Tribocorrosive Study of New and In Vivo Exposed Nickel Titanium and Stainless Steel Orthodontic Archwires
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Electrochemical Measurements
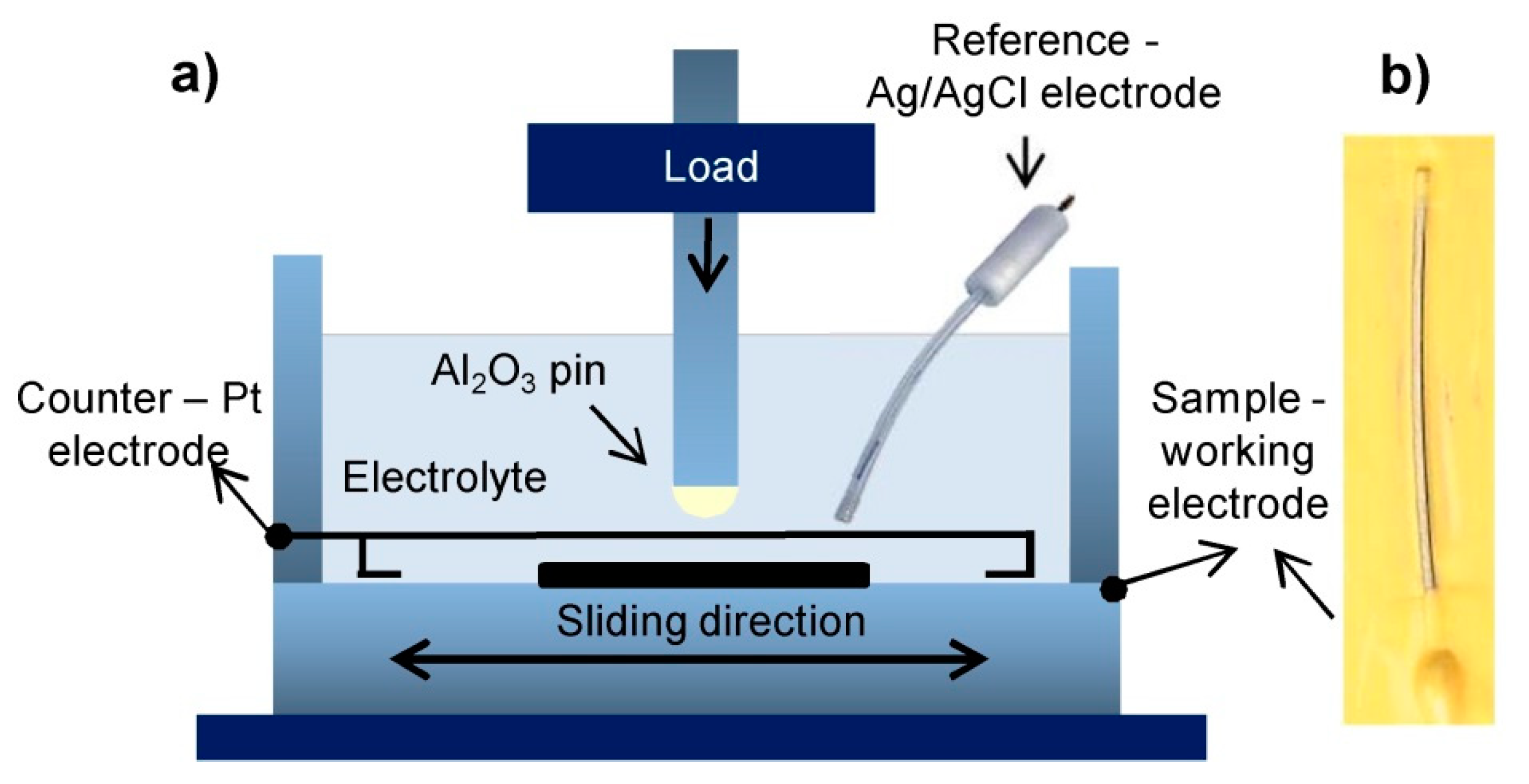
2.3. Wear Measurements
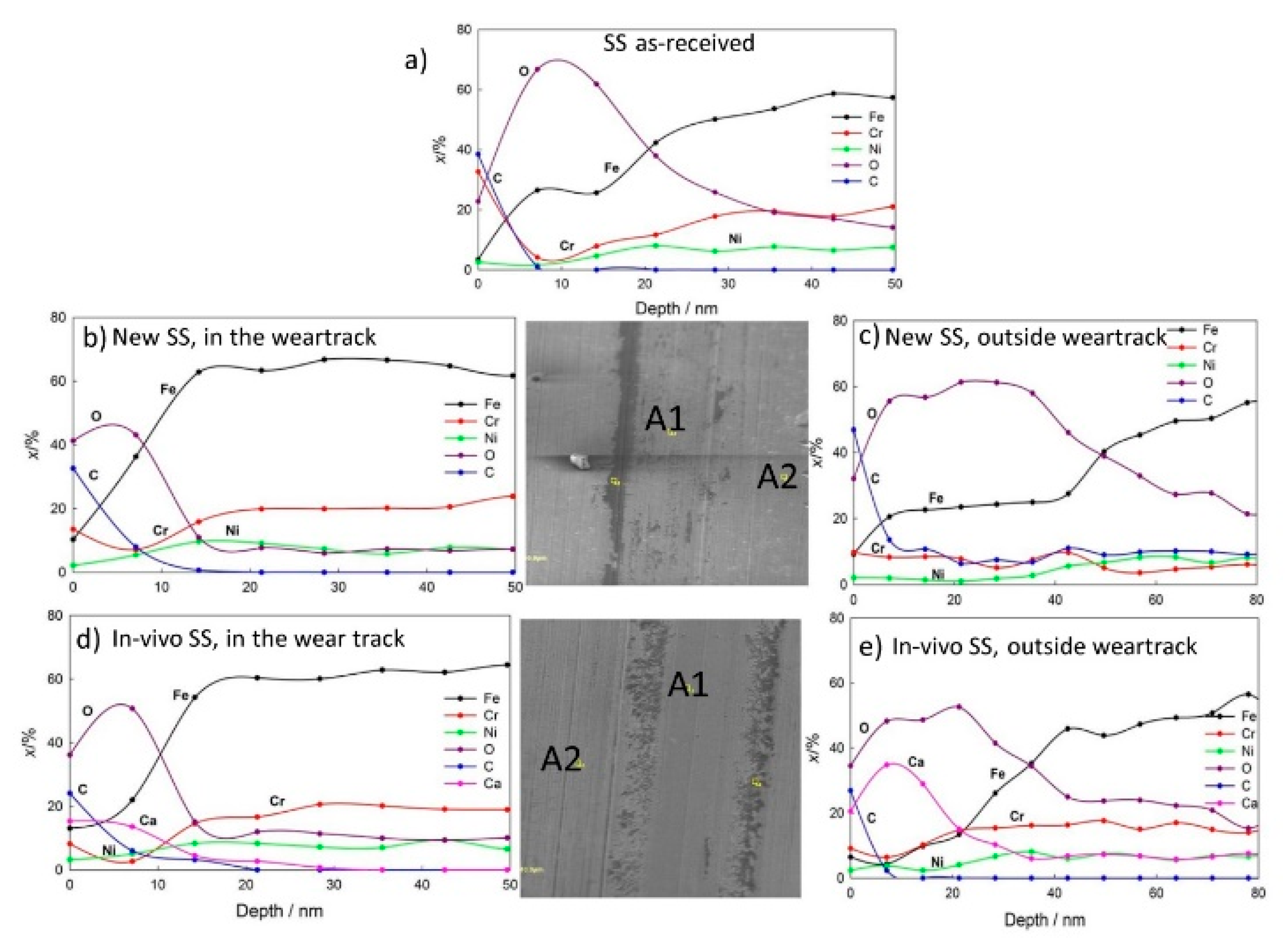
