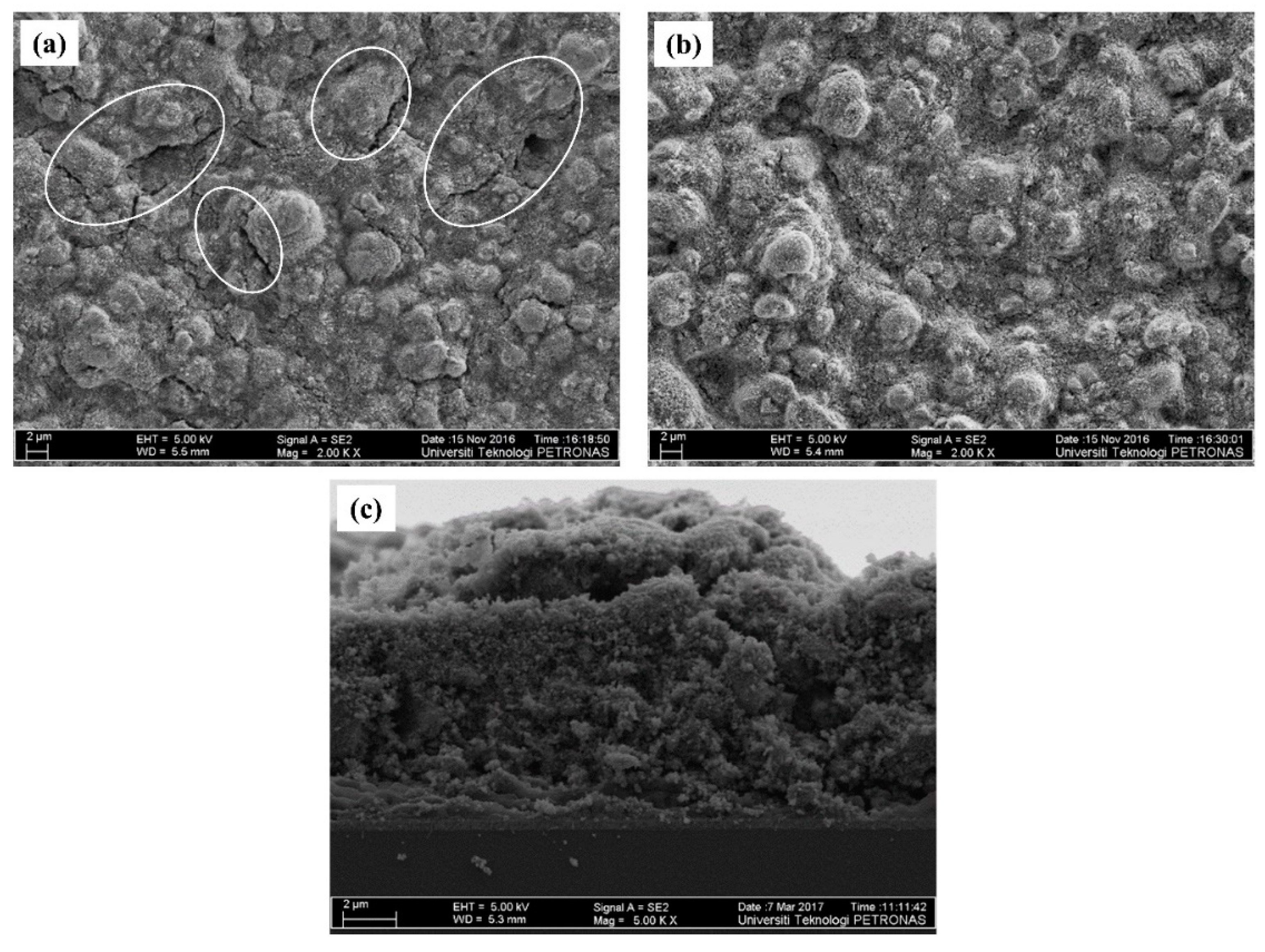
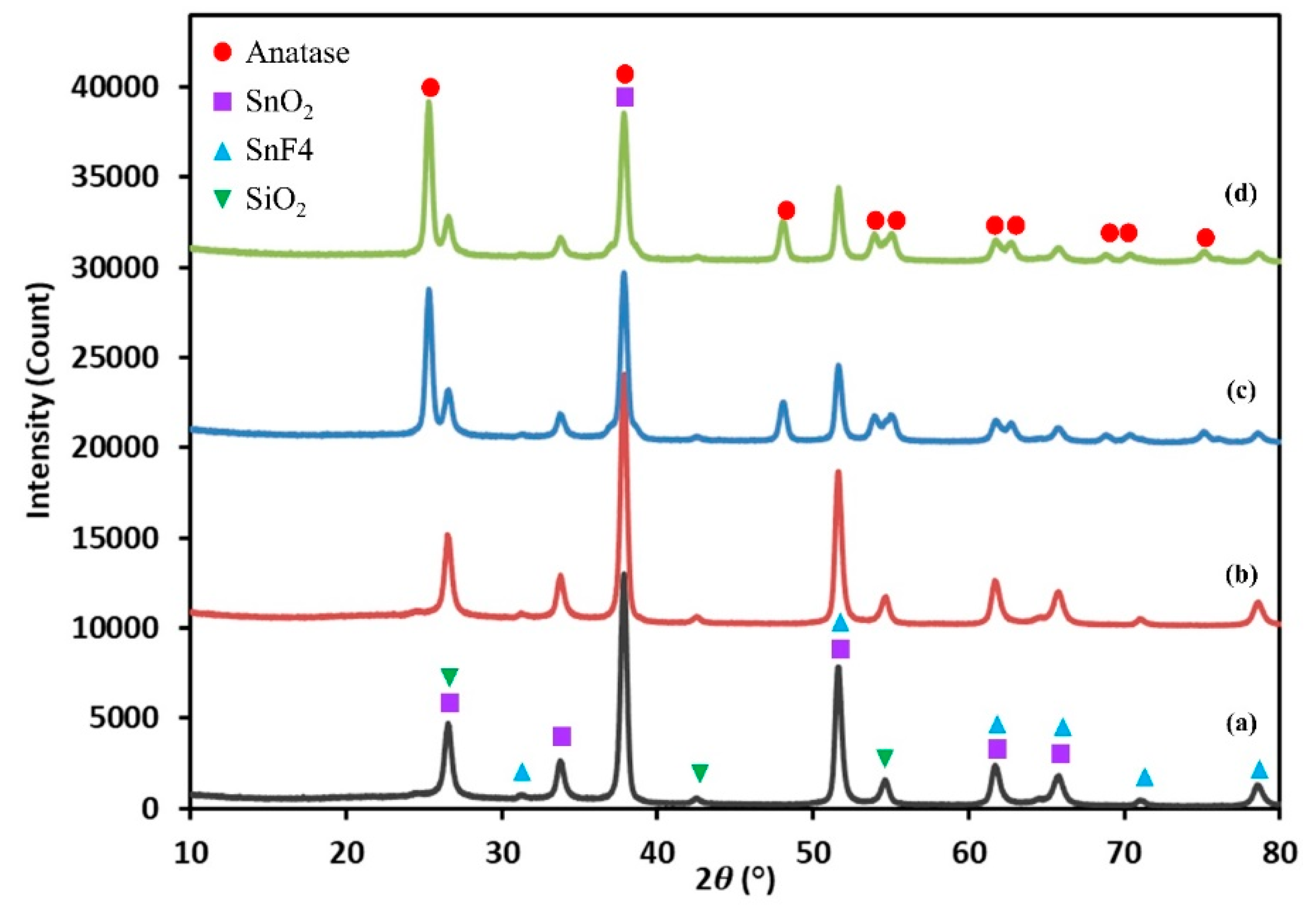
3.1. Morphology and Microstructure of Photoelectrode Film
3.2. Electrochemical Analysis
3.3. Photovoltaic Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar]
- Nazeeruddin, M.K.; Baranoff, E.; Grätzel, M. Dye-sensitized solar cells: A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- Lau, K.K.; Soroush, M. Overview of dye-sensitized solar cells in Dye-Sensitized Solar Cells; Academic Press: Cambridge, MA, USA, 2019; pp. 1–49. [Google Scholar]
- Karim, N.A.; Mehmood, U.; Zahid, H.F.; Asif, T. Nanostructured photoanode and counter electrode materials for efficient Dye-Sensitized Solar Cells (DSSCs). Sol. Energy 2019, 185, 165–188. [Google Scholar] [CrossRef]
- Jose, R.; Thavasi, V.; Ramakrishna, S. Metal oxide for dye-sensitized solar cells. J. Am. Ceram. Soc. 2009, 92, 289–301. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Numata, Y.; Han, L. Highly efficient dye-sensitized solar cells: progress and future challenges. Energy Environ. Sci. 2013, 6, 1443–1464. [Google Scholar] [CrossRef]
- Gong, J.; Liang, J.; Sumathy, K. Review on dye-sensitized solar cells (DSSCs): Fundamental concepts and novel materials. Renew. and Sustain. Energy Rev. 2012, 16, 5848–5860. [Google Scholar] [CrossRef]
- Afrooz, M.; Dehghani, H.; Khalili, S.S.; Firoozi, N. Effects of cobalt ion doped in the ZnS passivation layer on the TiO2 photoanode in dye sensitized solar cells based on different counter electrodes. Synth. Met. 2017, 226, 164–170. [Google Scholar] [CrossRef]
- Ako, R.T.; Ekanayake, P.; Tan, A.L.; Young, D.J. La modified TiO2 photoanode and its effect on DSSC performance: A comparative study of doping and surface treatment on deep and surface charge trapping. Mater. Chem. and Phys. 2016, 172, 105–112. [Google Scholar] [CrossRef]
- D'Souza, L.P.; Shwetharani, R.; Amoli, V.; Fernando, C.A.N.; Sinha, A.K.; Balakrishna, R.G. Photoexcitation of neodymium doped TiO2 for improved performance in dye-sensitized solar cell. Mater. & Design 2016, 104, 346–354. [Google Scholar]
