Preparation of Coated Corrugated Box for Controlled-Release of Chlorine Dioxide and Its Application in Strawberry Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating on Corrugated Boxes
2.2.1. Physical Properties of the Coated Corrugated Cardboard and Box
2.2.2. ClO2 Release Test
2.3. Application of Coated Corrugated Boxes in Strawberry Preservation
2.4. Physico-chemical Quality Changes of Strawberries
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Corrugated Cardboard and Box
3.1.1. Physical Properties of Corrugated Cardboard and Box
3.1.2. ClO2 Release Behavior from the Coated Corrugated Cardboard
3.2. Effects of the Coated Corrugated Box on Strawberry Preservation
3.2.1. Decay Rate
3.2.2. Weight Loss
3.2.3. Surface Color
3.2.4. Firmness
3.2.5. Content of AA, TA, and TSS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arango, J.; Rubino, M.I.; Auras, R.; Rachford, A.A.; Bai, Z.; Grzesiak, A.L.; Kijchavengkul, T. In situ quantification of chlorine dioxide gas consumption by fresh produce using UV–visible spectroscopy. J. Food Eng. 2014, 131, 75–81. [Google Scholar] [CrossRef]
- Benbettaieb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef] [PubMed]
- Praeger, U.; Herppich, W.B.; Hassenberg, K. Aqueous chlorine dioxide treatment of horticultural produce: Effects on microbial safety and produce quality—A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Floros, J.D.; Linton, R.H.; Nielsen, S.S.; Nelson, P.E. Response