2.4. Surface Analysis
3. Results
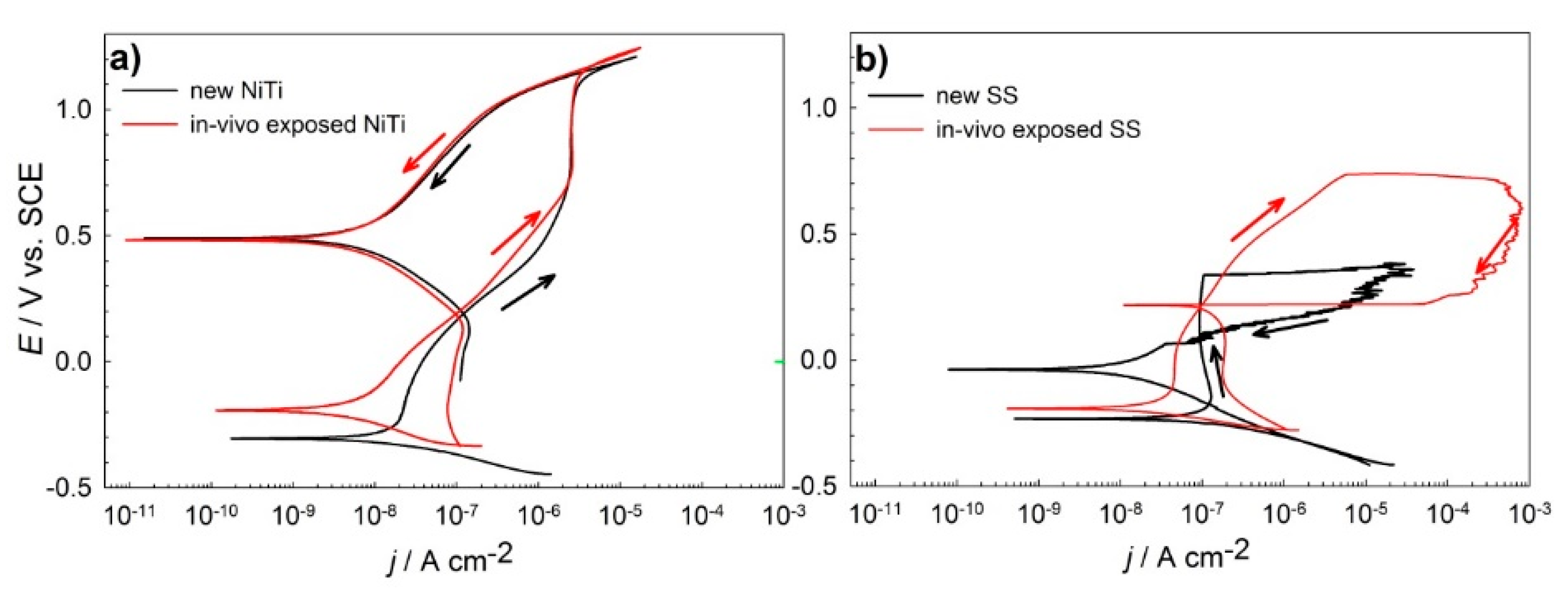
3.1. Electrochemical Properties
3.2. Tribo-Electrochemical and Surface Properties
4. Discussion
5. Conclusions
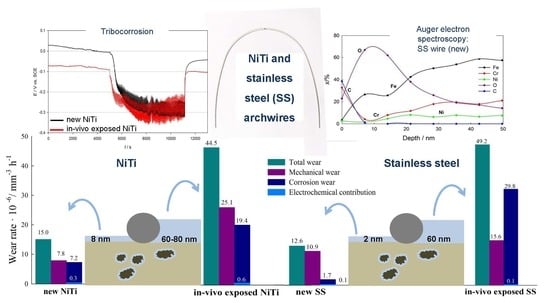
- AES surface analysis and depth profiling confirmed the formation of 8 nm thick TiO2 films on new NiTi archwires. After tribocorrosion tests, the TiO2 in the wear track was thinner. AES depth profiling showed up to 80 and 60 nm thick dental plaque on in vivo exposed NiTi and SS archwires, respectively. The passive film on new SS is mainly Cr2O3, with an estimated thickness of 1–2 nm.
