Corrosion and Wear Behavior of PEO Coatings on D16T Aluminum Alloy with Different Concentrations of Graphene
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of the Coatings
2.3. Characterization of the Coatings
2.4. Electrochemical Studies of the Coatings
2.5. Weight Loss Measurements
2.6. Wear Studies of the Coatings
3. Results and Discussion
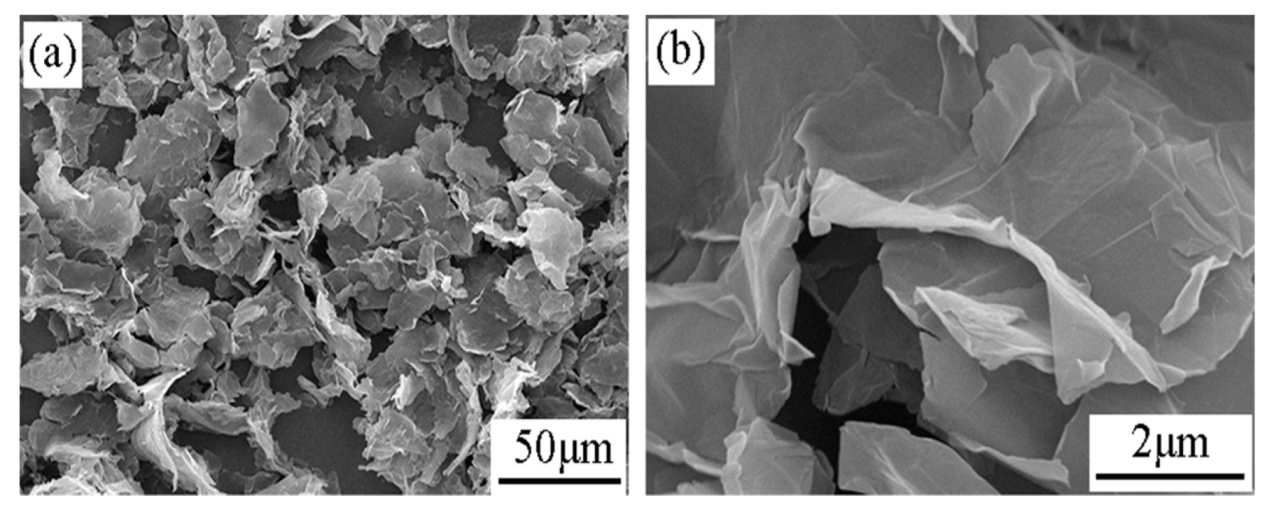
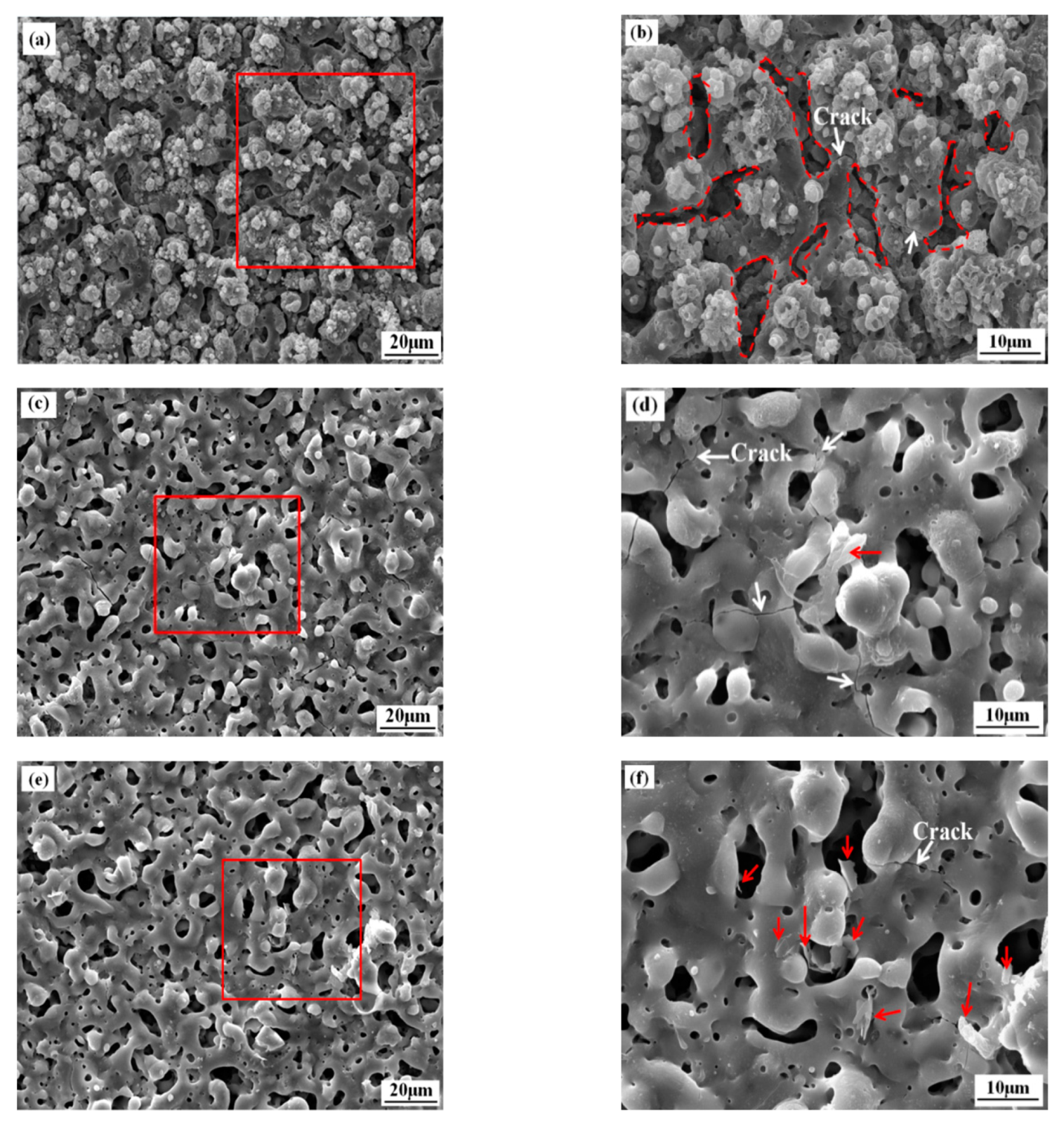
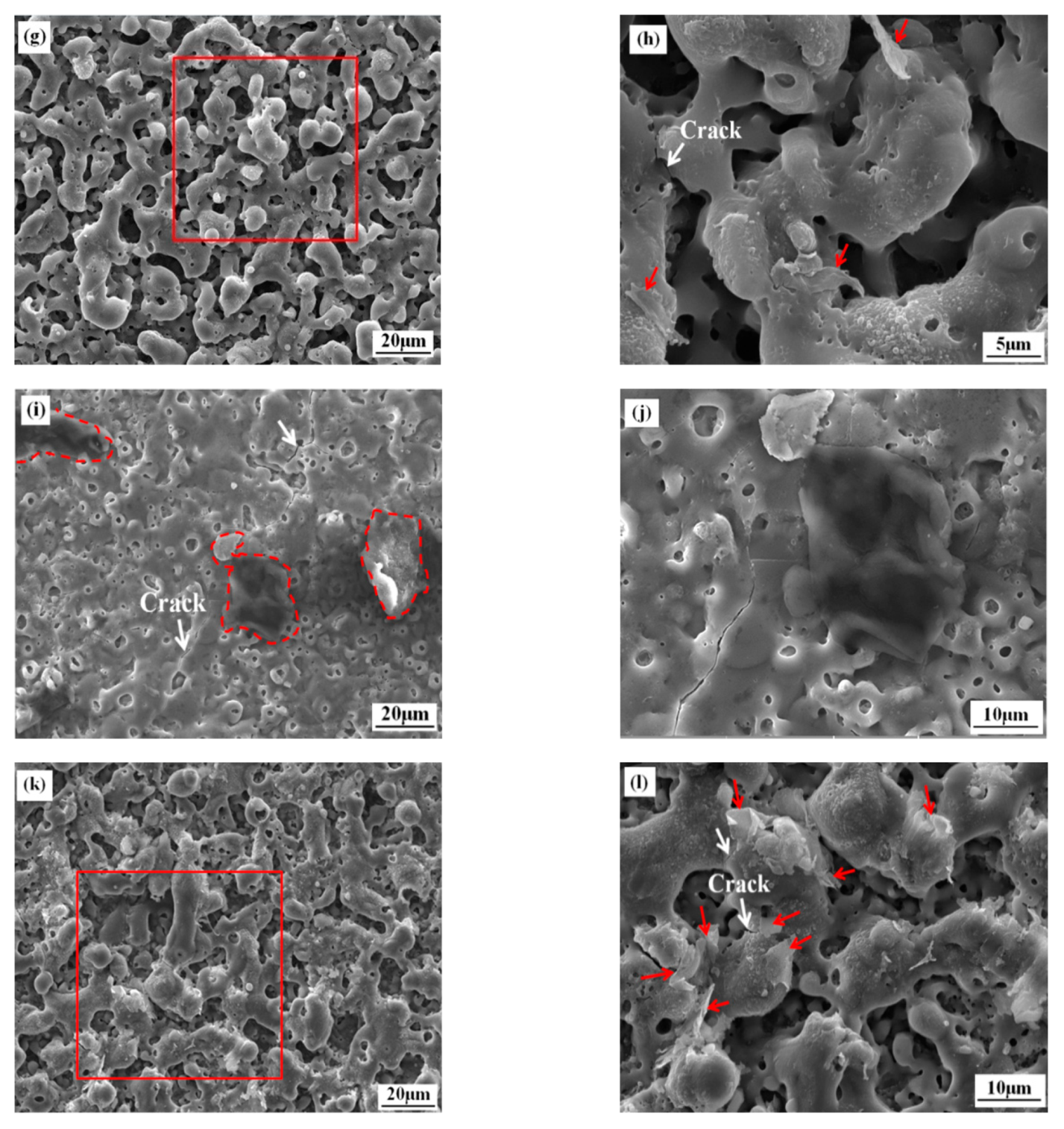
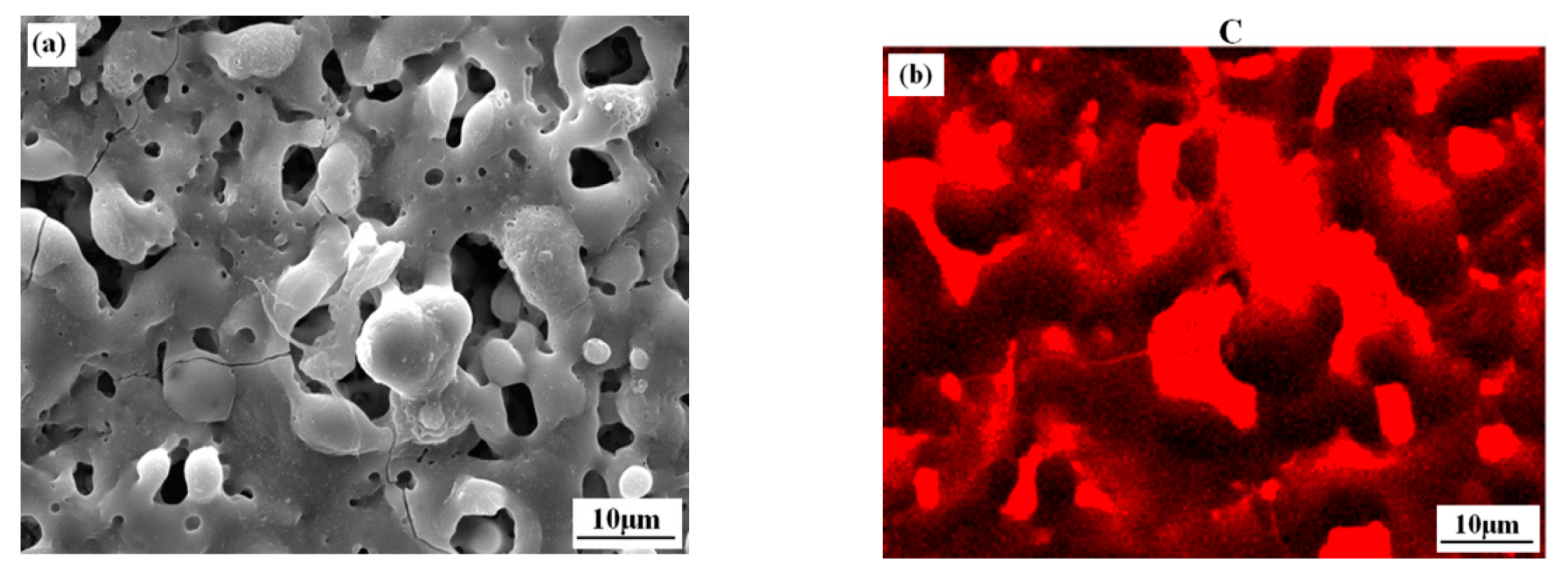
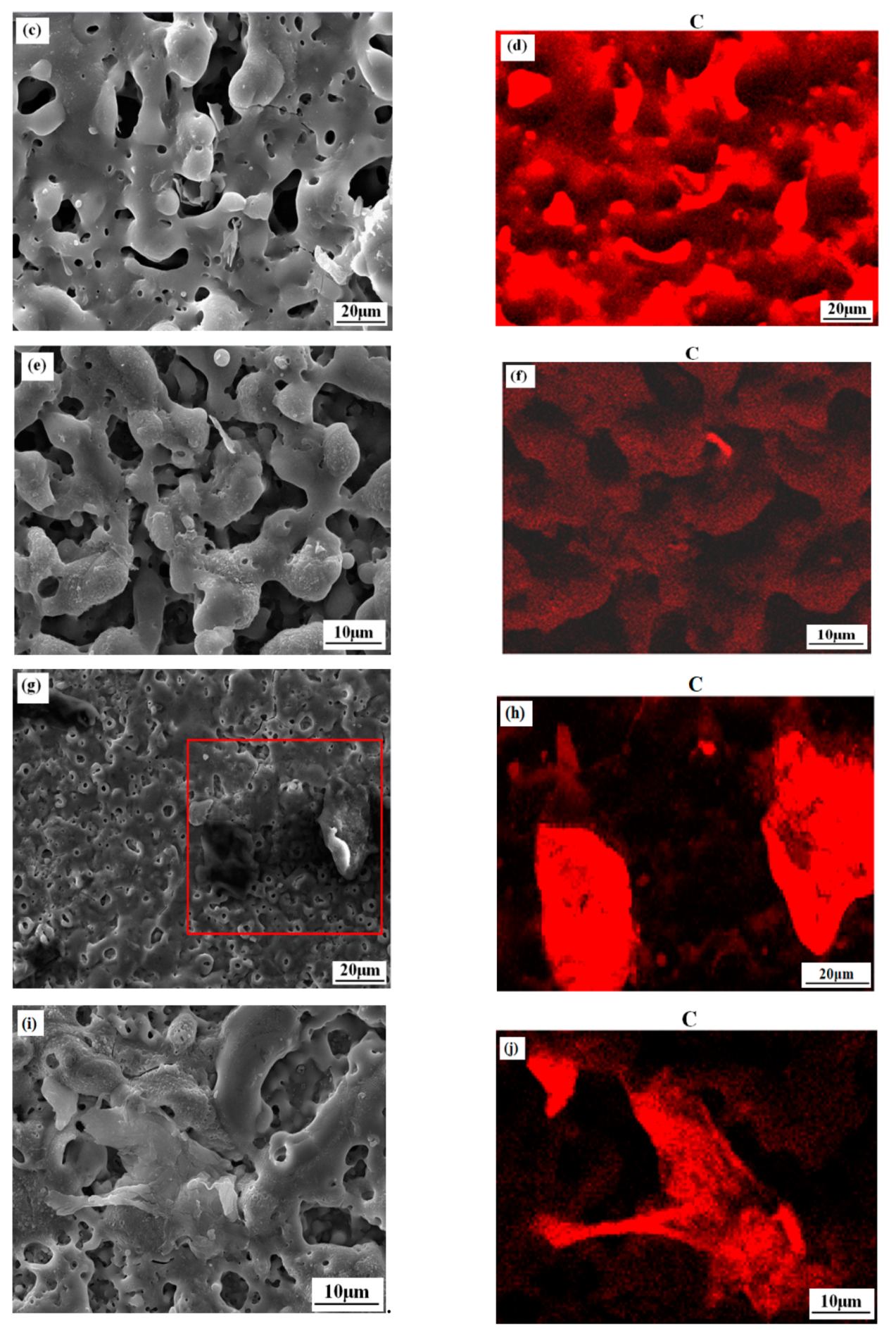
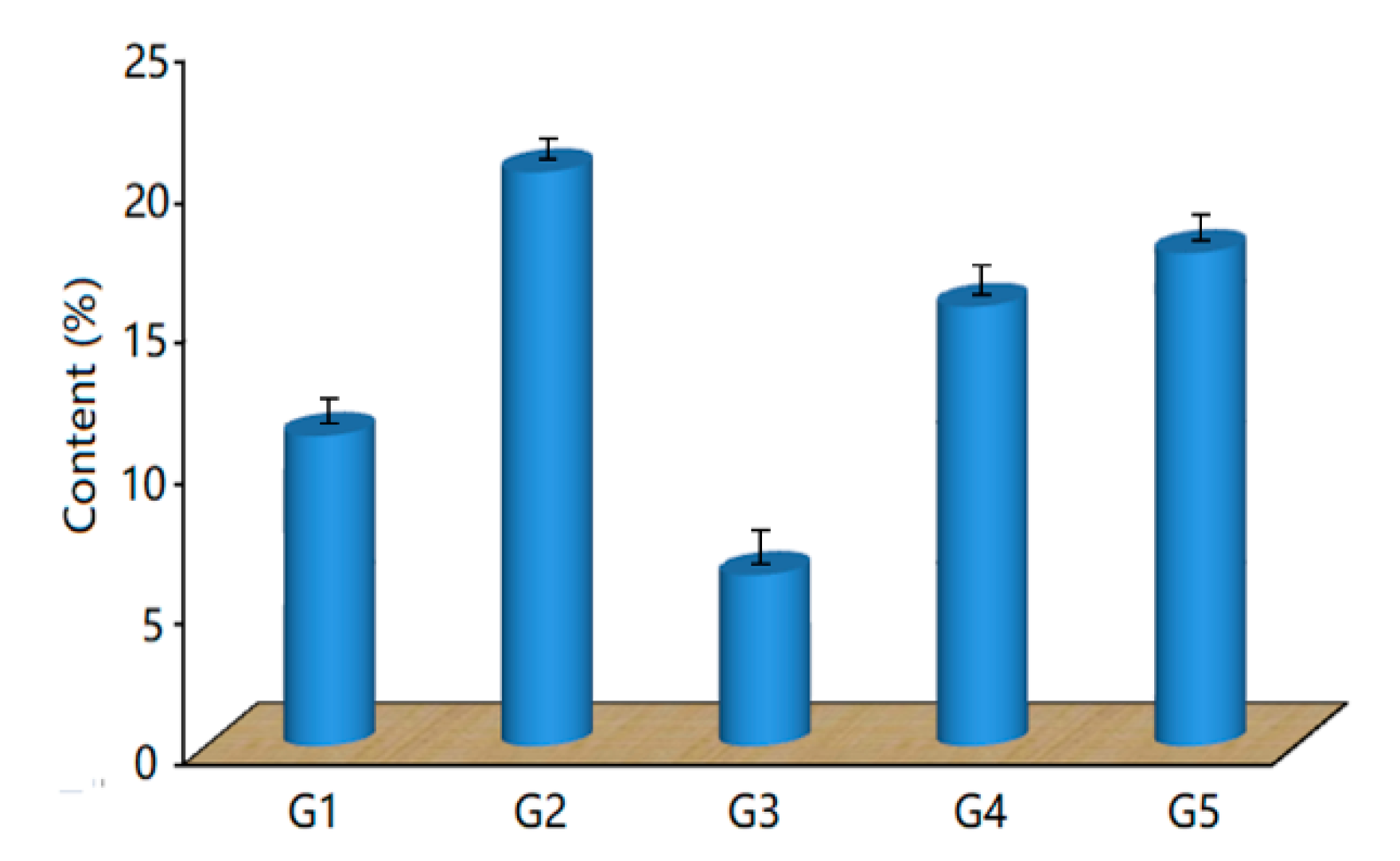
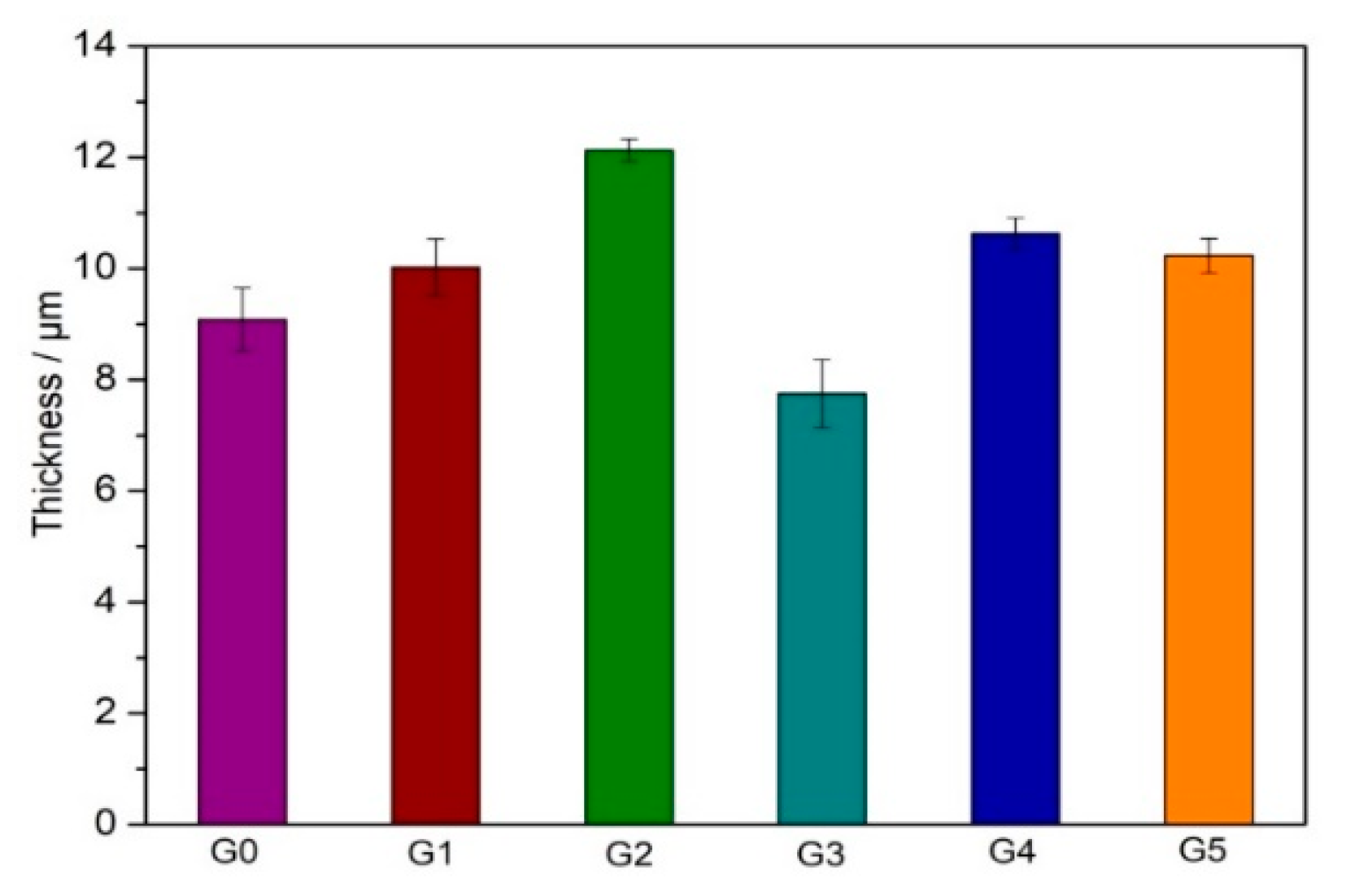
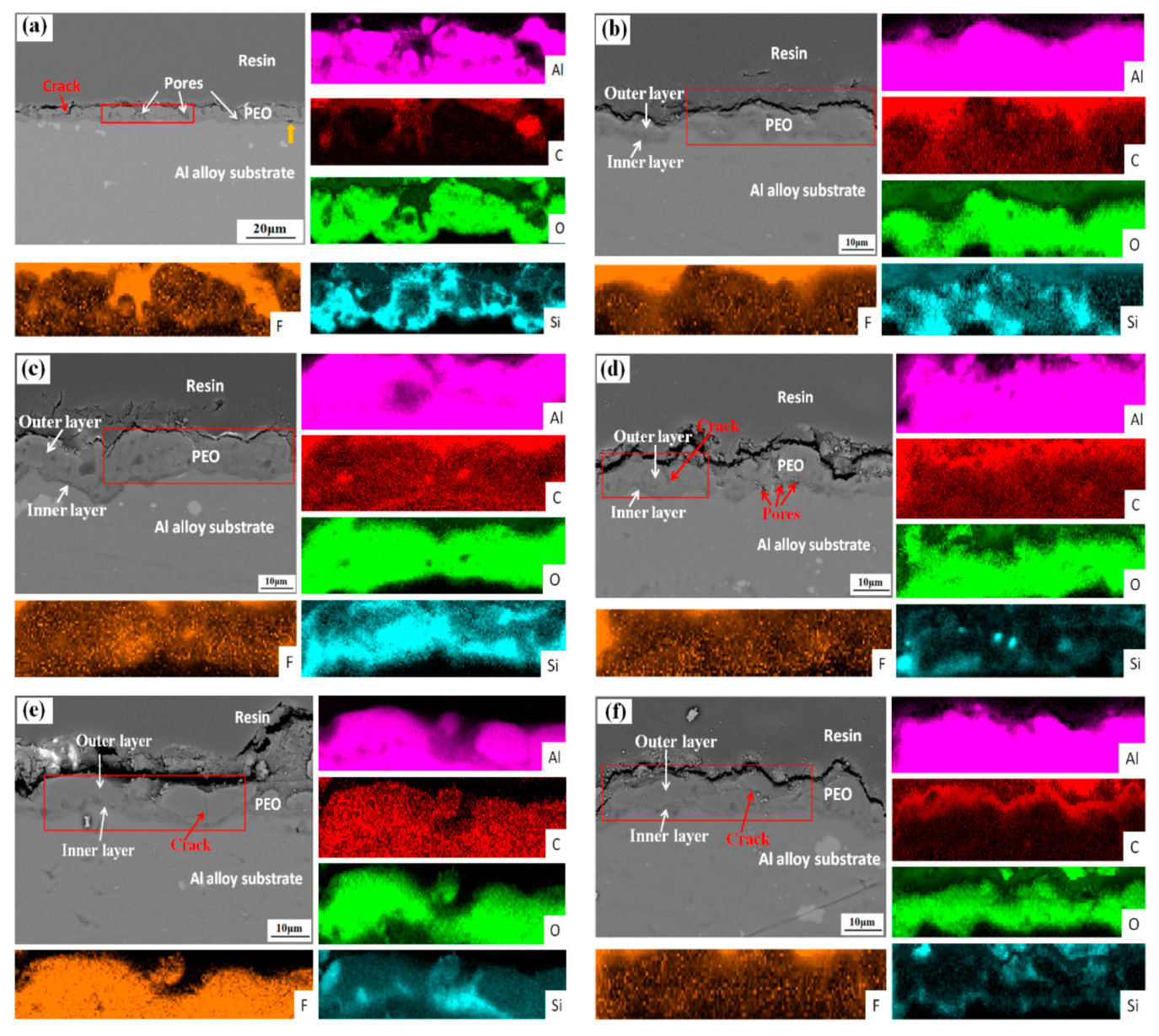
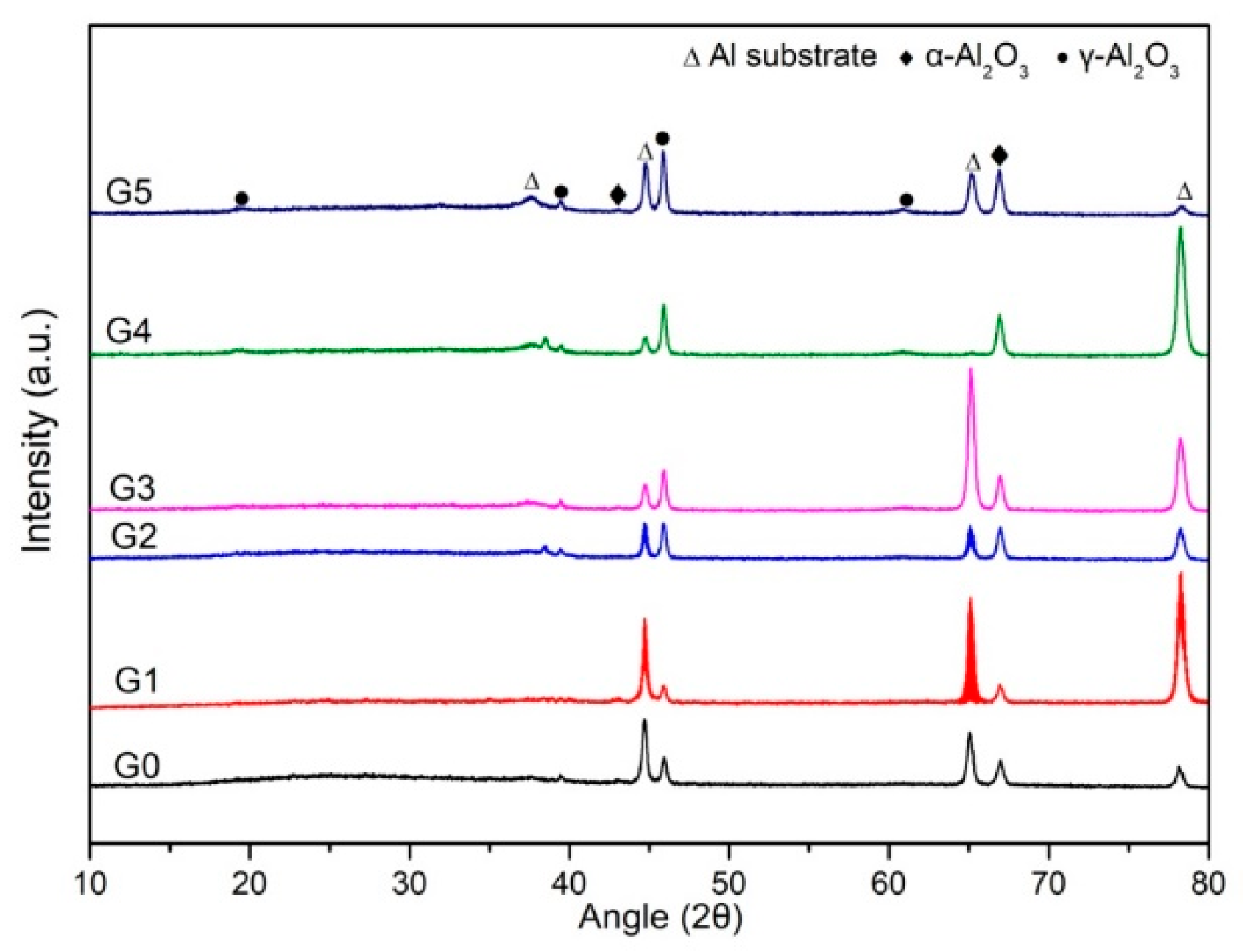
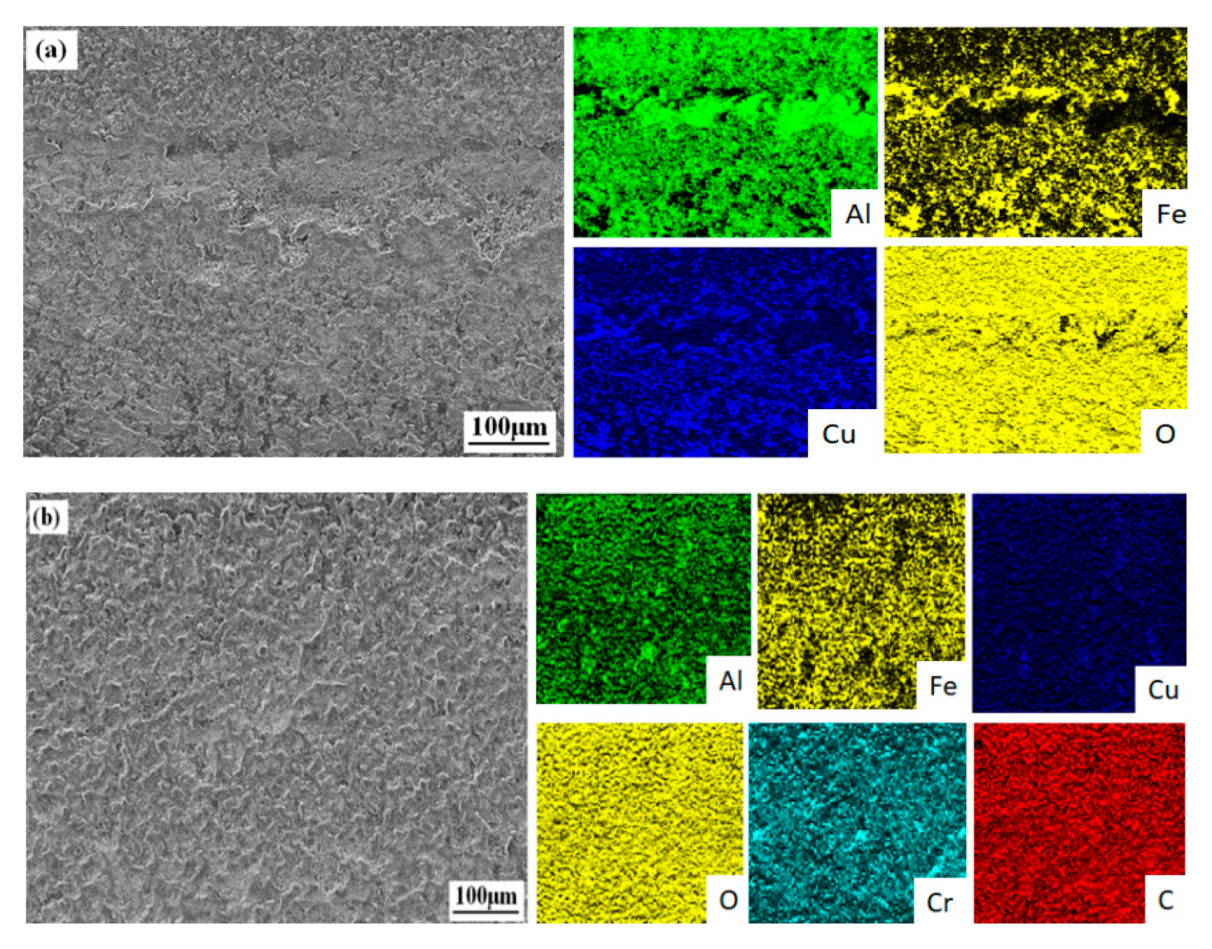
3.1. Microstructure and Composition of the Coatings
3.2. Phase Composition of the Coatings
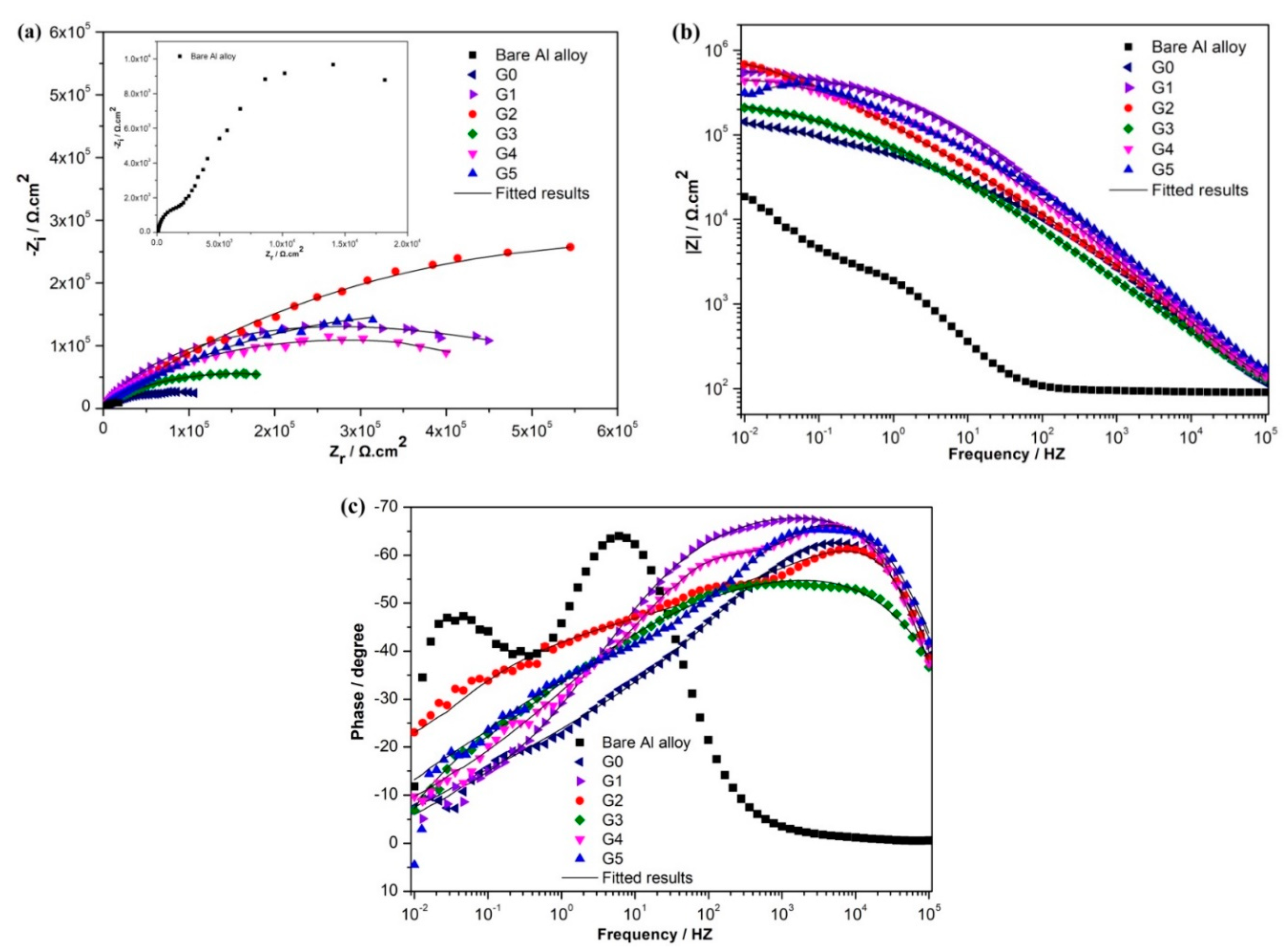
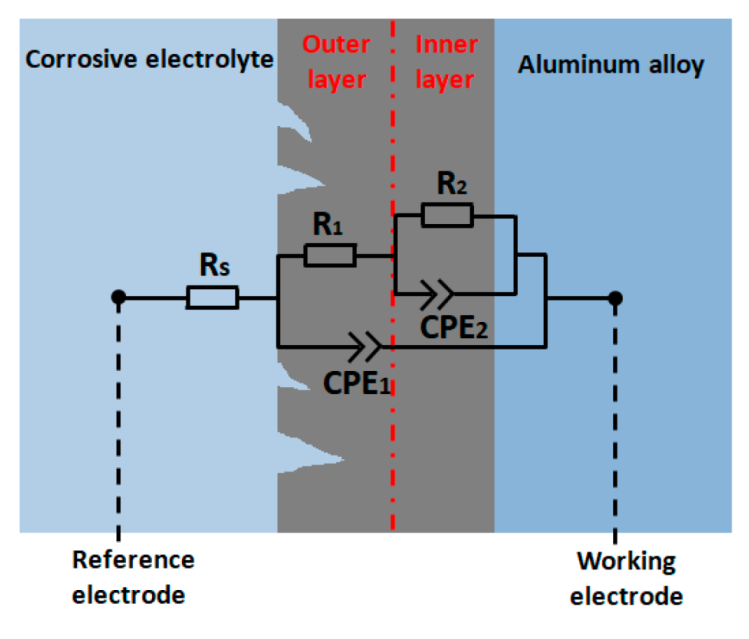
3.3. Electrochemical Behavior of the Coatings
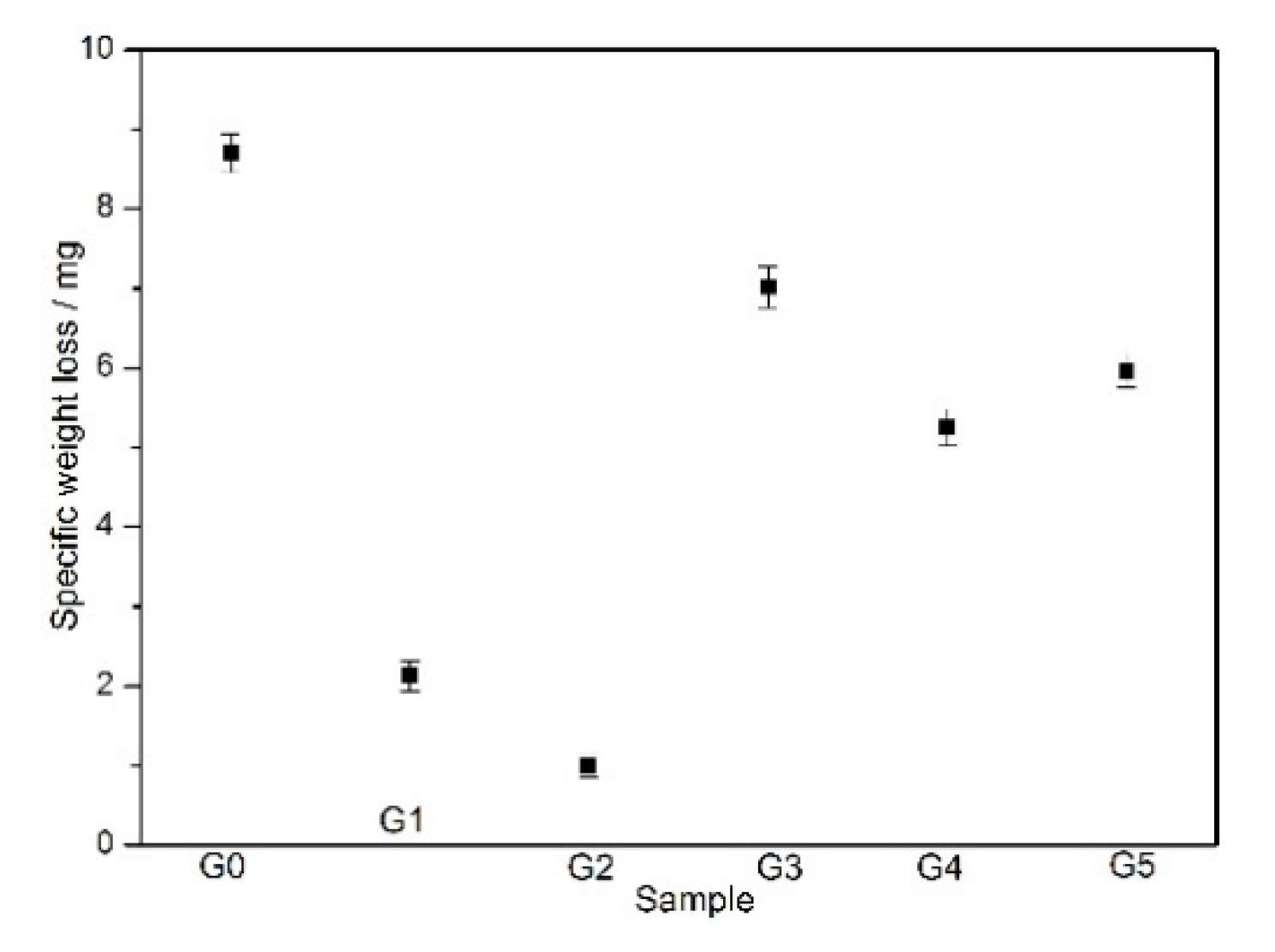
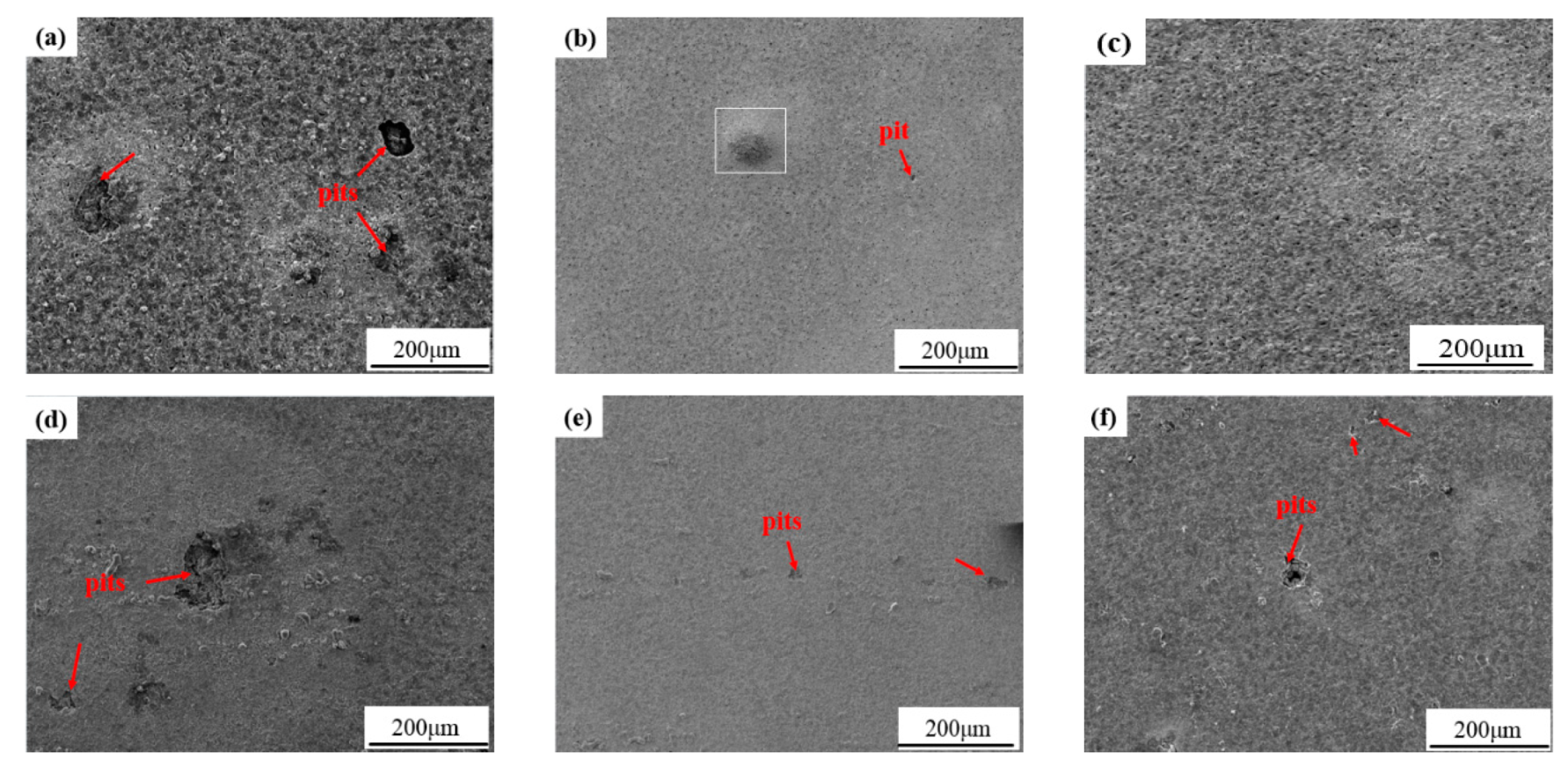
3.4. Weight Loss Measurements of the Coatings
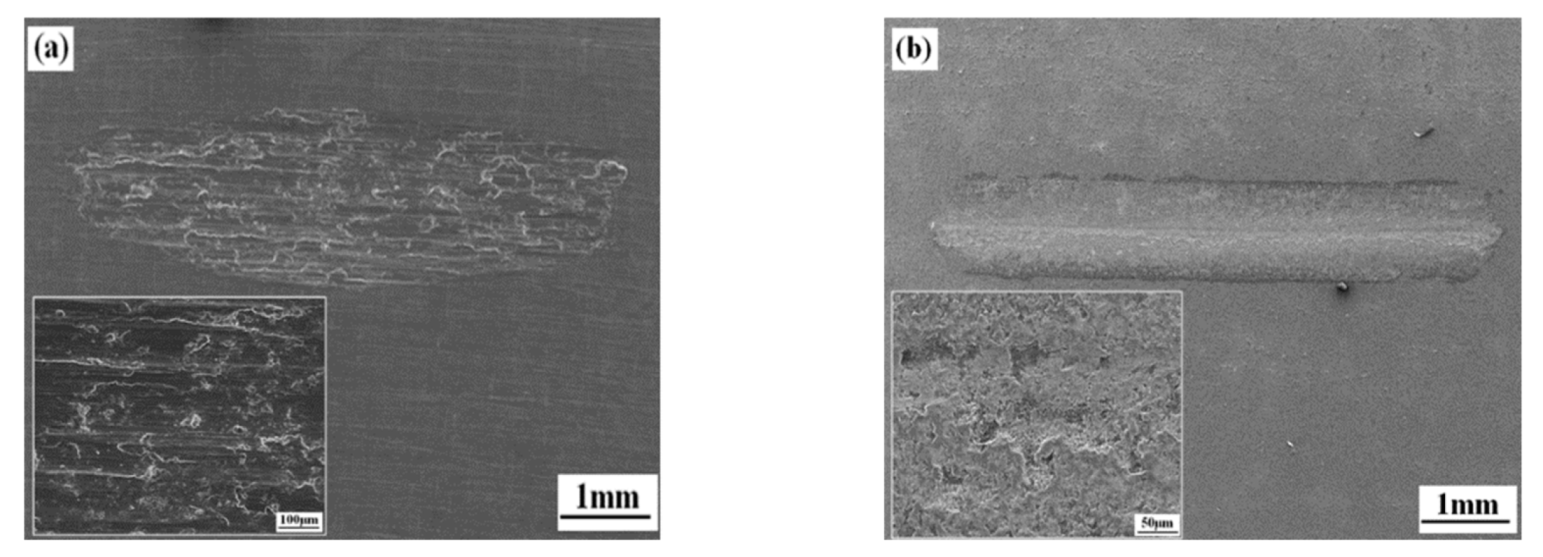
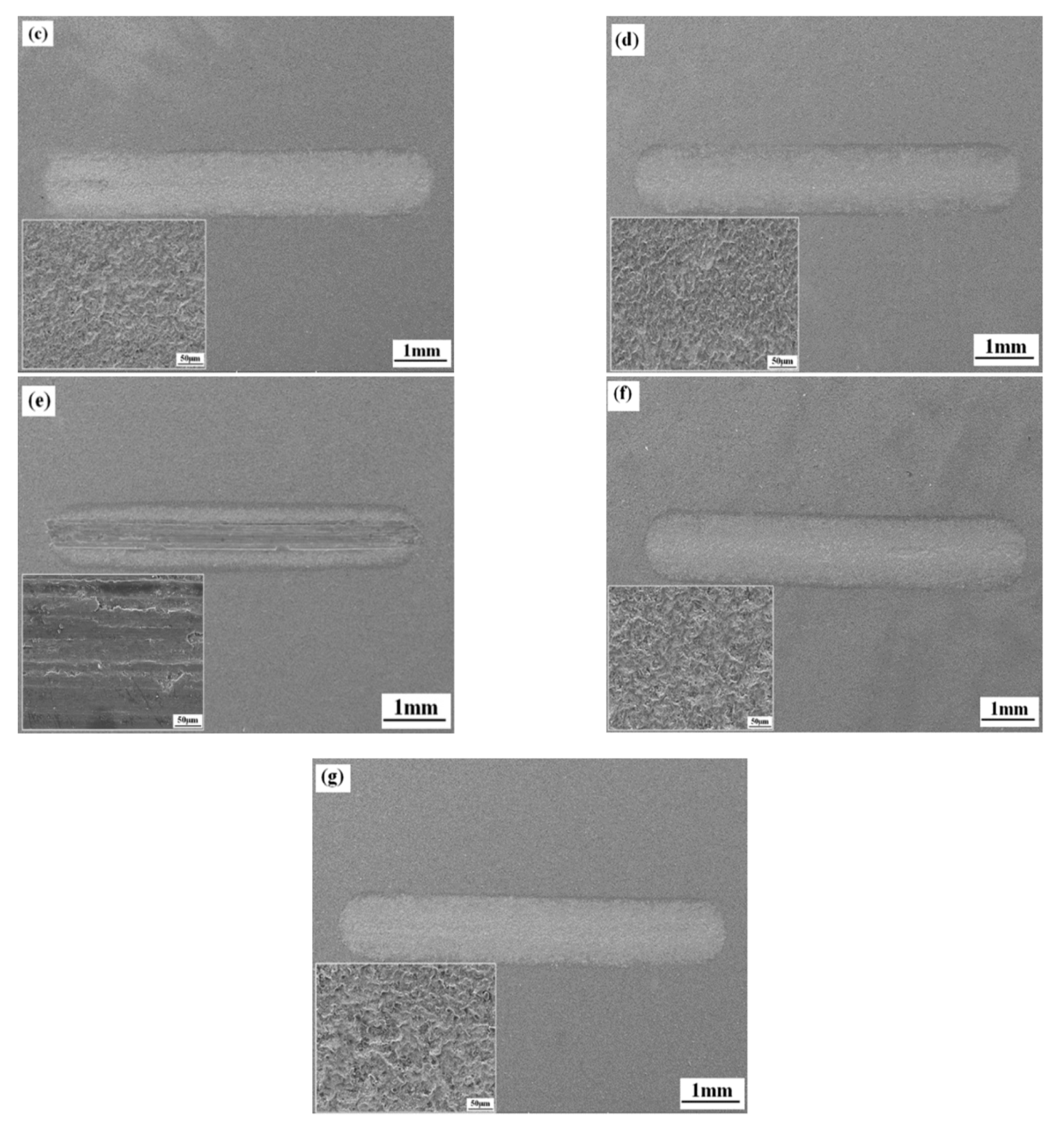
3.5. Wear Properties of the Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolescu, A.; Fisher, J.W.; Haq, M.A.; Bailey, T.F.; Lazarev, A. Drilling intervention-milling and fishing of high strength aluminum drill pipe. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 14–17 February 2012. [Google Scholar]
- Vakhrushev, A.V.; Gelfgat, M.Y.; Basovich, D.; Buyanovskiy, I.N. Aluminum drillpipe in an arcitic application-A well-known tool changing the development strategy. In Proceedings of the SPE Arctic and Extreme Environments Conference and Exhibition, Moscow, Russia, 15–17 October 2011. [Google Scholar]