surface modeling for the inactivation of Escherichia coli O157:H7 on green peppers (Capsicum annuum L.) by chlorine dioxide gas treatments. J. Food Prot. 2010, 67, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.E.; Berrang, M.E.; Kerr, W.L.; Harrison, M.A. Slow-release chlorine dioxide gas treatment as a means to reduce Salmonella contamination on spices. Innov. Food Sci. Emerg. Technol. 2019, 52, 256–261. [Google Scholar] [CrossRef]
- Eryilmaz, M.; Kaskatepe, B.; Kiymaci, M.E.; Erol, H.B.; Simsek, D.; Gumustas, A. In vitro antimicrobial activity of three new generation disinfectants. Trop. J. Pharm. Res. 2016, 15, 2191–2195. [Google Scholar] [CrossRef][Green Version]
- Lee, H.; Ryu, J.H.; Kim, H. Antimicrobial activity of gaseous chlorine dioxide against Aspergillus flavus on green coffee beans. Food Microbiol. 2020, 86, 103308. [Google Scholar] [CrossRef]
- Yan, R.; Chen, Q.; Fu, M. Chlorine dioxide generation method and it’s action mechanism for removing harmful substances and maintaining quality attributes of agricultural products. Food Bioprocess Technol. 2019, 12, 1110–1122. [Google Scholar]
- Qin, G.; Wu, B.; Peng, X.; Wang, J.; Li, Q.; Jing, J.; Ha, Y. Effects of chlorine dioxide treatment on respiration rate and ethylene synthesis of postharvest tomato fruit. Postharvest Biol. Technol. 2014, 93, 9–14. [Google Scholar]
