Hydrothermal Synthesis of Cobalt Ruthenium Sulfides as Promising Pseudocapacitor Electrode Materials
Abstract
1. Introduction
2. Materials and methods
2.1. Materials
2.2. Material Synthesis
Preparation of Cobalt Ruthenium Sulfide Materials
2.3. Material Characterization
2.4. Electrode Preparation and Electrochemical Measurements
3. Results and Discussion
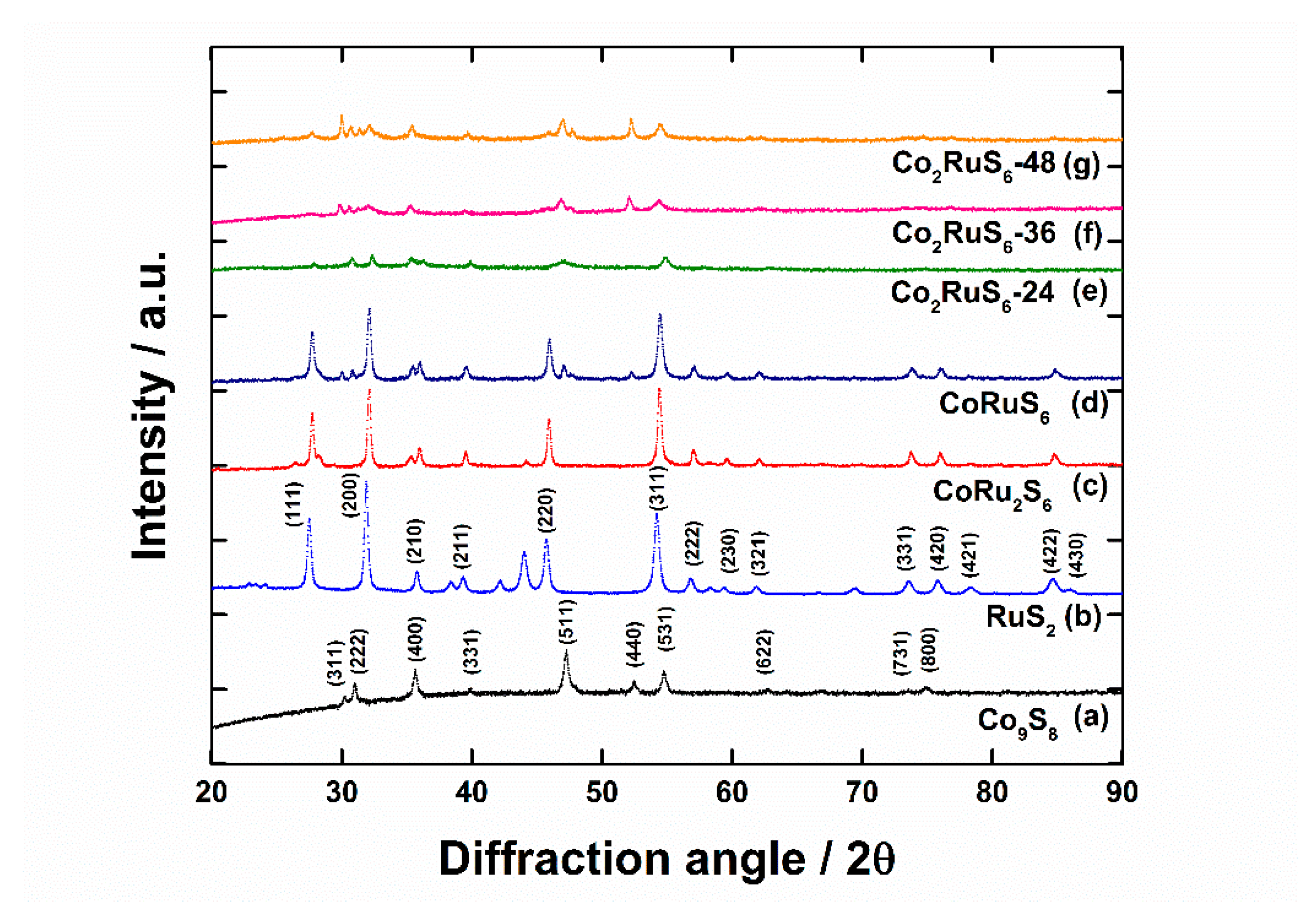
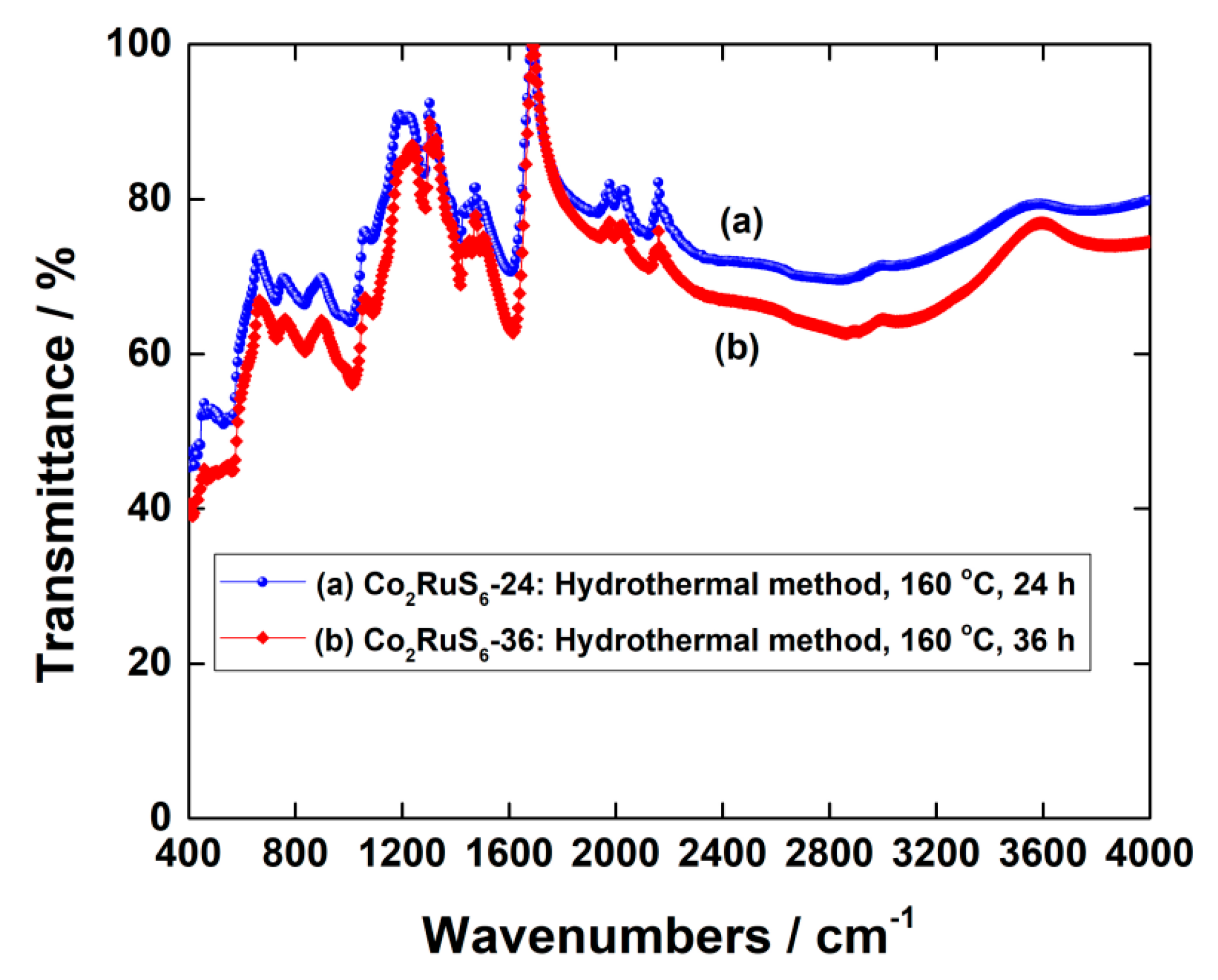
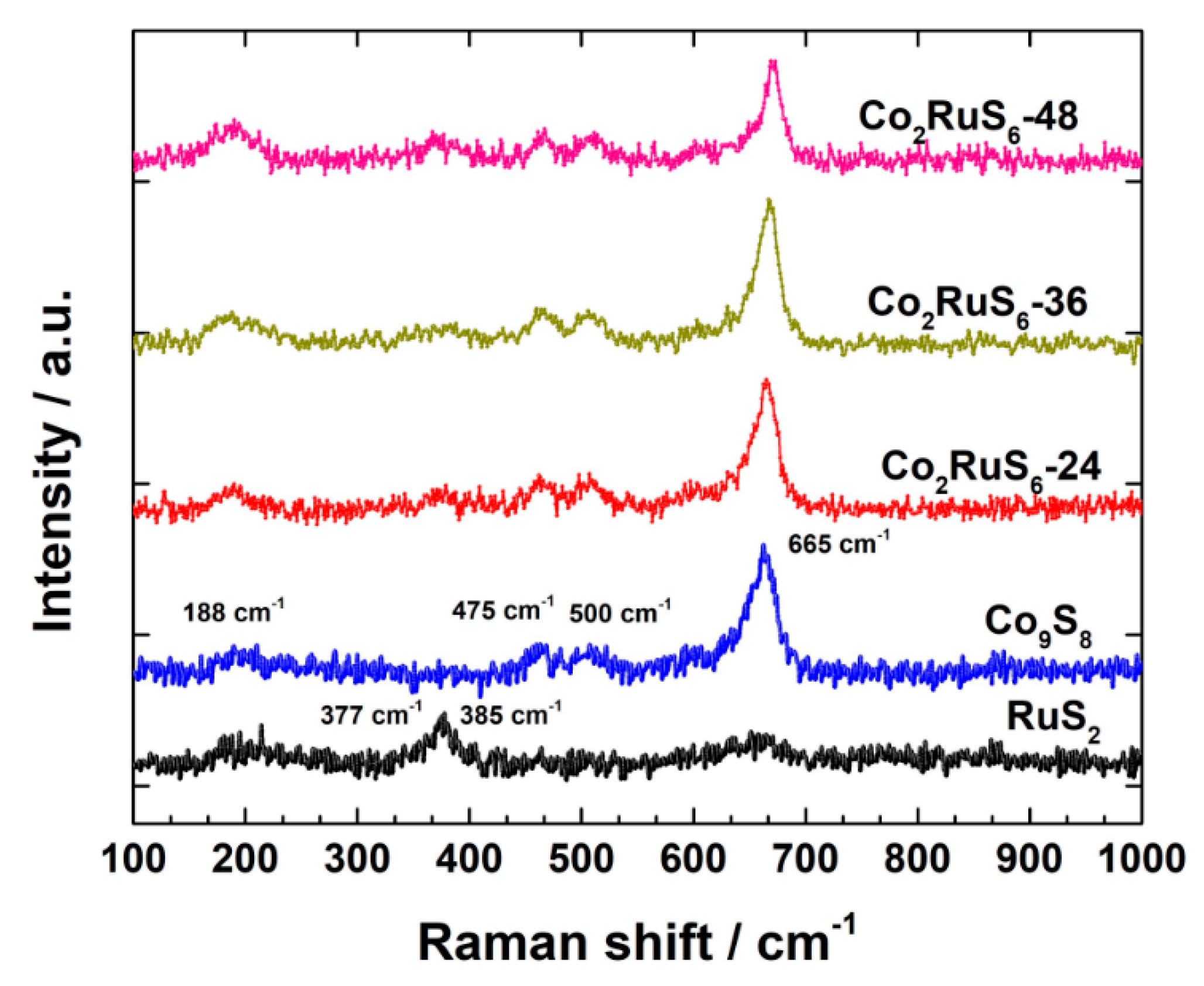
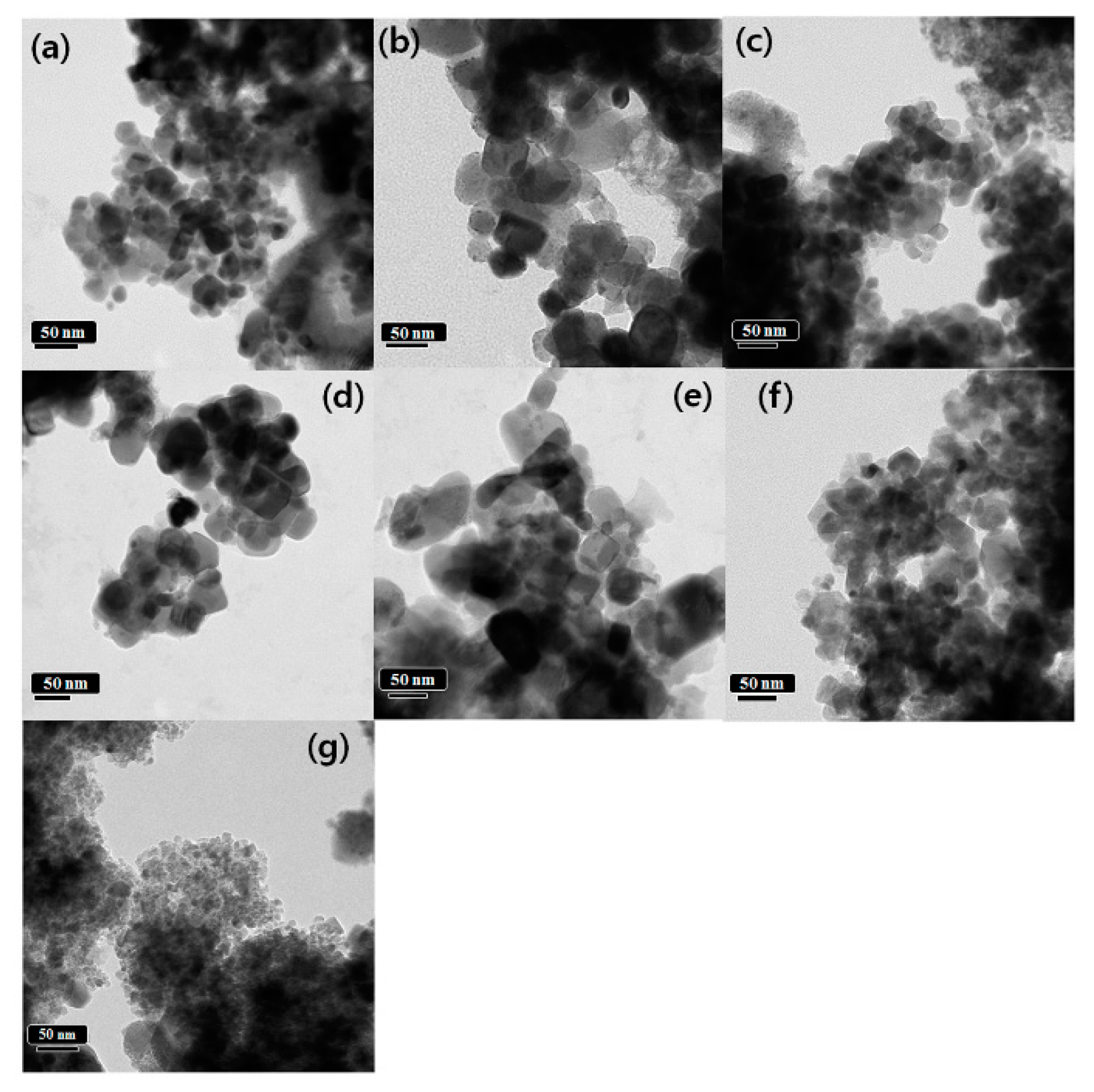
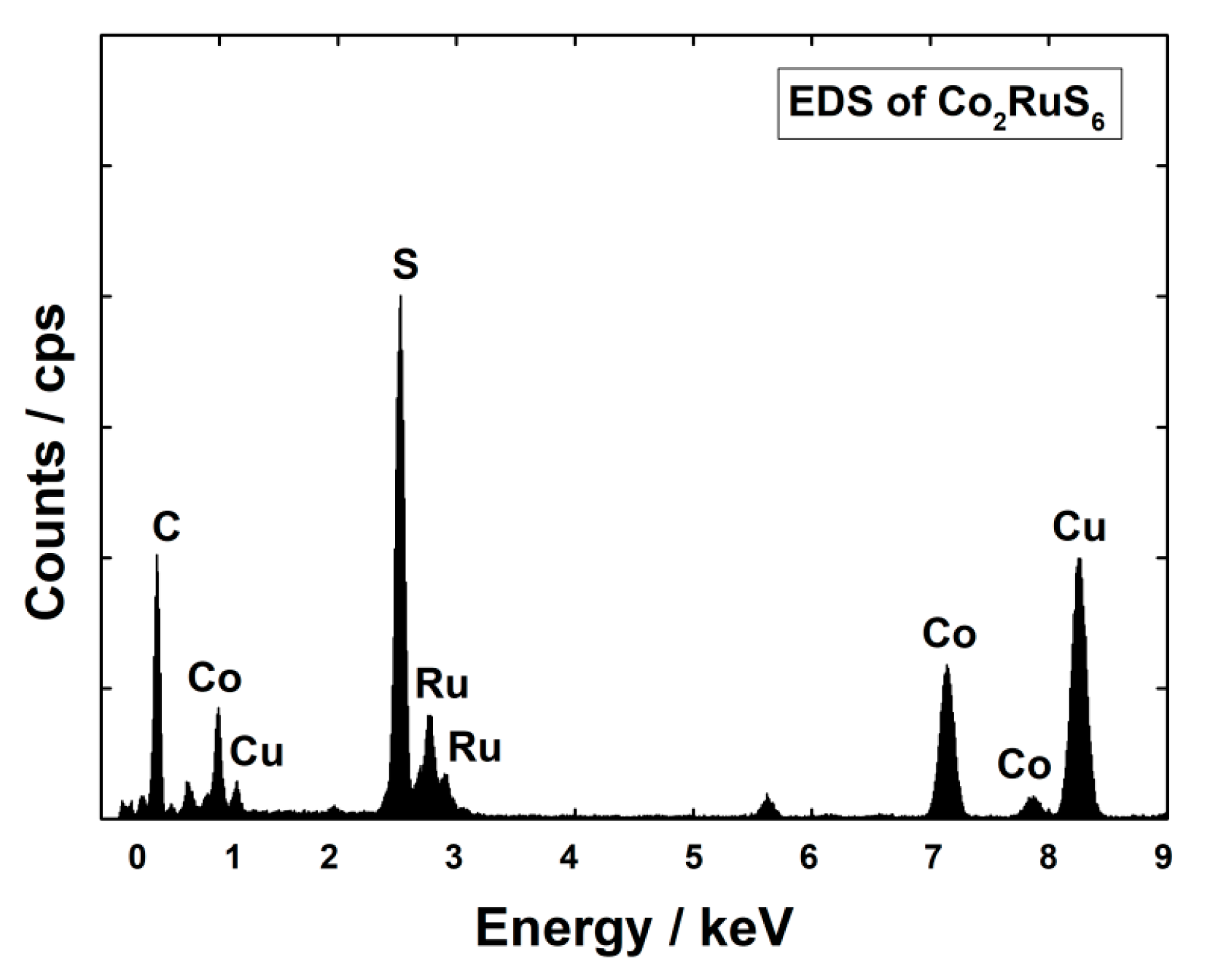
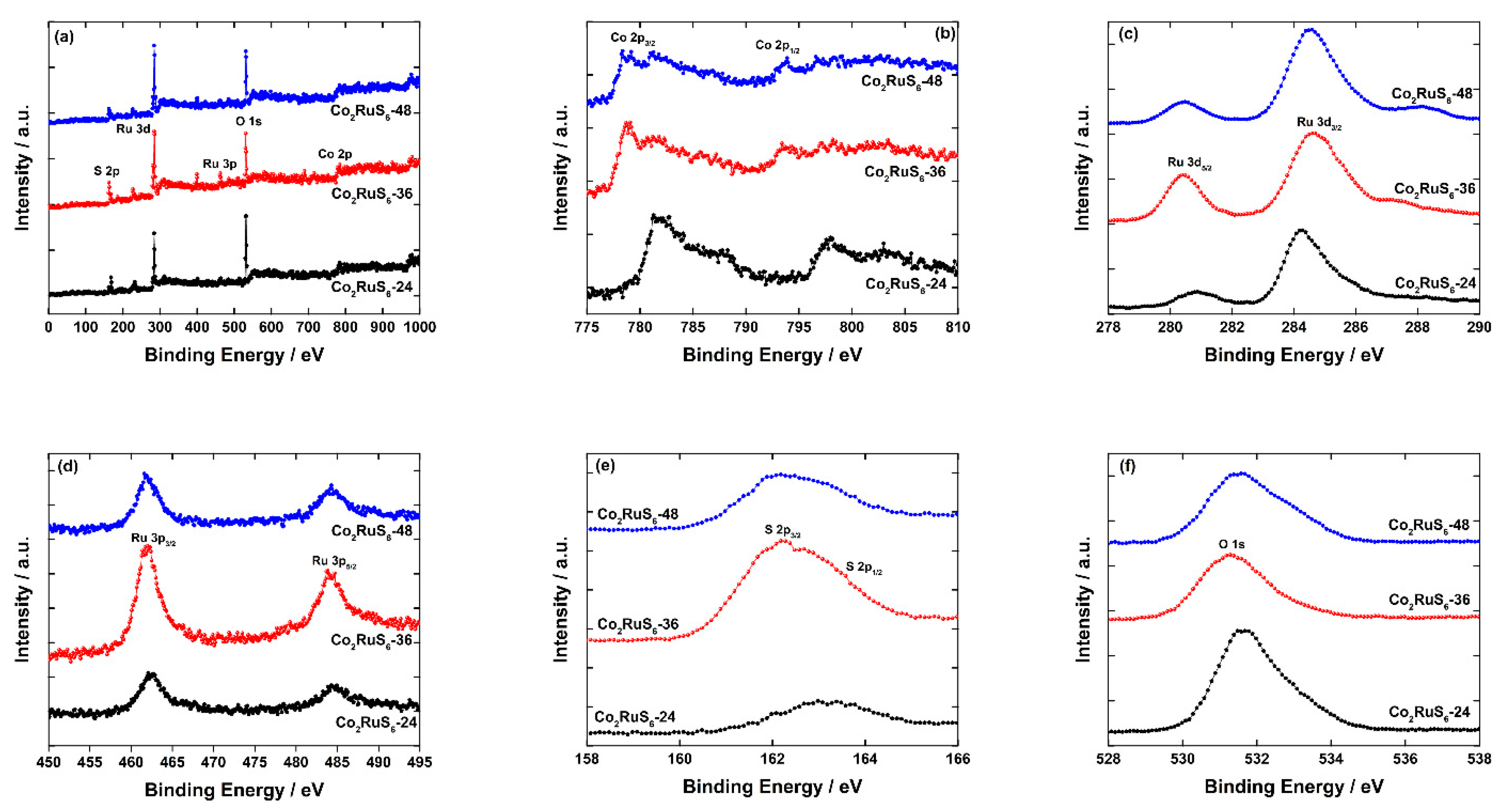
3.1. Characterization of Structure and Morphology
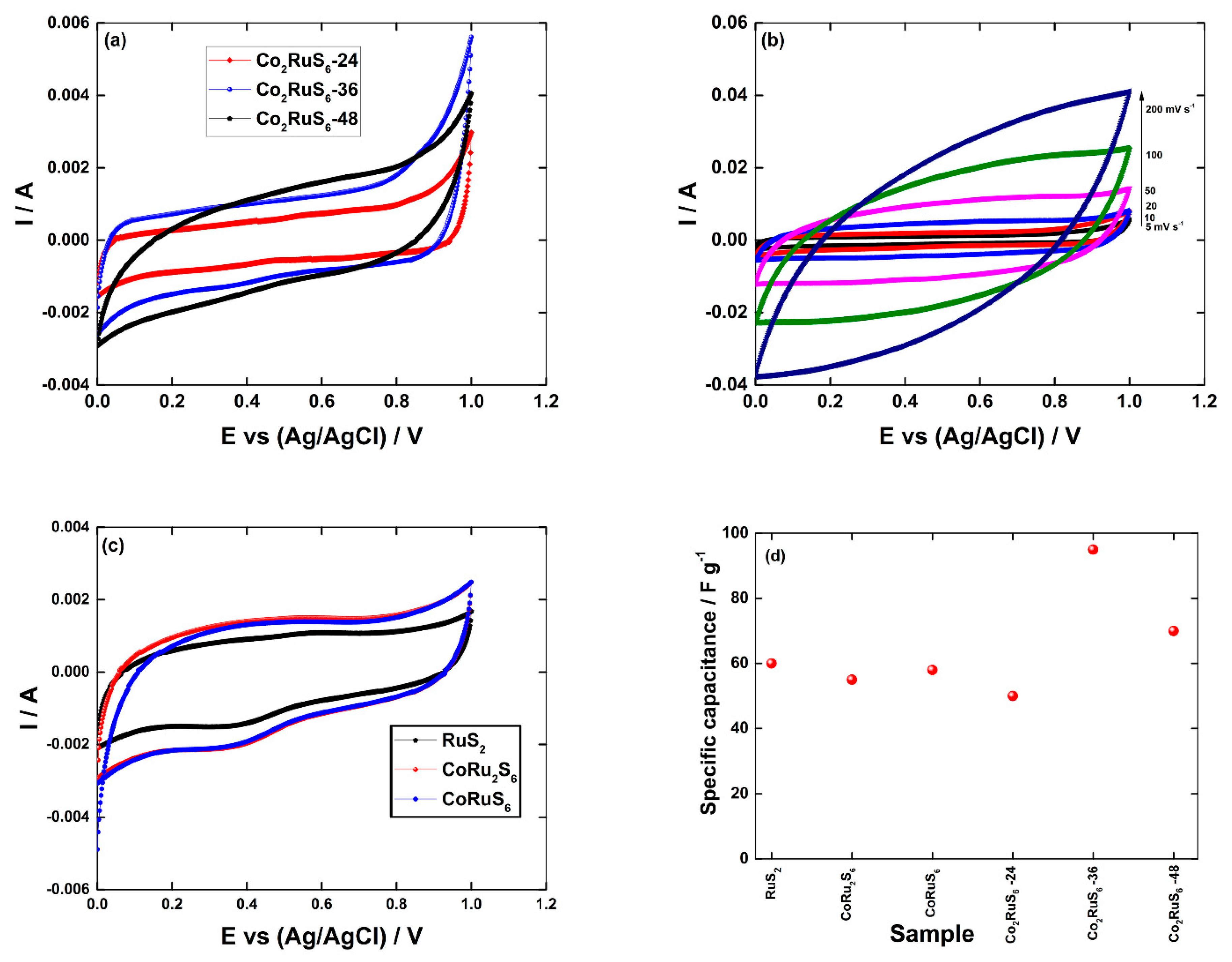
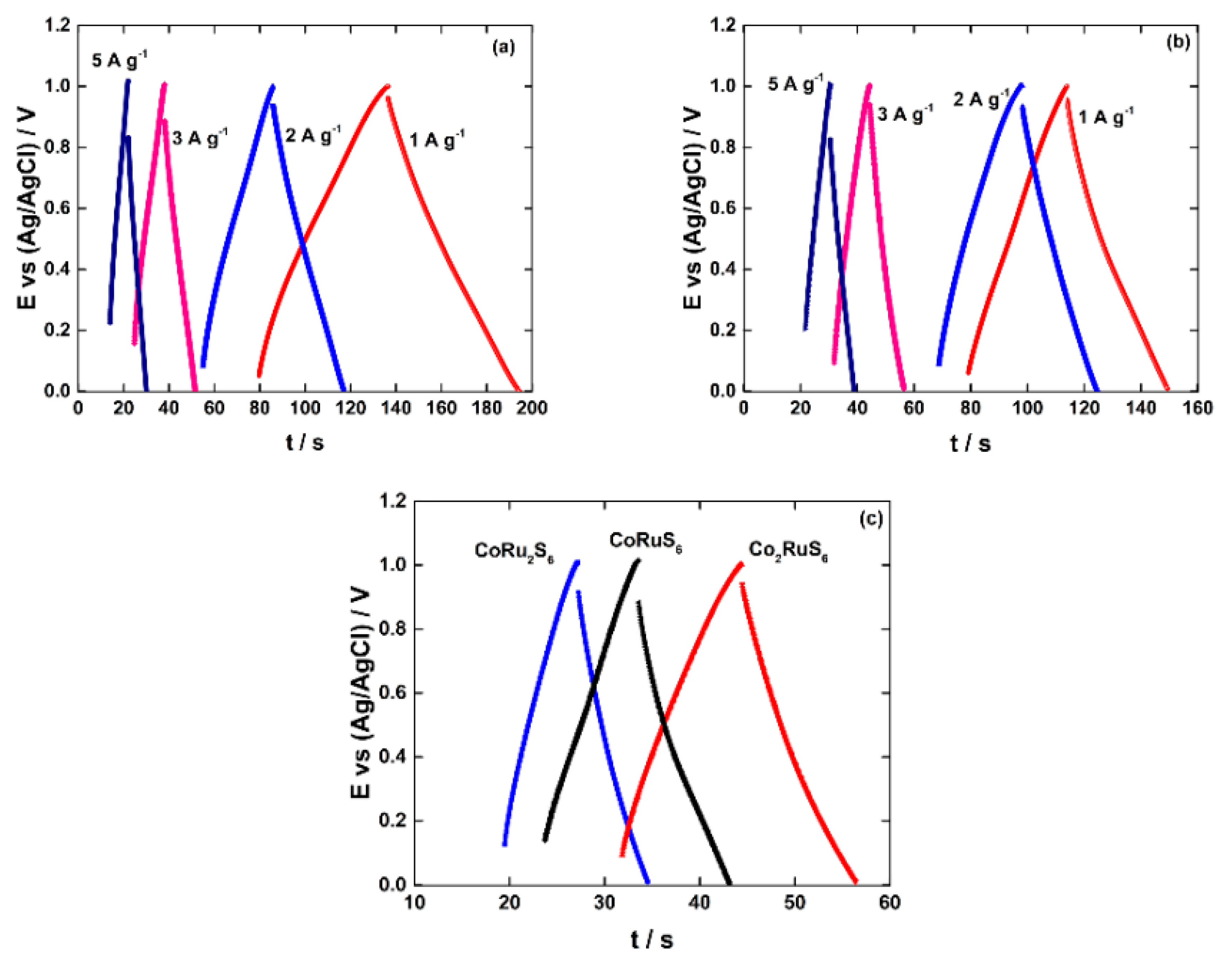