- Xue, W.B.; Wu, X.L.; Li, X.J.; Tian, H. Anti-corrosion film on 2024/SiC aluminum matrix composite fabricated by microarc oxidation in silicate electrolyte. J. Alloy. Compd. 2006, 425, 302–306. [Google Scholar] [CrossRef]
- Nan, X.; Song, R.G.; Zhang, J.J.; Song, R.X.; Lu, X.Y.; Su, X.P. Effects of current density on microstructure and properties of plasma electrolyte oxidation ceramic coatings formed on 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 806–813. [Google Scholar]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G.L. Anodizing treatments... alloys and their effect on corrosion resistance in various environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Lee, K.M.; Ko, Y.G.; Shin, D.H. Incorporation of carbon nanotubes into micro-coatings film formed on aluminum alloy via plasma electrolytic oxidation. Matter. Lett. 2011, 65, 2269–2273. [Google Scholar] [CrossRef]
- Shin, K.R.; Ko, Y.G.; Shin, D.H. Effect of electrolyte on surface properties of pure titanium coated by plasma electrolytic oxidation. J. Alloy. Compd. 2011, 509S, S478–S481. [Google Scholar] [CrossRef]
- Arrabal, R.; Mohedano, M.; Matykina, E.; Pardo, A.; Mingo, B.; Merino, M.C. Characterization and wear behavior of PEO coatings on 6082-T6 aluminum alloy with incorporated α-Al2O3 particles. Surf. Coat. Technol. 2015, 269, 64–73. [Google Scholar] [CrossRef]