- Chumyam, A.; Shank, L.; Uthaibutra, J.; Saengnil, K. Effects of chlorine dioxide on mitochondrial energy levels and redox status of ‘Daw’ longan pericarp during storage. Postharvest Biol. Technol. 2016, 116, 26–35. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, B.; Luo, Y.; Ference, C.; Baldwin, E.; Harrison, K.; Bai, J. Effect of controlled-release chlorine dioxide on the quality and safety of cherry/grape tomatoes. Food Control 2017, 82, 26–30. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhang, L.; Wang, J.; Huang, X.; Zhao, Y.; Liu, Y.; Li, C. Application of chlorine dioxide microcapsule sustained-release antibacterial films for preservation of mangos. J. Food Sci. Technol.-Mys. 2019, 56, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.R.; Zhang, X.M.; Jin, T.; Li, B.Q.; Zhang, Z.Q.; Tian, S.P. Inhibitory of grey mold on green pepper and winter jujube by chlorine dioxide (ClO2) fumigation and its mechanisms. LWT-Food Sci. Technol. 2019, 100, 335–340. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, J.; Huang, C.; Zhao, X.; Chen, H.; Sun, Z. Effects of combined aqueous chlorine dioxide and chitosan coatings on microbial growth and quality maintenance of fresh-cut bamboo shoots (Phyllostachys praecox f. prevernalis.) during storage. Food Bioprocess Technol. 2015, 8, 1011–1019. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Tiemur, A.; Wang, J.; Wu, B. Degradation of pesticide residues by gaseous chlorine dioxide on table grapes. Postharvest Biol. Technol. 2018, 137, 142–148. [Google Scholar] [CrossRef]