- Xiang, P.; Ma, W.; Xiao, T.; Jiang, L.; Tan, X.; Shu, T. Ta-doped hierarchical TiO2 spheres for dye-sensitized solar cells. J. Alloy Compd. 2016, 656, 45–50. [Google Scholar] [CrossRef]
- Zhou, L.; Wei, L.; Yang, Y.; Xia, X.; Wang, P.; Yu, J.; Luan, T. Improved performance of dye sensitized solar cells using Cu-doped TiO2 as photoanode materials: Band edge movement study by spectroelectrochemistry. Chem. Phys. 2016, 475, 1–8. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, C.S.; Okuyama, K.; Lee, H.M.; Jang, H.D.; Lee, S.E.; Kim, T.O. Copper and nitrogen doping on TiO2 photoelectrodes and their functions in dye-sensitized solar cells. J. Power Sources 2016, 306, 764–771. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J. Mol. Liq. 2018, 258, 354–365. [Google Scholar] [CrossRef]
- Rajaei, E.; Ravandi, S.A.H.; Valipouri, A. Electrochemical and photovoltaic properties of dye-sensitized solar cells based on Ag-doped TiO2 nanorods. Optik 2018, 158, 514–521. [Google Scholar] [CrossRef]
- Ünlü, B.; Çakar, S.; Özacar, M. The effects of metal doped TiO2 and dithizone-metal complexes on DSSCs performance. Sol. Energy 2018, 166, 441–449. [Google Scholar] [CrossRef]
- Tyona, M.D.; Jambure, S.B.; Lokhande, C.D.; Banpurkar, A.G.; Osuji, R.U.; Ezema, F.I. Dye-sensitized solar cells based on Al-doped ZnO photoelectrodes sensitized with rhodamine. Mater. Lett. 2018, 220, 281–284. [Google Scholar] [CrossRef]
- Kouhestanian, E.; Mozaffari, S.A.; Ranjbar, M.; SalarAmoli, H.; Armanmehr, M.H. Electrodeposited ZnO thin film as an efficient alternative blocking layer for TiCl4 pre-treatment in TiO2-based dye sensitized solar cells. Superlattices Microstruct. 2016, 96, 82–94. [Google Scholar] [CrossRef]