- Electrochemical investigations showed that NiTi dental alloys exhibited better general corrosion properties compared to stainless-steel alloys, where susceptibility to local types of corrosion was detected. In vivo exposed NiTi and SS archwires showed better electrochemical properties in simulated saliva than new archwires.
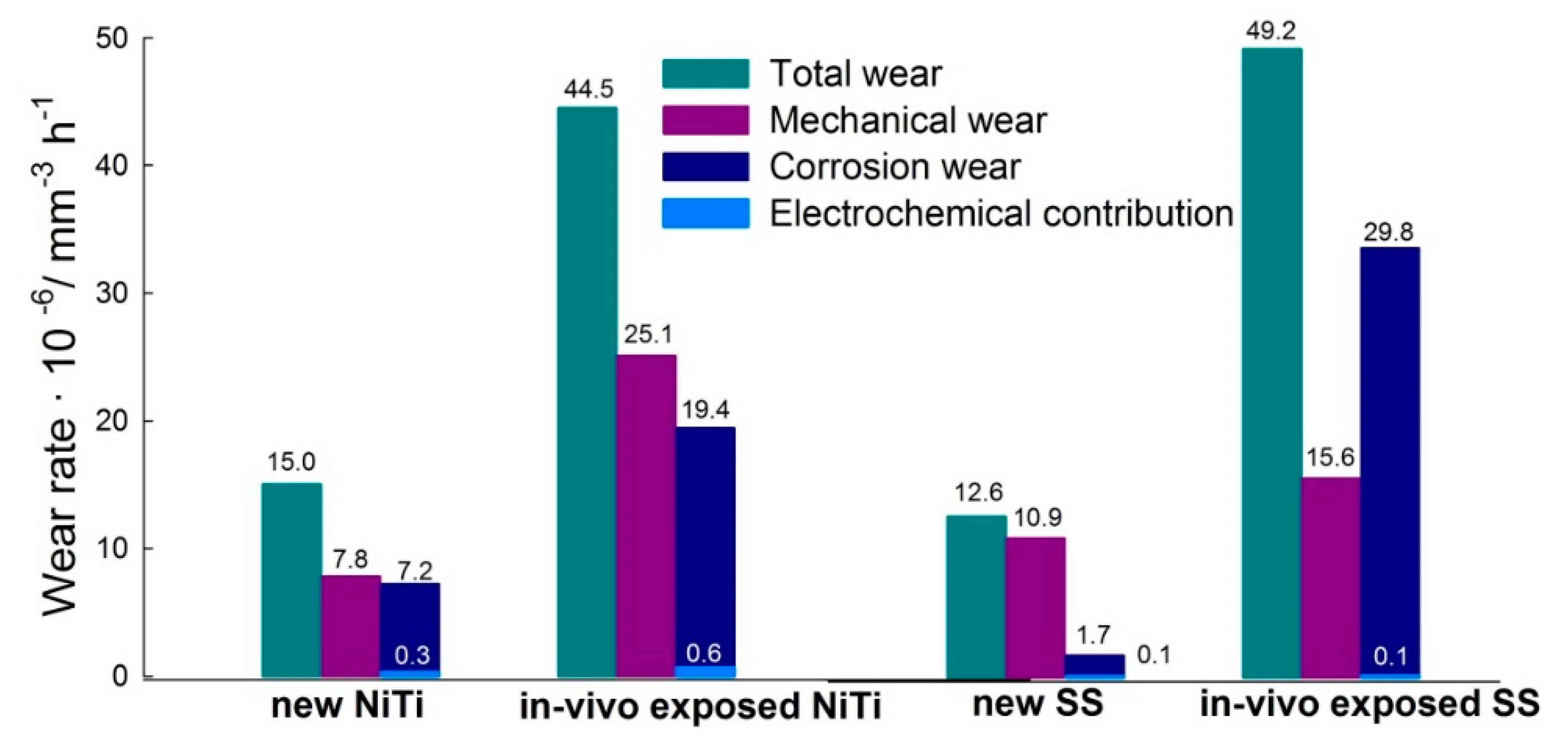
- The total wear was lower for the new NiTi and SS wires when compared to in vivo exposed wires. When mechanical loads were used in corrosive saliva, corrosion was accelerated in both types of archwires. The contribution of mechanical wear to the total wear was higher for the new archwires when compared to the in vivo exposed archwires, where corrosion wear was found to be predominant.