- Wang, Z.; Narciso, J.; Biotteau, A.; Plotto, A.; Baldwin, E.; Bai, J. Improving storability of fresh strawberries with controlled release chlorine dioxide in perforated clamshell packaging. Food Bioprocess Technol. 2014, 7, 3516–3524. [Google Scholar] [CrossRef]
- Sun, X.; Bai, J.; Ference, C.; Wang, Z.; Zhang, Y.; Narciso, J.; Zhou, K. Antimicrobial activity of controlled-release chlorine dioxide gas on fresh blueberries. J. Food Prot. 2014, 77, 1127–1132. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Yam, K. Encapsulation complex of chlorine dioxide in alpha-cyclodextrin: Structure characterization and release property. Food Control 2020, 107, 106783. [Google Scholar] [CrossRef]
- Saade, C.; Annous, B.A.; Gualtieri, A.J.; Schaich, K.M.; Liu, L.; Yam, K.L. System feasibility: Designing a chlorine dioxide self-generating package label to improve fresh produce safety part II: Solution casting approach. Innov. Food. Sci. Emerg. Technol. 2018, 47, 110–119. [Google Scholar] [CrossRef]
- Bai, Z.; Cristancho, D.E.; Rachford, A.A.; Reder, A.L.; Williamson, A.; Grzesiak, A.L. Controlled release of antimicrobial ClO2 gas from a two-layer polymeric film system. J. Agric. Food Chem. 2016, 64, 8647–8652. [Google Scholar] [CrossRef]