- Tang, M.Q.; Li, W.P.; Liu, H.C.; Zhu, L.Q. Influence of titania sol in the electrolyte on characteristics of the microarc oxidation coating formed on 2A70 aluminum alloy. Suf. Coat. Technol. 2011, 205, 4135–4140. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium. Electrochim. Acta 2009, 54, 6767–6778. [Google Scholar] [CrossRef]
- Jin, F.Y.; Chu, P.K.; Tong, H.H.; Zhao, J. Improvement of surface porosity and properties of alumina films by incorporation of Fe micrograins in micro-arc oxidation. Appl. Surf. Sci. 2006, 253, 863–868. [Google Scholar] [CrossRef]
- Sarbishei, S.; Sani, M.A.F.; Mohammadi, M.R. Effects of alumina nanoparticles concentration on microstructure and corrosion behavior of coatings formed on titanium substrate via PEO process. Ceram. Int. 2016, 42, 8789–8797. [Google Scholar] [CrossRef]
- Fatimah, S.; Kamil, M.P.; Kwon, J.H.; Kaseem, M.; Ko, Y.G. Dural incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloy. Compd. 2017, 707, 358–364. [Google Scholar] [CrossRef]
- Yazdani, B.; Xu, F.; Ahmad, I.; Hou, X.H.; Xia, Y.D.; Zhu, Y.Q. Tribological performance of graphene/carbon nanotube hybrid reinforced Al2O3. Compos. Sci. Rep. 2005, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Hvizdos, P.; Dusza, J.; Balazsi, C. Tribological properties of Si3N4-graphene nanocomposites. J. Eur. Ceram. Soc. 2013, 33, 2359–2364. [Google Scholar] [CrossRef]
- Belmonte, M.; Ramírez, C.; González-Julián, J.; Schneider, J.; Miranzo, P.; Osendi, M.I. The beneficial effect of graphene nanofillers on the tribological performance of ceramics. Carbon 2013, 61, 431–435. [Google Scholar] [CrossRef]
- Pezzato, L.; Angelini, V.; Brunelli, K.; Martini, C.; Dabala, M. Tribological and corrosion behavior of PEO coatings with graphite nanoparticles on AZ91 and AZ80 magnesium alloys. Trans. Nonferrous Met. Soc. China 2018, 28, 259–272. [Google Scholar] [CrossRef]