- The coefficient of friction was higher in the NiTi archwires than in the SS archwires and is similar for new and in vivo exposed archwires. Thereby, saliva and debris do not have detrimental effect on the clinical outcome.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Araújo, R.C.; Bichara, L.M.; Araujoand, A.M.; Normando, D. Debris and friction of self-ligating and conventional orthodontic brackets after clinical use. Angle Orthod. 2015, 85, 673–677. [Google Scholar] [CrossRef]
- Marques, I.S.; Araújo, A.M.; Gurgel, J.A.; Normando, D. Debris, roughness and friction of stainless steel archwires following clinical use. Angle Orthod. 2010, 80, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Kusy, R.P.; Whitley, J.Q. Resistance to sliding of orthodontic appliances in the dry and wet states: Influence of archwire alloy, interbracket distance, and bracket engagement. J. Biomed. Mater. Res. 2000, 52, 797–811. [Google Scholar] [CrossRef]
- Schiff, N.; Dalard, F.; Lissac, M.; Morgon, L.; Grosgogeat, B. Corrosion resistance of three orthodontic brackets: A comparative study of three fluoride mouthwashes. Eur. J. Orthod. 2005, 27, 541–549. [Google Scholar] [CrossRef] [PubMed]
- House, K.; Sernettz, F.; Dymock, D.; Sandy, J.R.; Ireland, A.J. Corrosion of orthodontic appliances--should we care? Am. J. Orthod. Dentofac. Orthop. 2008, 133, 584–592. [Google Scholar] [CrossRef]
- Mezeg, U.; Primožič, J. Influence of long-term in vivo exposure, debris accumulation and archwire material on friction force among different types of brackets and archwires couples. Eur. J. Orthod. 2017, 39, 673–679. [Google Scholar] [CrossRef]
- Franchi, L.; Baccetti, T.; Camporesi, M.; Barbato, E. Forces released during sliding mechanics with passive self-ligating brackets or nonconventional elastomeric ligatures. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 87–90. [Google Scholar] [CrossRef]
- Huang, H.-H. Surface characterizations and corrosion resistance of nickel-titanium orthodontic archwires in artificial saliva of various degrees of acidity. J. Biomed. Mater. Res. A 2005, 74A, 629–639. [Google Scholar] [CrossRef]
- Espinar, E.; Llamas, J.M.; Michiardi, A.; Ginebra, M.P.; Gil, F.J. Reduction of Ni release and improvement of the friction behaviour of NiTi orthodontic archwires by oxidation treatments. J. Mater. Sci. Mater. Med. 2011, 22, 1119–1125. [Google Scholar] [CrossRef]
- Rincic Mlinaric, M.; Kanizaj, L.; Zuljevic, D.; Katic, V.; Spalj, S.; Otmacic Curkovic, H. Effect of oral antiseptics on the corrosion stability of nickel-titanium orthodontic alloys. Mater. Corros. 2018, 69, 510–518. [Google Scholar] [CrossRef]
- Kuhta, M.; Pavlin, D.; Slaj, M.; Varga, S.; Laper-Varga, M.; Slaj, M. Type of Archwire and level of acidity: Effects on the release of Metal ions from Orthodontic appliances. Angle Orthod. 2009, 79, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wichai, W.; Anuwongnukroh, N.; Dechkunakorn, S. Comparison of Chemical Properties and Ni Release of Stainless Steel and Nickel Titanium Wires. Adv. Mater. Res. 2014, 884–885, 560–565. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A. Biological aspects of TiNi alloy surfaces. J. Phys. IV 1995, 5, 1199–1204. [Google Scholar] [CrossRef]