- Li, S.; Xiao, S.; Luo, Y. Effect of preservative paper coating on NaClO2 retention rate and ClO2 release rule. Packag. Eng. 2017, 38, 31–35. [Google Scholar]
- Cao, J.; Jiang, W.; Zhao, Y. Physiological and Biochemical Experiment Guidance of Postharvest Fruits and Vegetables, 1st ed.; China Light Industry Book Press: China, Beijing, 2007; pp. 37–39. [Google Scholar]
- Aghaeifard, F.; Babalar, M.; Fallahi, E.; Ahmadi, A. Influence of humic acid and salicylic acid on yield, fruit quality, and leaf mineral elements of strawberry (fragaria × ananassa duch.) cv. camarosa. J. Plant Nutr. 2015, 39, 1821–1829. [Google Scholar] [CrossRef]
- Fadiji, T.; Berry, T.M.; Coetzee, C.J.; Opara, U.L. Mechanical design and performance testing of corrugated paperboard packaging for the postharvest handling of horticultural produce. Biosys. Eng. 2018, 171, 220–244. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.; Bai, J. Applications of gaseous chlorine dioxide on postharvest handling and storage of fruits and vegetables—A review. Food Control 2019, 95, 18–26. [Google Scholar] [CrossRef]
- Arango, J.; Rubino, M.; Auras, R.; Gillett, J.; Schilder, A. Evaluation of chlorine dioxide as an antimicrobial against Botrytis cinerea in California strawberries. Food Packaging Shelf 2016, 9, 45–54. [Google Scholar] [CrossRef]
- Nguyen, V.T.B.; Nguyen, D.H.H.; Nguyen, H.V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria × ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