- Lv, G.H.; Chen, H.; Gu, W.C.; Feng, W.R.; Li, L.; Niu, E.W.; Zhang, X.H.; Yang, S.Z. Effects of graphite additives in the electrolytes on the microstructure and corrosion resistance of alumina PEO coatings. Curr. Appl. Phys. 2009, 9, 324–328. [Google Scholar] [CrossRef]
- Zhao, J.M.; Xie, X.; Zhang, C. Effect of the graphene oxide additive on the corrosion resistance of the plasma electrolytic oxidation coating of the AZ31 magnesium alloy. Corros. Sci. 2017, 114, 146–155. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, Z.; Xin, S.; Wu, X. Electrochimical impedance spectroscopy of ceramic coatings on Ti-6Al-4V by micro-plasma oxidation. Electrochim. Acta 2005, 50, 3273–3279. [Google Scholar] [CrossRef]
- Lu, X.P.; Blawert, C.; Kainer, K.U.; Zheludkevich, M.L. Investigation of the formation mechanisms of plasma electrolytic oxidation coatings on Mg alloy AM50 using particles. Electrochim. Acta 2016, 196, 680–691. [Google Scholar] [CrossRef]
- Dai, L.B.; Li, W.F.; Zhang, G.G.; Fu, N.Q.; Duan, Q. Anti-corrosion and wear properties of plasma electrolytic oxidation coating formed on high Si content Al alloy by sectionalized oxidation mode. Mater. Sci. Eng. 2017, 167, 012063. [Google Scholar] [CrossRef]
- Han, B.; Yang, Y.; Huang, Z.; You, L.; Huang, H.; Wang, K. A composite anodic coating containing graphene on AZ31 magnesium alloy. Int. J. Electrochem. Sci. 2017, 12, 9829–9843. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Lin, Y.; Zhang, Z.; Feng, S.; Talha, M.; Shi, Y.; Shi, T. Effects of graphene on structure and corrosion resistance of plasma electrolytic oxidation coatings formed on D16T Al alloy. Appl. Surf. Sci. 2019, 475, 645–659. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Fan, Z.; Yan, J.; Wei, T. Preparation of graphene nanosheet/alumina composites by spark plasma Sintering. Mater. Res. Bull. 2011, 46, 315–318. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.Y.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Liu, W.; Blawert, C.; Zheludkevich, M.L.; Lin, Y.; Talha, M.; Shi, Y.; Chen, L. Effects of graphene nanosheets on the ceramic coatings formed on Ti6Al4V alloy drill pipe by plasma electrolytic oxidation. J. Alloy. Compd. 2019, 789, 996–1007. [Google Scholar] [CrossRef]
- Wei, T.B.; Yan, F.Y.; Tian, J. Characterization and wear- and corrosion-resistance of microarc oxidation ceramic coatings on aluminum alloy. J. Alloy. Compd. 2005, 389, 169–176. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Wong, M.H.; Cheng, F.T.; Man, H.C. Characterization and corrosion studies of fluoride conversion coating on degradable Mg implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]
- Du, N.; Wang, S.X.; Zhao, Q.; Zhu, W.H. Microstructure and tribological properties of microarc oxidation composite coating containing Cr2O3 particles on TC4 titanium alloy. Rare Met. Mater. Eng. 2013, 42, 621–624. (In Chinese) [Google Scholar]
- Erarslan, Y. Wear performance of in-situ aluminum matrix composite after micro-arc oxidation. Trans. Nonferrous Met. Soc. China 2013, 23, 347–352. [Google Scholar] [CrossRef]
- Bian, D.; Aradhyula, T.V.; Guo, Y.X.; Zhao, Y.W. Improving tribological performance of chemically bonded phosphate ceramic coatings reinforced by graphene nano-platelets. Ceram. Int. 2017, 43, 12466–12471. [Google Scholar] [CrossRef]