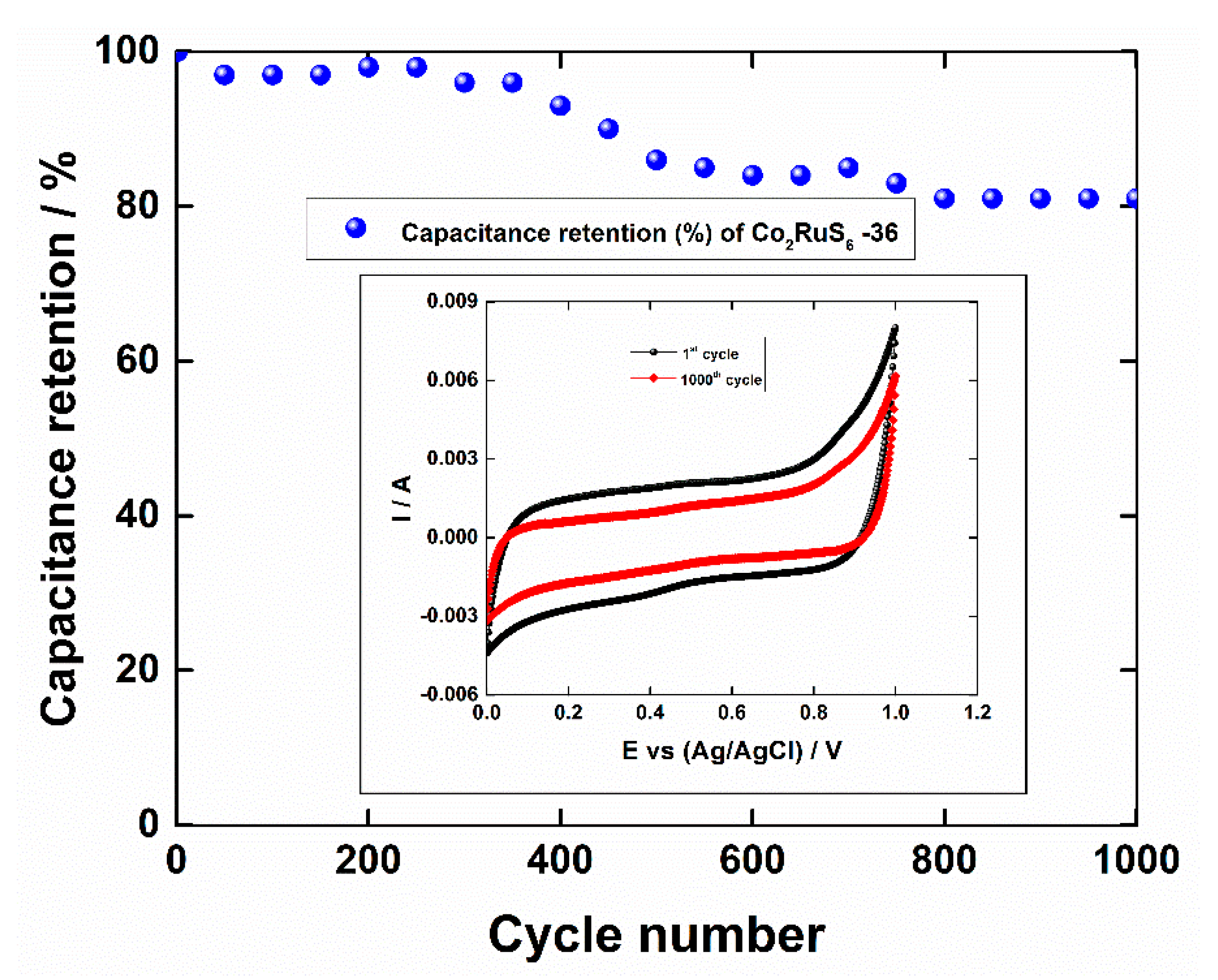
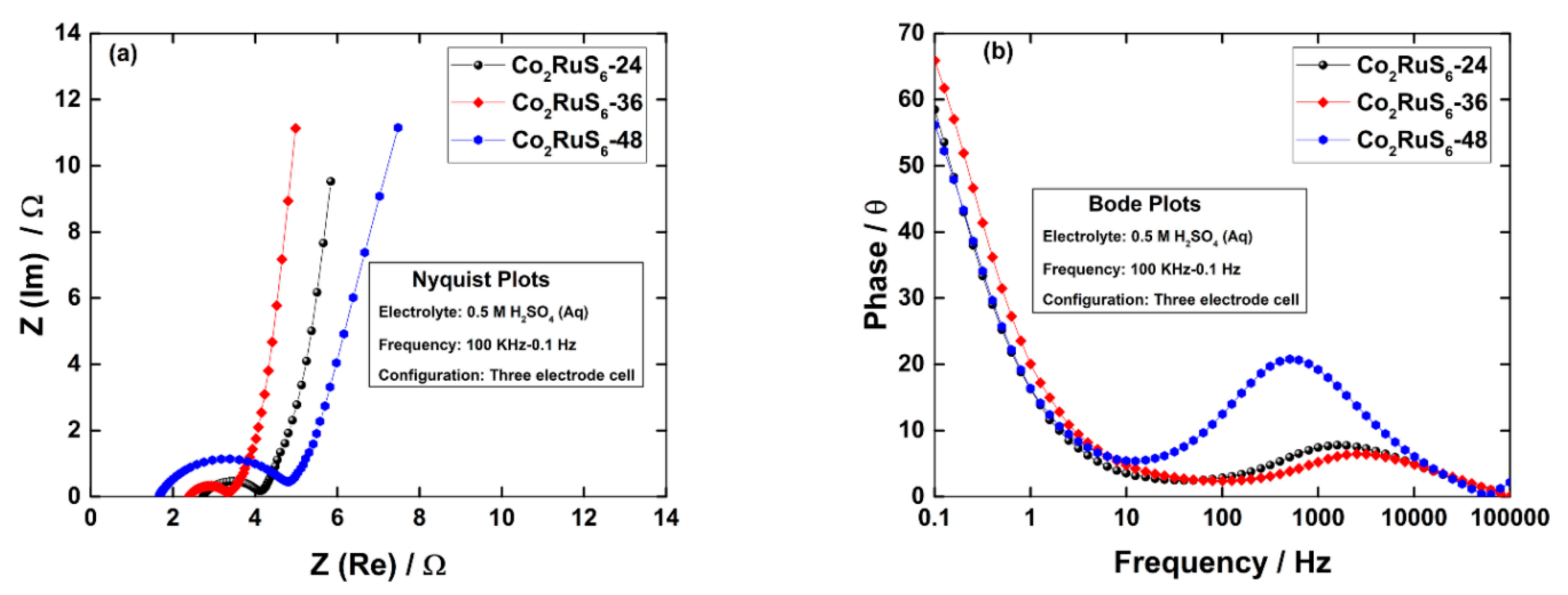
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, T.; Chen, I.W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef]
- Gonzalez, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sust. Energ. Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.K.; Rao, G.R. Fabrication of NiCo2S4 nanoball embedded nitrogen doped mesoporous carbon on nickel foam as an advanced charge storage material. Electrochim. Acta 2018, 268, 139–149. [Google Scholar] [CrossRef]
- Lu, Y.; Li, B.; Zheng, S.; Xu, Y.; Xue, H.; Pang, H. Syntheses and energy storage applications of MxSy(M= Cu, Ag, Au) and their composites: rechargeable batteries and supercapacitors. Adv. Funct. Mater. 