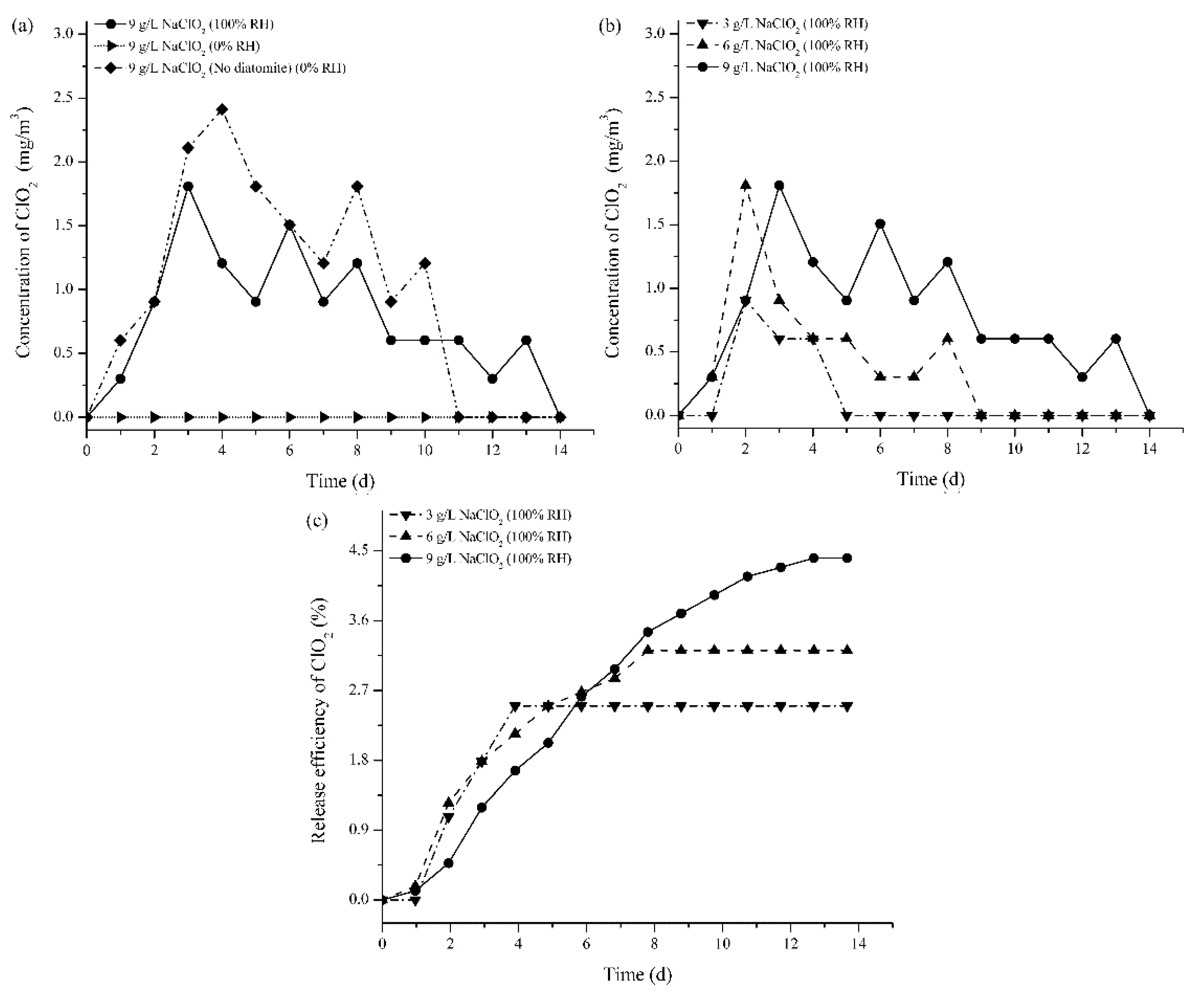
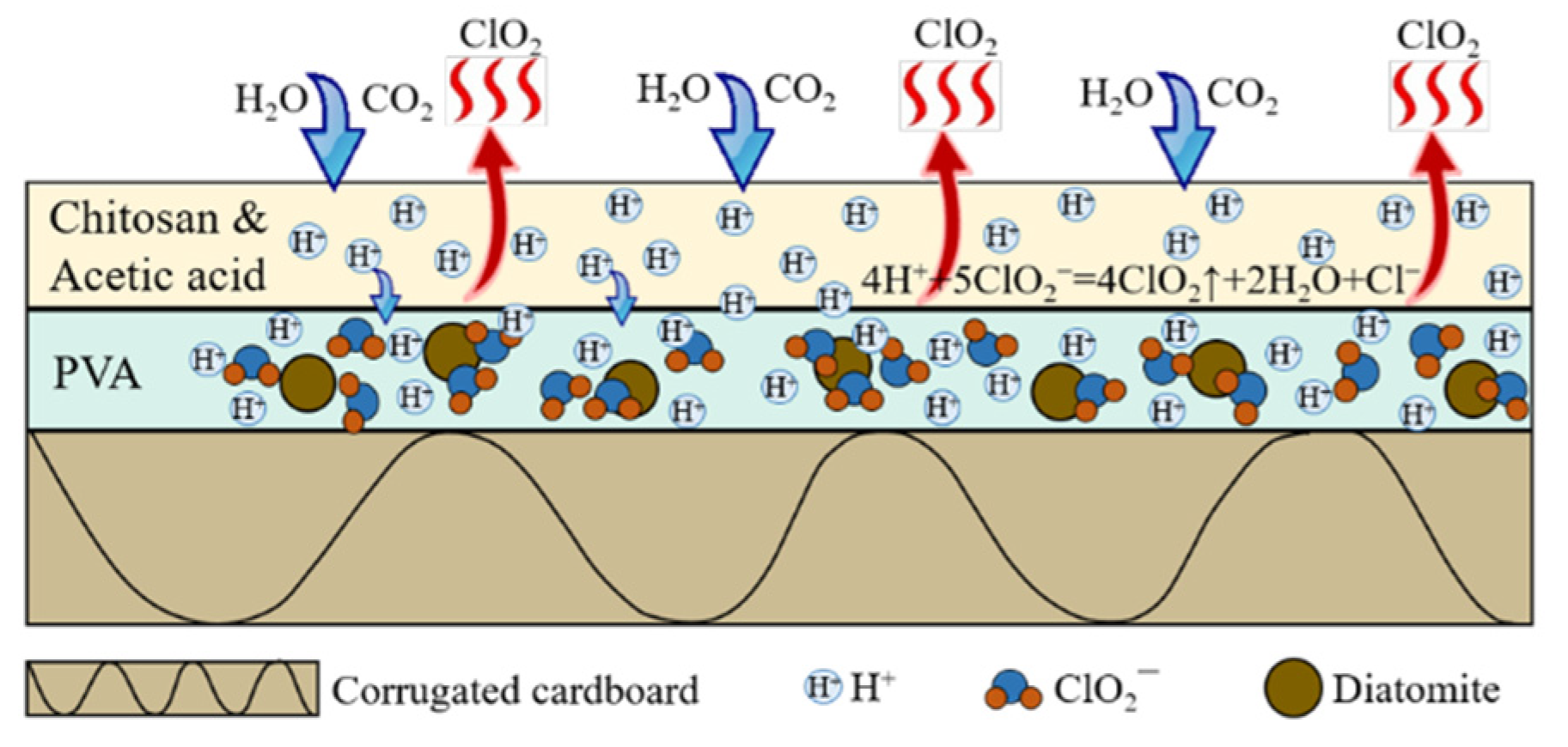

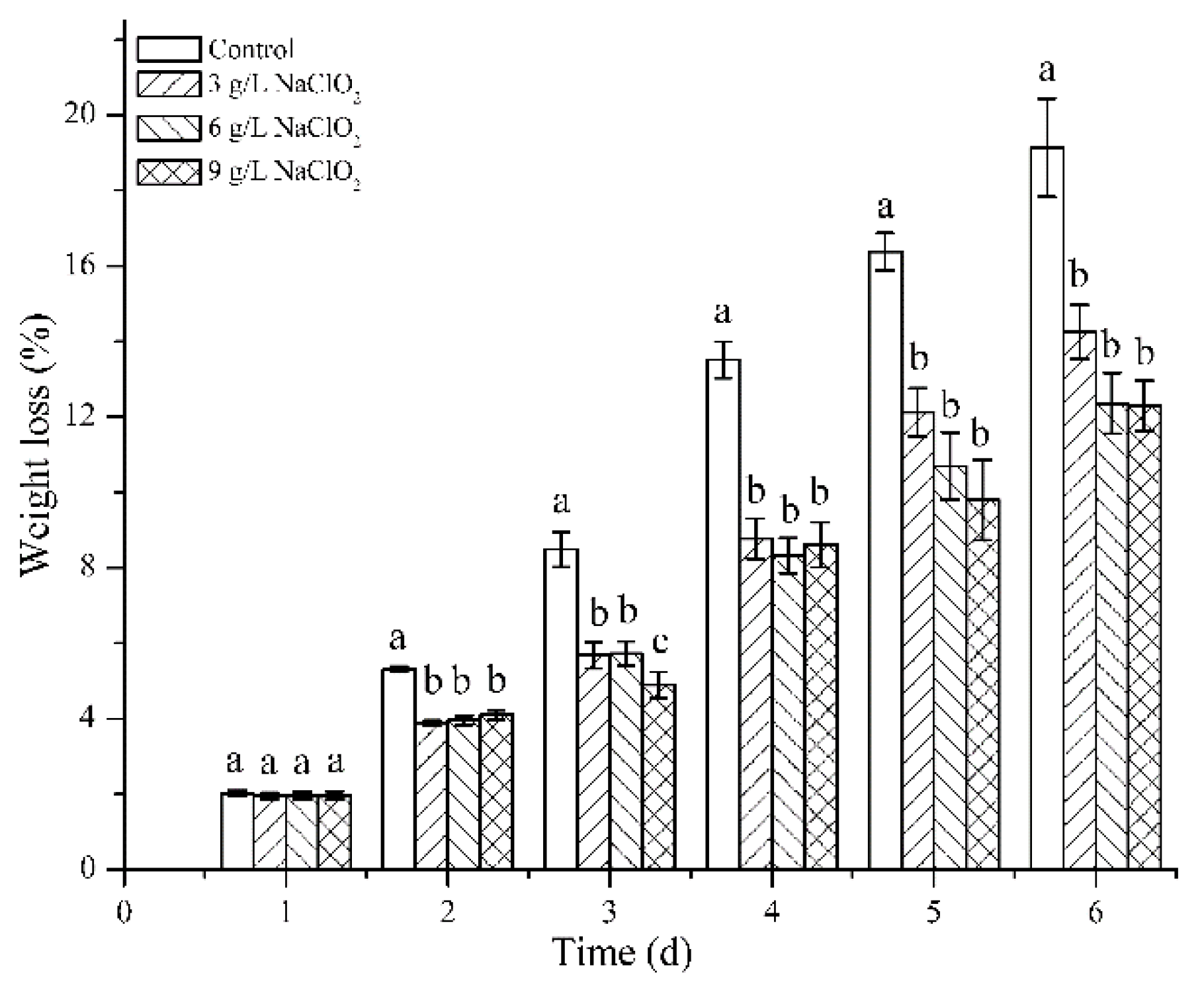
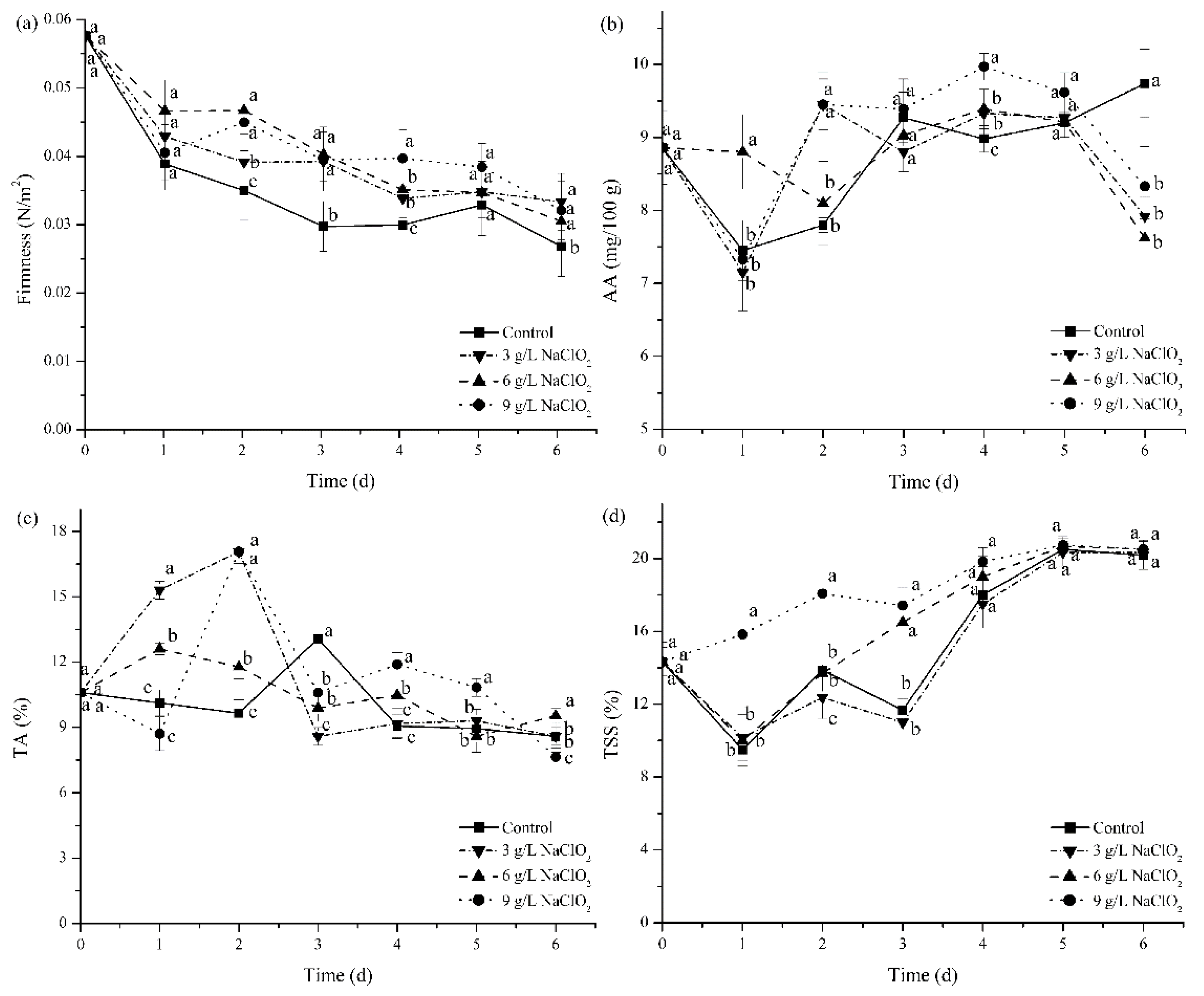
| Samples | Thickness (mm) | Moisture Content (%) | PS (J) | ECS (kN/m) | FCS (kPa) | BCS (N) |
|---|---|---|---|---|---|---|
| Control | 1.59 ± 0.02b | 12.69 ± 0.36b | 4.75 ± 0.03a | 1.25 ± 0.12a | 65.40 ± 3.44a | 587.22 ± 59.97a |
| 3 g/L NaClO2 | 1.66 ± 0.01a | 16.17 ± 0.67a | 4.58 ± 0.06b | 0.91 ± 0.10b | 52.77 ± 5.24b | 382.71 ± 41.47b |
| 6 g/L NaClO2 | 1.66 ± 0.02a | 16.11 ± 0.53a | 4.57 ± 0.05b | 0.90 ± 0.08b | 53.41 ± 3.09b | 366.93 ± 21.90b |
| 9 g/L NaClO2 | 1.64 ± 0.01a | 16.17 ± 0.45a | 4.67 ± 0.05b | 0.90 ± 0.08b | 52.01 ± 3.27b | 363.65 ± 22.20b |
| Color Parameter | Storage Time (d) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | ||
| L* | Control | 31.84 ± 3.98aA | 28.59 ± 2.29bB | 26.87 ± 1.78bB | 21.09 ± 3.40abC |
| 3 g/L NaClO2 | 31.84 ± 3.98aA | 29.13 ± 1.84abB | 27.20 ± 2.16abB | 20.62 ± 2.04bC | |
| 6 g/L NaClO2 | 31.84 ± 3.98aA | 29.26 ± 2.18abB | 28.75 ± 1.45aB | 21.04 ± 2.63abC | |
| 9 g/L NaClO2 | 31.84 ± 3.98aA | 30.29 ± 2.11aAB | 28.98 ± 1.77aB | 22.76 ± 0.99aC | |
| a* | Control | 37.94 ± 1.27aA | 32.55 ± 1.97bB | 31.41 ± 2.18bB | 31.51 ± 3.69cB |
| 3 g/L NaClO2 | 37.94 ± 1.27aA | 34.49 ± 1.72aB | 33.22 ± 1.68aC | 35.08 ± 1.59aB | |
| 6 g/L NaClO2 | 37.94 ± 1.27aA | 33.26 ± 2.90abB | 33.85 ± 1.96aB | 33.40 ± 4.51bB | |