- Feng, C.J.; Hu, S.L.; Jiang, Y.F.; Zhou, Y. Effects of micro-arc oxidation of Ti6Al4V alloy on adhesion property to electroless Ni-P-ZrO2 composite platings and their wear resistance. Rare Met. Mater. Eng. 2013, 42, 2427–2432. [Google Scholar]
- Kaseem, M.; Kamil, M.P.; Kwon, J.H.; Ko, Y.G. Effect of sodium benzoate on corrosion behavior of 6061 Al alloy processed by plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 283, 268–273. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.J.; Li, S.Y.; Wang, Y.M.; Han, Z.W.; Ren, L.Q. Fabrication of a superhydrophobic graphene surface with excellent mechanical abrasion and corrosion resistance on an aluminum alloy substrate. RSC Adv. 2014, 4, 45389–45396. [Google Scholar] [CrossRef]
- Ma, Y.; Di, H.H.; Yu, Z.X.; Liang, L.; Lv, L.; Pan, Y.; Zhang, Y.Y.; Yin, D. Fabrication of silica-decorated graphene oxide nanohybrids and the properties of composite epoxy coatings research. Appli. Surf. Sci. 2016, 360, 936–945. [Google Scholar] [CrossRef]
- Richard Prabakar, S.J.; Hwang, Y.H.; Bae, E.G.; Lee, D.K.; Pyo, M. Graphene oxide as a corrosion inhibitor for the aluminum current collector in lithium ion batteries. Carbon 2013, 52, 128–136. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Adawy, A.I.; Abbas, M.A.; Zakaria, K. New Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic medium: Weight loss, electrochemical and SEM characterization techniques. Res. Chem. Intermed. 2016, 42, 3385–3411. [Google Scholar] [CrossRef]