- Ranasinghe, C.S.K.; Jayaweera, E.N.; Kumara, G.R.A.; Rajapakse, R.M.G.; Onwona-Agyeman, B.; Perera, A.G.U.; Tennakone, K. Tin oxide based dye-sensitized solid-state solar cells: surface passivation for suppression of recombination. Mater. Sci. Semicond. Process. 2015, 40, 890–895. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Wang, W.; Qian, Y.; Li, Z. Improved performance of dye-sensitized solar cell based on TiO2 photoanode with FTO glass and film both treated by TiCl4. Phys. B Condens. Matter 2016, 500, 48–52. [Google Scholar]
- Xiang, Y.; Yu, J.; Zhang, T.; Wen, Q.; Zhuang, J.; Guo, J. Improved performance of nanoporous TiO2 film in dye-sensitized solar cells via ZrCl4 and TiCl4 surface co-modifications. Mater. Sci. Semicond. Process. 2016, 49, 48–53. [Google Scholar] [CrossRef]
- Choi, H.; Nahm, C.; Kim, J.; Moon, J.; Nam, S.; Jung, D.R.; Park, B. The effect of TiCl4-treated TiO2 compact layer on the performance of dye-sensitized solar cell. Curr. Appl. Phys. 2012, 12, 737–741. [Google Scholar] [CrossRef]
- Gopakumar, G.; Ashok, A.; Vijayaraghavan, S.N.; Nair, S.V.; Shanmugam, M. MoO3 surface passivation on TiO2: An efficient approach to minimize loss in fill factor and maximum power of dye sensitized solar cell. Appl. Surf. Sci. 447, 554–560. [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8326–8349. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, A.R.; Bourgeois, L.; Duffy, N.W.; Xiang, W.; Cheng, Y.B. Surface state recombination and passivation in nanocrystalline TiO2 dye-sensitized solar cells. J. Phys. Chem. C. 2013, 117, 25118–25126. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, K.H.; Kang, P.; Kim, H.J.; Choi, Y.S.; Lee, W.I. Effect of layer-by-layer assembled SnO2 interfacial layers in photovoltaic properties of dye-sensitized solar cells. Langmuir 2012, 28, 10620–10626. [Google Scholar] [CrossRef]
- Park, J.; Lee, M. Performance enhancement of dye-sensitized solar cell with a TiCl4-treated TiO2 compact layer. Electron. Mater. Lett. 2015, 11, 271–275. [Google Scholar] [CrossRef]
- Lee, S.W.; Ahn, K.S.; Zhu, K.; Neale, N.R.; Frank, A.J. Effects of TiCl4 treatment of nanoporous TiO2 films on morphology, light harvesting, and charge-carrier dynamics in dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 21285–21290. [Google Scholar] [CrossRef]