| 9 g/L NaClO2 | 37.94 ± 1.27aA | 34.32 ± 2.10aB | 31.25 ± 1.58bC | 33.24 ± 1.09bB | |
| b* | Control | 36.29 ± 4.23aA | 22.43 ± 1.74cB | 18.47 ± 1.76cC | 17.63 ± 1.44aC |
| 3 g/L NaClO2 | 36.29 ± 4.23aA | 24.65 ± 1.91aB | 20.55 ± 1.73aB | 14.08 ± 1.44cC | |
| 6 g/L NaClO2 | 36.29 ± 4.23aA | 22.43 ± 1.85cB | 19.63 ± 1.92bBC | 16.73 ± 1.72bC | |
| 9 g/L NaClO2 | 36.29 ± 4.23aA | 23.07 ± 1.74bB | 18.83 ± 1.82cC | 16.35 ± 1.83bC | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ren, D.; Xu, D. Preparation of Coated Corrugated Box for Controlled-Release of Chlorine Dioxide and Its Application in Strawberry Preservation. Coatings 2020, 10, 242. https://doi.org/10.3390/coatings10030242
Li Y, Ren D, Xu D. Preparation of Coated Corrugated Box for Controlled-Release of Chlorine Dioxide and Its Application in Strawberry Preservation. Coatings. 2020; 10(3):242. https://doi.org/10.3390/coatings10030242
Chicago/Turabian StyleLi, Yuanyuan, Dan Ren, and Dan Xu. 2020. "Preparation of Coated Corrugated Box for Controlled-Release of Chlorine Dioxide and Its Application in Strawberry Preservation" Coatings 10, no. 3: 242. https://doi.org/10.3390/coatings10030242
APA StyleLi, Y., Ren, D., & Xu, D. (2020). Preparation of Coated Corrugated Box for Controlled-Release of Chlorine Dioxide and Its Application in Strawberry Preservation. Coatings, 10(3), 242. https://doi.org/10.3390/coatings10030242