- Briceño, J.; Romeu, A.; Espinar, E.; Llamas, J.M.; Gil, F.J. Influence of the microstructure on electrochemical corrosion and nickel release in NiTi orthodontic archwires. Mater. Sci. Eng. C 2013, 33, 4989–4993. [Google Scholar] [CrossRef]
- Močnik, P.; Kosec, T.; Kovač, J.; Bizjak, M. The effect of pH, fluoride and tribocorrosion on the surface properties of dental archwires. Mat. Sci. Eng. C, Mat. Biol. Appl. 2017, 78, 682–689. [Google Scholar] [CrossRef]
- Milošev, I.; Kapun, B. The corrosion resistance of Nitinol alloy in simulated physiological solutions: Part 1: The effect of surface preparation. Mat. Sci. Eng. C 2012, 32, 1087–1096. [Google Scholar] [CrossRef]
- Anuradha, P.; Varma, N.K.S.; Balakrishnan, A. Reliability performance of titanium sputter coated Ni–Ti arch wires: Mechanical performance and nickel release evaluation. Biomed. Mater. Eng. 2015, 26, 67–77. [Google Scholar] [CrossRef]
- Undisz, A.; Schrempel, F.; Wesch, W.; Rettenmayr, M. Mechanism of oxide layer growth during annealing of NiTi. J. Biomed. Mat. Res. 2012, 7, 1743–1750. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.-Y.; Nazarzadeh Zare, E.; Borzacchiello, A.; Niu, L.; Tay, F. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020. [Google Scholar] [CrossRef]
- Zare, E.N.; Jamaledin, R.; Naserzadeh, P.; Afjeh-Dana, E.; Ashtari, B.; Hosseinzadeh, M.; Vecchione, R.; Wu, A.; Tay, F.R.; Borzacchiell, A.; et al. Metal-based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity and Their Biomedical Applications. Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Huang, H.H.; Chiu, Y.H.; Lee, T.H.; Wu, S.C.; Yang, H.W.; Su, K.H.; Hsu, C.C. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585–3592. [Google Scholar] [CrossRef]
- Wichelhaus, A.; Geserick, M.; Hibst, R.; Sander, F.G. The effect of surface treatment and clinical use on friction in NiTi orthodontic wires. Dental Materials: Off. Publ. Acad. Dent. Mater. 2005, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Mezeg, U. Evaluation of Surface Properties of Orthodontic Brackets, Archwires and Their Degradation Processes in the Oral Cavity. Ph.D. Thesis, Medical Faculty at University of Ljubljana, Ljubljana, Slovenia, 2018. [Google Scholar]
- Ovsenik, M.; Godec, M.; Kovač, J.; Dolinar, D.; Oblak, Č.; Kosec, T.; Jenko, M. Characterization of the surface chemistry and microstructure of new and in-vivo-exposed titanium-nickel shape-memory alloy and stainless steel AISI 304 archwires. In Proceedings of the 26th International Conference on Materials and Technology, Portorož, Slovenia, 3–5 October 2018; p. 105. [Google Scholar]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Trib. Inter. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Celis, J.-P.; Ponthiaux, P.; Wenger, F. Tribo-corrosion of materials: Interplay between chemical, electrochemical and mechanical reactivity of surfaces. Wear 2006, 261, 939–946. [Google Scholar] [CrossRef]
- Abedini, M.; Ghasemi, H.M.; Nili Ahmadabadi, M. Tribological behavior of NiTi alloy in martensitic and austenitic states. Mat. Des. 2009, 30, 4493–4497. [Google Scholar] [CrossRef]
- Figueira, N.; Silva, T.M.; Carmezim, M.J.; Fernandes, J.C.S. Corrosion behaviour of NiTi alloy. Electrochim. Acta 2009, 59, 921–926. [Google Scholar] [CrossRef]