- Charoensirithavorn, P.; Ogomi, Y.; Sagawa, T.; Hayase, S.; Yoshikawa, S. Improvement of dye-sensitized solar cell through TiCl4-treated TiO2 nanotube arrays. J. Electrochem. Soc. 2010, 157, B354–B356. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z. Dye-sensitized TiO2 nanotube solar cells with markedly enhanced performance via rational surface engineering. Chem. Mater. 2010, 22, 579–584. [Google Scholar]
- Fuke, N.; Katoh, R.; Islam, A.; Kasuya, M.; Furube, A.; Fukui, A.; Chiba, Y.; Komiya, R.; Yamanaka, R.; Han, L. Influence of TiCl4 treatment on back contact dye-sensitized solar cells sensitized with black dye. Energy & Environ. Sci. 2009, 2, 1205–1209. [Google Scholar]
- Knorr, F.J.; Zhang, D.; McHale, J.L. Influence of TiCl4 treatment on surface defect photoluminescence in pure and mixed-phase nanocrystalline TiO2. Langmuir 2007, 23, 8686–8690. [Google Scholar] [CrossRef] [PubMed]
- O'Regan, B.C.; Durrant, J.R.; Sommeling, P.M.; Bakker, N.J. Influence of the TiCl4 treatment on nanocrystalline TiO2 films in dye-sensitized solar cells. 2. Charge density, band edge shifts, and quantification of recombination losses at short circuit. J. Phys. Chem. 2007, 111, 14001–14010. [Google Scholar] [CrossRef]
- Sommeling, P.M.; O'Regan, B.C.; Haswell, R.R.; Smit, H.J.P.; Bakker, N.J.; Smits, J.J.T.; Kroon, J.M.; Van Roosmalen, J.A.M. Influence of a TiCl4 post-treatment on nanocrystalline TiO2 films in dye-sensitized solar cells. J. Phys. Chem. B vol. 2006, 110, 19191–19197. [Google Scholar] [CrossRef] [PubMed]
- Roy-Mayhew, J.D.; Aksay, I.A. Graphene materials and their use in dye-sensitized solar cells. Chem. Rev. 2014, 114, 6323–6348. [Google Scholar] [CrossRef]
- Chen, T.; Hu, W.; Song, J.; Guai, G.H.; Li, C.M. Interface functionalization of photoelectrodes with graphene for high performance dye-sensitized solar cells. Adv. Funct. Mater. 2012, 22, 5245–5250. [Google Scholar] [CrossRef]