- Dennis, R.V.; Robert, V.D.; Lasantha, T.V.; Anil, V.; Tapan, K.R.; Sarbajit, B. Graphene nanocomposite coatings for protecting low-alloy steels from corrosion. Am. Ceram. Soc. Bull. 2013, 92, 18–24. [Google Scholar]
- Krishnamurthy, A.; Gadhamshetty, V.; Mukherjee, R.; Chen, Z.; Ren, W.; Cheng, H.M.; Koratkar, N. Passivation of microbial corrosion using a graphene coating. Carbon 2013, 56, 45–49. [Google Scholar] [CrossRef]
- Mondal, A.K.; Chandra Rao, B.S.S.; Kumar, S. Wear behaviour of AE42 + 20% saffil Mg-MMC. Tribol. Int. 2007, 40, 290–296. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Xie, H.J.; Cheng, Y.L.; Li, S.X.; Cao, J.H.; Cao, L. Wear and corrosion resistant coatings on surface of cast A356 aluminum alloy by plasma electrolytic oxidation in moderately concentrated aluminate electrolytes. Trans. Nonferrous Met. Soc. China 2017, 27, 336–351. [Google Scholar] [CrossRef]



| Element | Cu | Mg | Mn | Fe | Ti | Si | Zn | Al |
|---|---|---|---|---|---|---|---|---|
| Content | 3.85 | 1.29 | 0.65 | 0.11 | 0.049 | 0.068 | 0.054 | Balance |
| Sample | Electrolyte |
|---|---|
| G0 | Na2SiO3 (12 g/L), NaOH (4 g/L), KF (2 g/L), glycerol (4 mL/L) |
| G1 | Base electrolyte + 1 g/L graphene |
| G2 | Base electrolyte + 2 g/L graphene |
| G3 | Base electrolyte + 3 g/L graphene |
| G4 | Base electrolyte + 4 g/L graphene |
| G5 | Base electrolyte + 5 g/L graphene |
| Sample | Rs (Ω·cm2) | R1 (Ω·cm2) | CPE1 | R2 (Ω·cm2) | CPE2 | ||
|---|---|---|---|---|---|---|---|
| Y1 (Ω−1·cm−2 Sn) | n1 | Y2 (Ω−1·cm−2 Sn) | n2 | ||||
| G0 | 39.67 | 1.86 × 104 | 5.84 × 105 | 0.54 | 9.39 × 105 | 4.79 × 105 | 0.48 |
| G1 | 66.56 | 1.34 × 105 | 4.05 × 105 | 0.77 | 3.68 × 105 | 1.05× 105 | 0.75 |
| G2 | 76.53 | 1.70 × 105 | 1.42 × 105 | 0.84 | 1.29 × 105 | 2.79× 105 | 0.65 |