- Duffo, G.S.; Castillo, Q. Development of artificial saliva solution for studying the corrosion behaviour of dental alloys. Corrosion 2004, 60, 594–602. [Google Scholar] [CrossRef]
- Kosec, T.; Močnik, P.; Legat, A. The tribocorrosion behaviour of NiTi alloy. App. Surf. Sci. 2014, 288, 727–735. [Google Scholar] [CrossRef]
- Tait, S.W. An Introduction to Electrochemical Corrosion Testing for Practicing Engineers and Scientists; PairODocs Publications: Racine, WI, USA, 1994. [Google Scholar]
- Mathew, M.T.; Runa, M.J.; Laurent, M.; Jacobs, J.J.; Rocha, L.A.; Wimmer, M.A. Tribocorrosion behavior of CoCrMo alloy for hip prosthesis as a function of loads: A comparison between two testing systems. Wear 2011, 271, 1210–1219. [Google Scholar] [CrossRef]
- Kocijan, A.; Donik, Č.; Jenko, M. Electrochemical and XPS studies of passive film formed on stainless steels in borate buffer and chloride solutions. Corros. Sci. 2007, 49, 2083–2098. [Google Scholar] [CrossRef]
- Cumpson, P.J. The thickogram:a method for easy film thickness measurement in XPS. Surf. Interface Anal. 2000, 29, 403–406. [Google Scholar] [CrossRef]
- Virtanen, S. Corrosion and passivity of metals and coatings. In Tribocorrosion of Passive Metals and Coatings, 1st ed.; Landolt, D., Mischler, S., Eds.; Woodhead Publishing in Materials: Cambridge, UK, 2011; pp. 3–28. [Google Scholar]
- Alfonso, M.V.; Espina, E.; Llamas, J.M.; Rupérez de Gracia, E.; Manero Planella, J.M.; Barrera, J.M.; Solano, E.; Gil, M.; Javier, F. Friction coefficients and wear rates of different orthodontic archwires in artificial saliva. J. Mat. Sci.: Mat. Med. 2013, 24, 1327–1332. [Google Scholar] [CrossRef] [PubMed]


| Archwire | Vickers Hardness | Ra |
|---|---|---|
| New NiTi archwire | 376 ± 50 | 115 ± 5 |
| In vivo exposed NiTi archwire | 105 ± 3 | |
| New SS archwire | 597 ± 20 | 15 ± 2 |
| In vivo exposed SS archwire | 14 ± 2 |
| Archwire | Ecorr (V) | jcorr(nA/cm2) | Eb(V) | Erp(V) |
|---|---|---|---|---|
| New NiTi archwire | −0.306 | 19 | 1.08 | |
| In vivo exposed NiTi archwire | −0.193 | 10 | 1.17 | |
| New SS archwire | −0.234 | 106 | 0.340 | 0.083 |
| In vivo exposed SS archwire | −0.194 | 27 | 0.748 | 0.217 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosec, T.; Močnik, P.; Mezeg, U.; Legat, A.; Ovsenik, M.; Jenko, M.; Grant, J.T.; Primožič, J. Tribocorrosive Study of New and In Vivo Exposed Nickel Titanium and Stainless Steel Orthodontic Archwires. Coatings 2020, 10, 230. https://doi.org/10.3390/coatings10030230
Kosec T, Močnik P, Mezeg U, Legat A, Ovsenik M, Jenko M, Grant JT, Primožič J. Tribocorrosive Study of New and In Vivo Exposed Nickel Titanium and Stainless Steel Orthodontic Archwires. Coatings. 2020; 10(3):230. https://doi.org/10.3390/coatings10030230
Chicago/Turabian StyleKosec, Tadeja, Petra Močnik, Uroš Mezeg, Andraž Legat, Maja Ovsenik, Monika Jenko, John T. Grant, and Jasmina Primožič. 2020. "Tribocorrosive Study of New and In Vivo Exposed Nickel Titanium and Stainless Steel Orthodontic Archwires" Coatings 10, no. 3: 230. https://doi.org/10.3390/coatings10030230
APA StyleKosec, T., Močnik, P., Mezeg, U., Legat, A., Ovsenik, M., Jenko, M., Grant, J. T., & Primožič, J. (2020). Tribocorrosive Study of New and In Vivo Exposed Nickel Titanium and Stainless Steel Orthodontic Archwires. Coatings, 10(3), 230. https://doi.org/10.3390/coatings10030230