2017, 27, 1703949. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Cottineau, T.; Toupin, M.; Delahaye, T.; Brousse, T.; Belanger, D. Nanostructured transition metal oxides for aqueous hybrid electrochemical supercapacitors. Appl. Phys. A. 2006, 82, 599–606. [Google Scholar] [CrossRef]
- Deng, W.; Ji, X.; Chen, Q.; Banks, C.E. Electrochemical capacitors utilising transition metal oxides: an update of recent developments. Rsc Adv. 2011, 1, 1171–1178. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Bolagam, R.; Boddula, R.; Srinivasan, P. Synthesis of highly crystalline polyaniline with the use of (Cyclohexylamino)-1-propanesulfonic acid for supercapacitor. J. Appl. Electrochem. 2015, 45, 51–56. [Google Scholar] [CrossRef]
- Rajender, B.; Palaniappan, S. Organic solvent soluble methyltriphenylphosphonium peroxodisulfate: a novel oxidant for the synthesis of polyaniline and the thus prepared polyaniline in high performance supercapacitors. New J. Chem. 2015, 39, 5382–5388. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Bolagam, R.; Boddula, R.; Srinivasan, P. One-step preparation of sulfonated carbon and subsequent preparation of hybrid material with polyaniline salt: a promising supercapacitor electrode material. J. Solid State Electrochem. 2017, 21, 1313–1322. [Google Scholar] [CrossRef]