- Tang, B.; Hu, G. Two kinds of graphene-based composites for photoanode applying in dye-sensitized solar cell. J. Power Sources 2012, 220, 95–102. [Google Scholar] [CrossRef]
- Durantini, J.; Boix, P.P.; Gervaldo, M.; Morales, G.M.; Otero, L.; Bisquert, J.; Barea, E.M. Photocurrent enhancement in dye-sensitized photovoltaic devices with titania–graphene composite electrodes. J. Electroanal. Chem. 2012, 683, 43–46. [Google Scholar] [CrossRef]
- Zhang, D.W.; Li, X.D.; Li, H.B.; Chen, S.; Sun, Z.; Yin, X.J.; Huang, S.M. Graphene-based counter electrode for dye-sensitized solar cells Carbon 2011, 49, 5382–5388. Carbon 2011, 49, 5382–5388. [Google Scholar] [CrossRef]
- Kim, S.R.; Parvez, M.K.; Chhowalla, M. UV-reduction of graphene oxide and its application as an interfacial layer to reduce the back-transport reactions in dye-sensitized solar cells. Chem. Phys. Lett. 2009, 483, 124–127. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Nazeeruddin, M.K.; Liska, P.; Péchy, P.; Grätzel, M. Fabrication of screen-printing pastes from TiO2 powders for dye-sensitised solar cells. Prog. in Photovolt. Res. Appl. 2007, 15, 603–612. [Google Scholar] [CrossRef]
- Agarwala, S.; Kevin, M.; Wong, A.S.W.; Peh, C.K.N.; Thavasi, V.; Ho, G.W. Mesophase ordering of TiO2 film with high surface area and strong light harvesting for dye-sensitized solar cell. ACS Appl. Mater. & Interfaces 2010, 2, 1844–1850. [Google Scholar]
- Wang, Q.; Ito, S.; Grätzel, M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J.; Bessho, T.; Imai, H. Characteristics of high efficiency dye-sensitized solar cells. J. Phys. Chem. B 2006, 110, 25210–25221. [Google Scholar] [CrossRef]
- Adachi, M.; Sakamoto, M.; Jiu, J.; Ogata, Y.; Isoda, S. Determination of parameters of electron transport in dye-sensitized solar cells using electrochemical impedance spectroscopy. J. Phys. Chem. B 2006, 110, 13872–13880. [Google Scholar] [CrossRef]