| G3 | 54.43 | 3.33 × 105 | 3.29 × 105 | 0.64 | 2.03× 105 | 4.29 × 105 | 0.62 |
| G4 | 57.67 | 9.30 × 105 | 6.23 × 105 | 0.74 | 3.45× 105 | 1.82 × 105 | 0.72 |
| G5 | 87.09 | 1.87 × 105 | 1.63 × 105 | 0.81 | 7.87 × 105 | 2.05× 105 | 0.45 |
| Sample | Al | Cu | Fe | Cr | O | C |
|---|---|---|---|---|---|---|
| Bare Al alloy | 88.42 | 6.43 | 0.49 | - | 4.43 | - |
| G0 | 41.81 | 2.76 | 7.35 | - | 18.63 | - |
| G1 | 21.76 | 0.56 | 39.62 | 0.64 | 21.57 | 8.69 |
| G2 | 17.35 | - | 48.32 | 0.89 | 29.88 | 12.48 |
| G3 | 69.77 | 5.84 | 0.84 | - | 8.52 | 4.93 |
| G4 | 28.89 | 0.93 | 36.74 | 0.58 | 20.75 | 7.69 |
| G5 | 34.08 | 1.22 | 30.59 | 0.34 | 19.72 | 8.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Pu, Y.; Liao, H.; Lin, Y.; He, W. Corrosion and Wear Behavior of PEO Coatings on D16T Aluminum Alloy with Different Concentrations of Graphene. Coatings 2020, 10, 249. https://doi.org/10.3390/coatings10030249
Liu W, Pu Y, Liao H, Lin Y, He W. Corrosion and Wear Behavior of PEO Coatings on D16T Aluminum Alloy with Different Concentrations of Graphene. Coatings. 2020; 10(3):249. https://doi.org/10.3390/coatings10030249
Chicago/Turabian StyleLiu, Wanying, Yi Pu, Hongcheng Liao, Yuanhua Lin, and Wanying He. 2020. "Corrosion and Wear Behavior of PEO Coatings on D16T Aluminum Alloy with Different Concentrations of Graphene" Coatings 10, no. 3: 249. https://doi.org/10.3390/coatings10030249
APA StyleLiu, W., Pu, Y., Liao, H., Lin, Y., & He, W. (2020). Corrosion and Wear Behavior of PEO Coatings on D16T Aluminum Alloy with Different Concentrations of Graphene. Coatings, 10(3), 249. https://doi.org/10.3390/coatings10030249