- MORA-SERO, I.V.A.N. Characterization of capacitance, transport and recombination parameters in hybrid perovskite and organic solar cells in Unconventional Thin Film Photovoltaics; The Royal Society of Chemistry: London, UK, 2016; Chapter 3; pp. 57–106. [Google Scholar]
- Bisquert, J. Theory of the impedance of charge transfer via surface states in dye-sensitized solar cells. J. Electroanal. Chem. 2010, 646, 43–51. [Google Scholar] [CrossRef]
- Bisquert, J. Theory of the impedance of electron diffusion and recombination in a thin layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef]
- Bisquert, J. Chemical capacitance of nanostructured semiconductors: Its origin and significance for nanocomposite solar cells. Phys. Chem. Chem. Phys. 2003, 5, 5360–5364. [Google Scholar] [CrossRef]
- Bisquert, J.; Zaban, A.; Greenshtein, M.; Mora-Seró, I. Determination of rate constants for charge transfer and the distribution of semiconductor and electrolyte electronic energy levels in dye-sensitized solar cells by open-circuit photovoltage decay method. J. Am. Ceram. Soc 2004, 126, 13550–13559. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Randriamahazaka, H.; Zaban, A.; Garcia-Canadas, J.; Garcia-Belmonte, G.; Bisquert, J. Chemical capacitance of nanoporous-nanocrystalline TiO2 in a room temperature ionic liquid. Phys. Chem. Chem. Phys. 2006, 8, 1827–1833. [Google Scholar] [CrossRef]
- Bisquert, J.; Mora-Seró, I. Simulation of steady-state characteristics of dye-sensitized solar cells and the interpretation of the diffusion length. J. Phys. Chem. Lett. 2010, 1, 450–456. [Google Scholar] [CrossRef]
- Agosta, R.; Giannuzzi, R.; De Marco, L.; Manca, M.; Belviso, M.R.; Cozzoli, P.D.; Gigli, G. Electrochemical assessment of the band-edge positioning in shape-tailored TiO2-nanorod-based photoelectrodes for dye solar cells. J. Phys. Chem. C 2013, 117, 2574–2583. [Google Scholar] [CrossRef][Green Version]
- Agosta, R.; Giannuzzi, R.; De Marco, L.; Manca, M.; Belviso, M.R.; Cozzoli, P.D.; Gigli, G. Valence band edge shifts and charge-transfer dynamics in Li-doped NiO based p-type DSSCs. Electrochim. Acta 2016, 188, 309–316. [Google Scholar]
- Zaban, A.; Greenshtein, M.; Bisquert, J. Determination of the electron lifetime in nanocrystalline dye solar cells by open-circuit voltage decay measurement. Chem. Phys. Chem. 2003, 4, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J.; Fabregat-Santiago, F.; Mora-Sero, I.; Garcia-Belmonte, G.; Gimenez, S. Electron lifetime in dye-sensitized solar cells: Theory and interpretation of measurements. J. Phys. Chem. C 2009, 113, 17278–17290. [Google Scholar] [CrossRef]
- Manjceevan, A.; Bandara, J. Robust surface passivation of trap sites in PbS q-dots by controlling the thickness of CdS layers in PbS/CdS quantum dot solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 157–163. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, X.; Xu, Y.; Sun, P.; Huang, N.; Sun, Y. Mixed P25 nanoparticles and large rutile particles as a top scattering layer to enhance performance of nanocrystalline TiO2 based dye-sensitized solar cell. Appl. Surf. Sci. 2015, 337, 188–194. [Google Scholar] [CrossRef]
- Ivanovska, T.; Saponjic, Z.; Radoicic, M.; Ortolani, L.; Morandi, V.; Ruani, G. Improvement of dye solar cell efficiency by photoanode post-treatment. Int. J. Photoenergy 2014, 835760. [Google Scholar]
- Ondersma, J.W.; Hamann, T.W. Impedance investigation of dye-sensitized solar cells employing outer-sphere redox shuttles. J. Phys. Chem. C 2010, 114, 638–645. [Google Scholar] [CrossRef]
- Ondersma, J.W.; Hamann, T.W. Recombination and redox couples in dye-sensitized solar cells. Coord. Chem. Rev. 2013, 257, 1533–1543. [Google Scholar] [CrossRef]


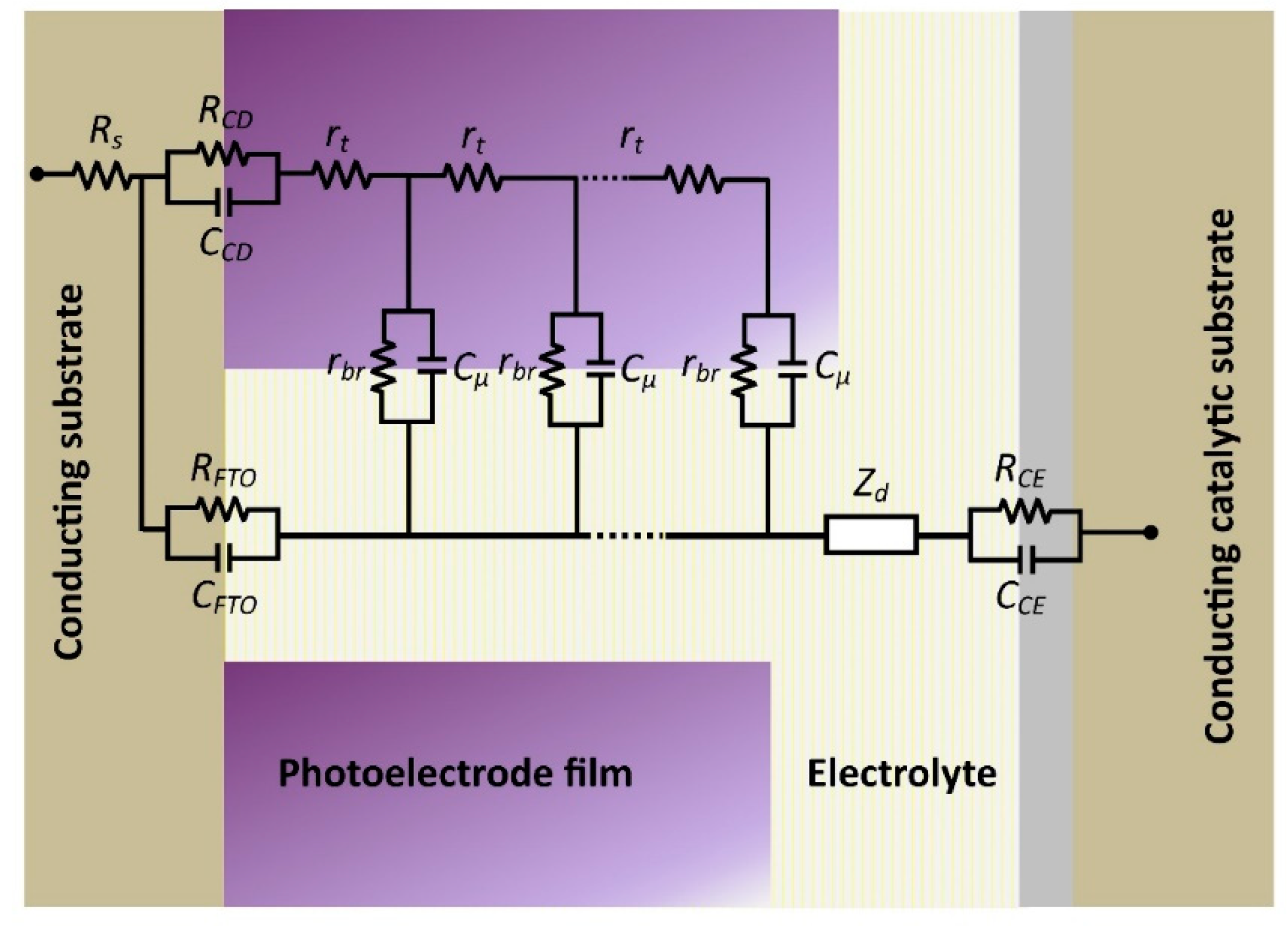
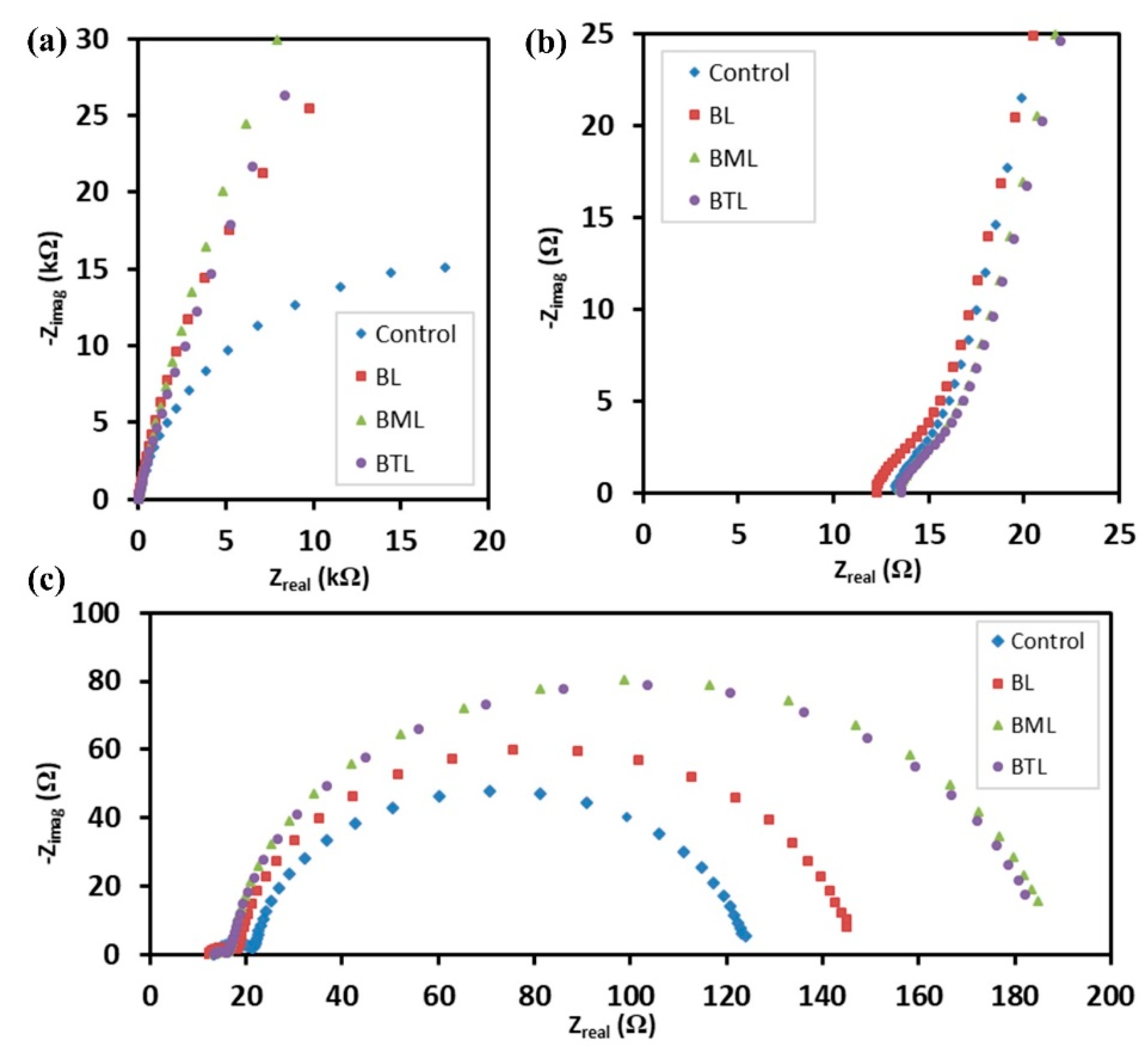
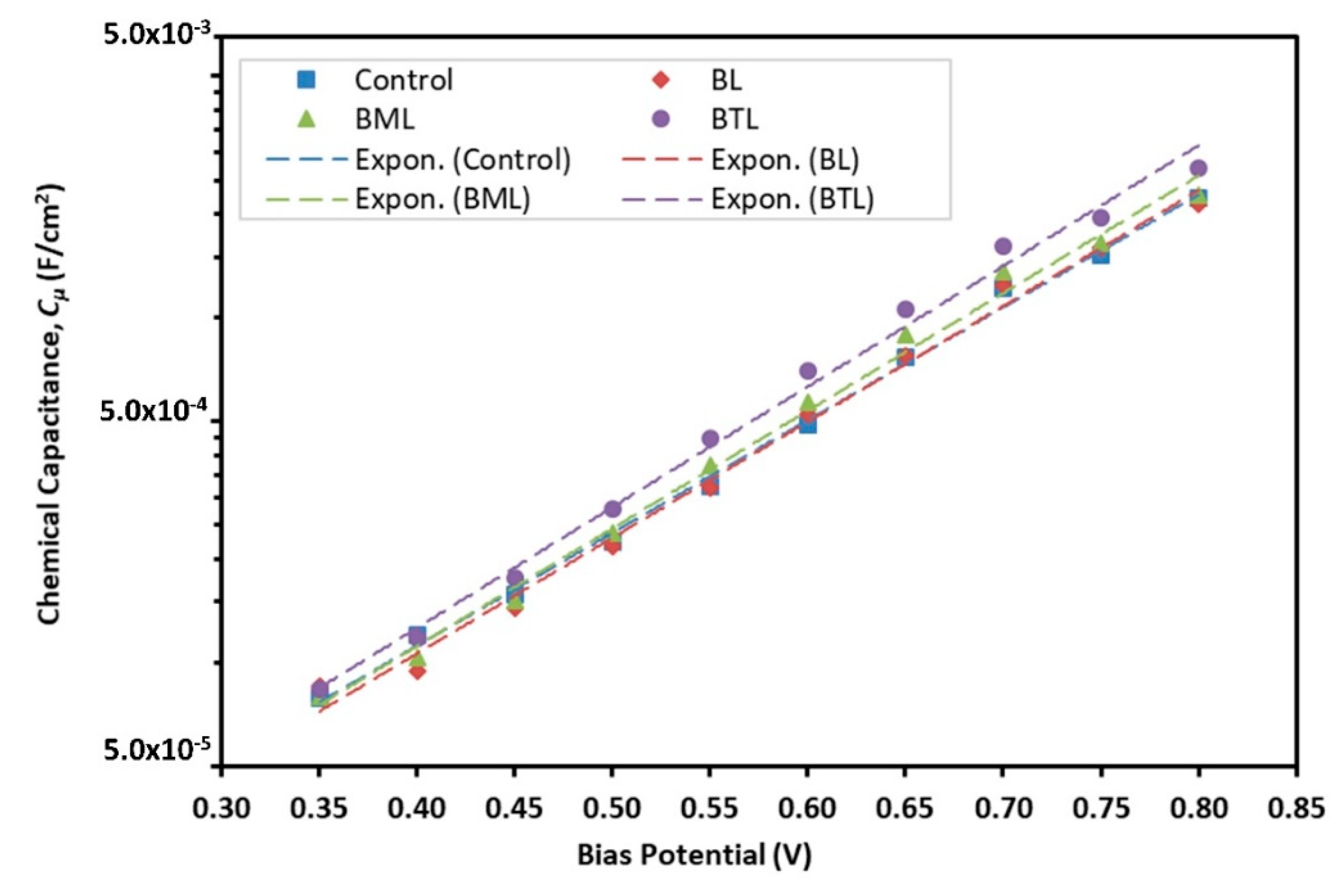
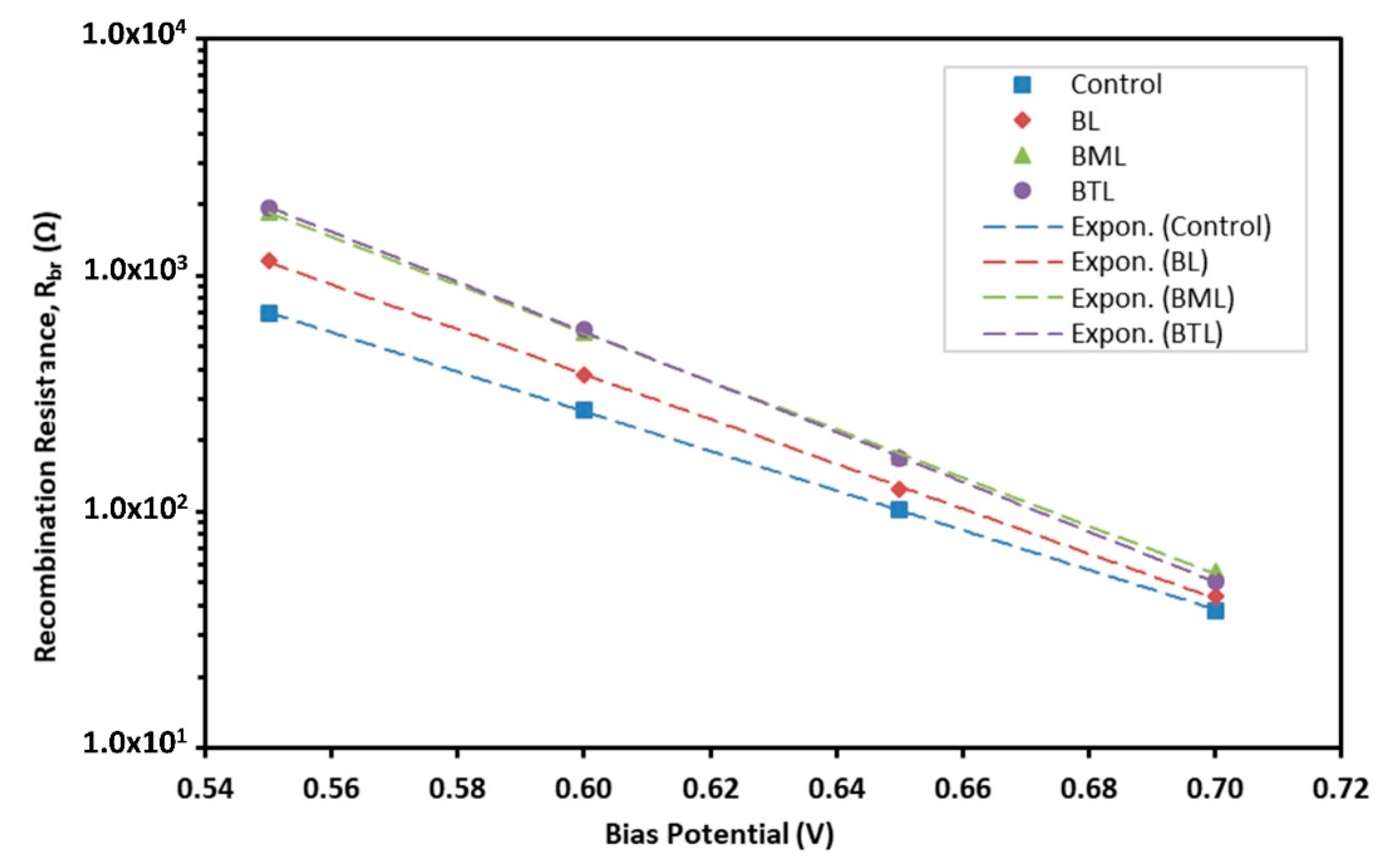
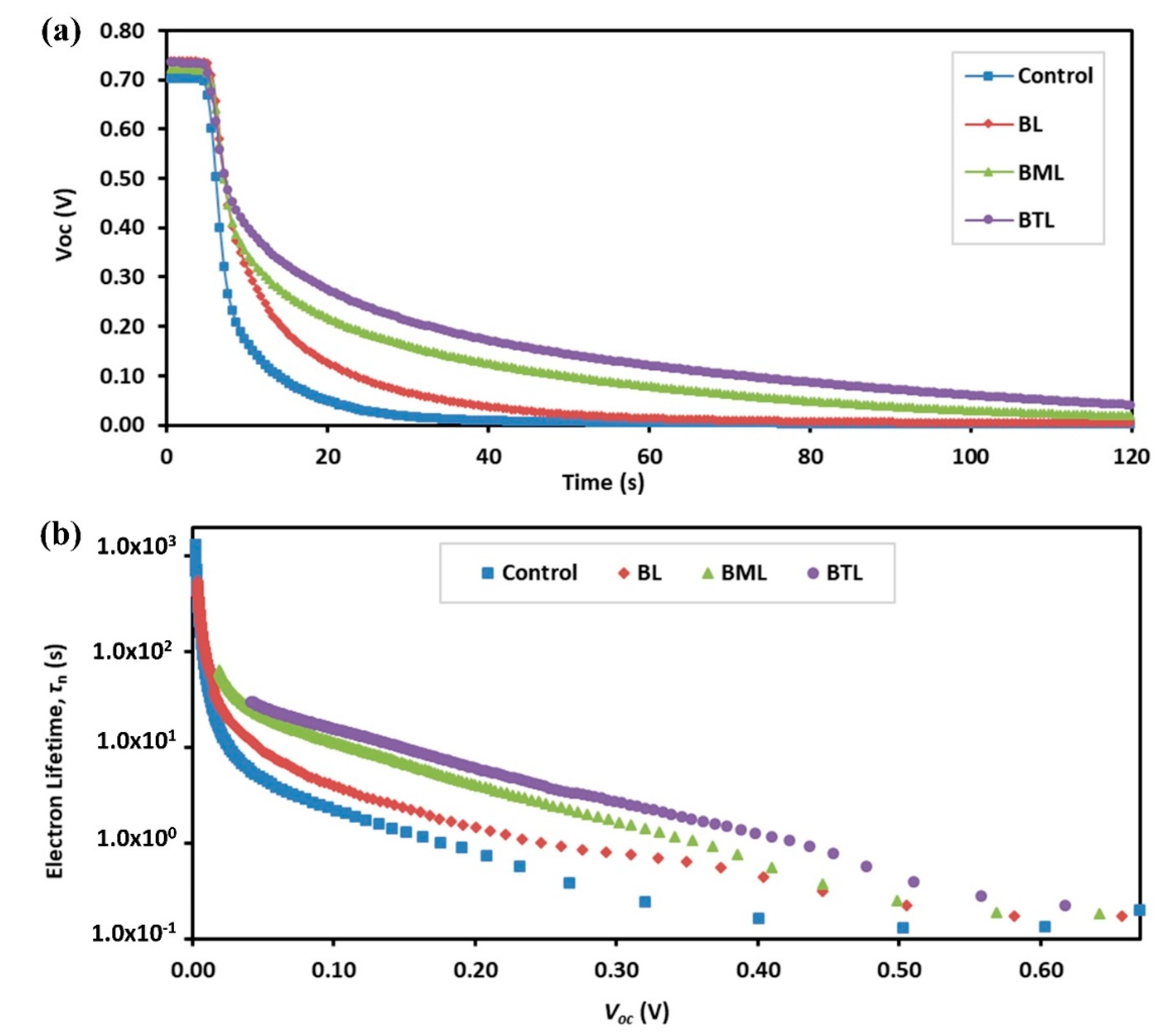
| Sample | Goodness of Fit | Rt (Ω) | Rbr (Ω) | Cµ(F∙cm−2) | τn (s) | k (s−1) | Dn (cm2∙s−1) | Con (Ω∙cm∙s−1) | ns (cm−3) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1.35 × 10−4 | 3.455 | 102.1 | 7.61 × 10−4 | 0.078 | 12.88 | 3.81 × 10−6 | 1.512 | 1.07 × 1017 |
| BL | 1.28 × 10−4 | 4.833 | 123.7 | 8.21 × 10−4 | 0.102 | 9.84 | 2.52 × 10−6 | 1.400 | 1.15 × 1017 |
| BML | 1.50 × 10−4 | 4.749 | 170.4 | 8.89 × 10−4 | 0.152 | 6.60 | 2.37 × 10−6 | 1.293 | 1.25 × 1017 |
| BTL | 1.40 × 10−4 | 5.033 | 168.4 | 1.06 × 10−3 | 0.178 | 5.63 | 1.88 × 10−6 | 1.090 | 1.48 × 1017 |
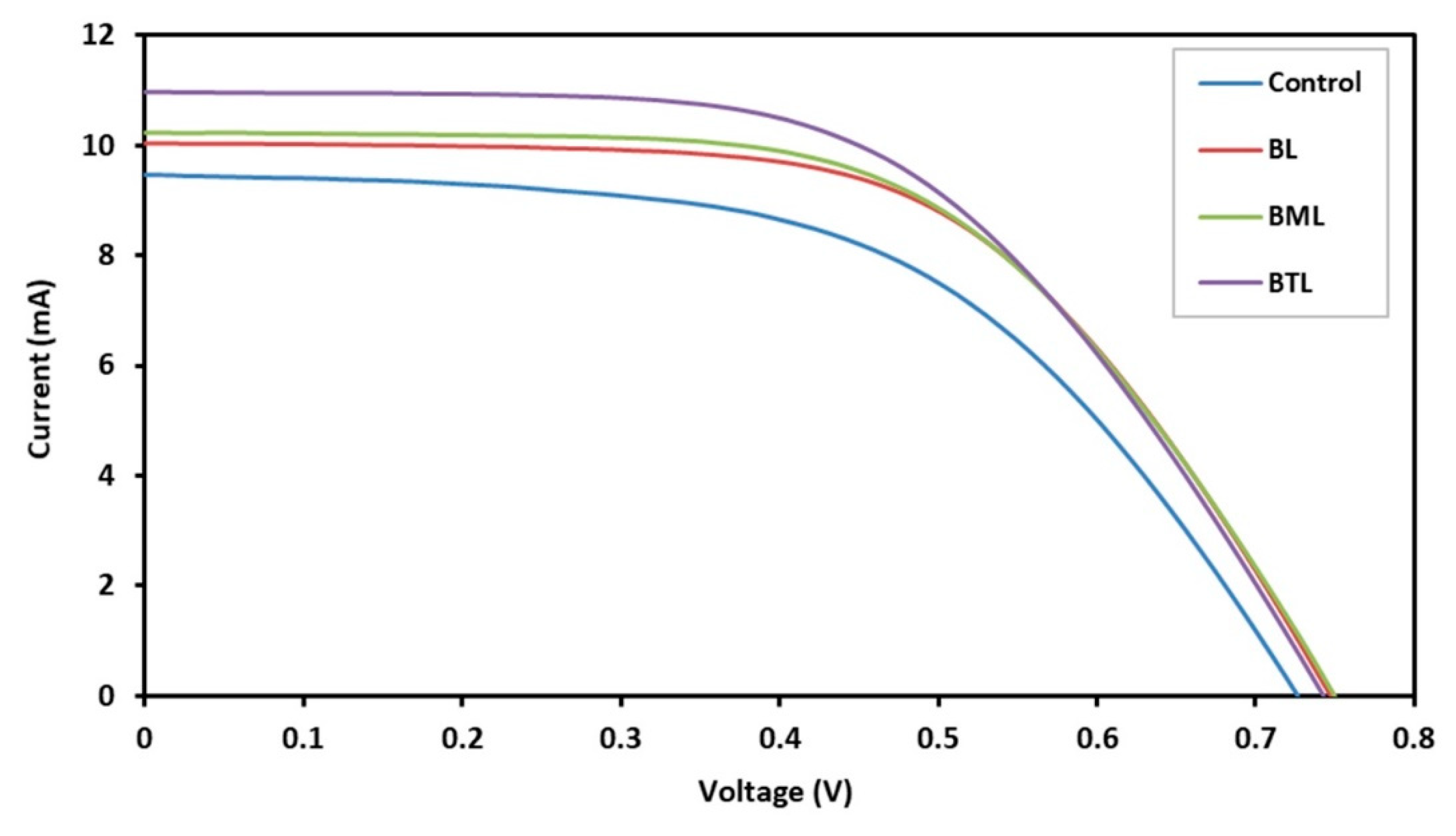
| Sample | Isc (mA) | Voc (V) | FF | 𝜂 (%) |
|---|---|---|---|---|
| Control | 9.458 | 0.728 | 0.546 | 3.756 |
| BL | 10.042 | 0.748 | 0.587 | 4.408 |
| BML | 10.217 | 0.751 | 0.577 | 4.425 |
| BTL | 10.965 | 0.743 | 0.562 | 4.581 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaine, S.N.A.; Mohamed, N.M.; Khatani, M.; Samsudin, A.E.; Shahid, M.U. Trap State and Charge Recombination in Nanocrystalline Passivized Conductive and Photoelectrode Interface of Dye-Sensitized Solar Cell. Coatings 2020, 10, 284. https://doi.org/10.3390/coatings10030284
Zaine SNA, Mohamed NM, Khatani M, Samsudin AE, Shahid MU. Trap State and Charge Recombination in Nanocrystalline Passivized Conductive and Photoelectrode Interface of Dye-Sensitized Solar Cell. Coatings. 2020; 10(3):284. https://doi.org/10.3390/coatings10030284
Chicago/Turabian StyleZaine, Siti Nur Azella, Norani Muti Mohamed, Mehboob Khatani, Adel Eskandar Samsudin, and Muhammad Umair Shahid. 2020. "Trap State and Charge Recombination in Nanocrystalline Passivized Conductive and Photoelectrode Interface of Dye-Sensitized Solar Cell" Coatings 10, no. 3: 284. https://doi.org/10.3390/coatings10030284
APA StyleZaine, S. N. A., Mohamed, N. M., Khatani, M., Samsudin, A. E., & Shahid, M. U. (2020). Trap State and Charge Recombination in Nanocrystalline Passivized Conductive and Photoelectrode Interface of Dye-Sensitized Solar Cell. Coatings, 10(3), 284. https://doi.org/10.3390/coatings10030284