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Bolagam, R.; Srinivasan, P. Use of oil in the polymerization of aniline to polyaniline salt containing dual dopants, sulfuric acid, and castor oil: material for high-performance supercapacitor. Ionics 2017, 23, 1277–1284. [Google Scholar] [CrossRef]
- Yu, X.Y.; Lou, X.W. Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 2018, 8, 1701592. [Google Scholar] [CrossRef]
- Sahoo, S.; Pazhamalai, P.; Krishnamoorthy, K.; Kim, S.J. Hydrothermally prepared α-MnSe nanoparticles as a new pseudocapacitive electrode material for supercapacitor. Electrochim. Acta 2018, 268, 403–410. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Yu, L.; Lou, X.W.D. Metal sulfide hollow nanostructures for electrochemical energy storage. Adv. Energy Mater. 2016, 6, 1501333. [Google Scholar] [CrossRef]
- Rui, X.; Tan, H.; Yan, Q. Nanostructured metal sulfides for energy storage. Nanoscale 2014, 6, 9889–9924. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Yu, L.; Shen, L.; Song, X.; Chen, H.; Lou, X.W.D. General formation of MS (M= Ni, Cu, Mn) box-in-box hollow structures with enhanced pseudocapacitive properties. Adv. Funct. Mater. 2014, 24, 7440–7446. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Kim, S.J. Ruthenium sulfide nanoparticles as a new pseudocapacitive material for supercapacitor. Electrochim. Acta 2017, 227, 85–94. [Google Scholar] [CrossRef]
- Bolagam, R.; Um, S. l-cysteine-assisted synthesis of ruthenium sulfide/thermally reduced graphene oxide nanocomposites: Promising electrode materials for high-performance energy storage applications. Electrochim. Acta 2018, 281, 571–581. [Google Scholar] [CrossRef]
- Patil, S.J.; Kim, J.H.; Lee, D.W. Graphene-nanosheet wrapped cobalt sulphide as a binder free hybrid electrode for asymmetric solid-state supercapacitor. J. Power Sources 2017, 342, 652–665. [Google Scholar] [CrossRef]
- Luo, F.; Li, J.; Yuan, H.; Xiao, D. Rapid synthesis of three-dimensional flower-like cobalt sulfide hierarchitectures by microwave assisted heating method for high-performance supercapacitors. Electrochim. Acta 2014, 123, 183–189. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, W.; Sun, Y.; Dai, M. Ruthenium nanoparticles stabilized by mercaptan and acetylene derivatives with supercapacitor application. Electrochim. Acta 2018, 270, 284–293. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Holze, R.; Jadhav, H.S.; Lokhande, C.D.; Park, C.J. Solution-based binder-free synthetic approach of RuO2 thin films for all solid state supercapacitors. Electrochim. Acta 2015, 103, 103–109. [Google Scholar] [CrossRef]
- Kulkarni, P.; Nataraj, S.K.; Balakrishna, R.G.; Nagaraju, D.H.; Reddy, M.V. Nanostructured binary and ternary metal sulfides: synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A. 2017, 5, 22040–22094. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Holze, R.; Kim, D.H.; Gomez-Romero, P. Ultrathin Mesoporous RuCo2O4 Nanoflakes: An Advanced Electrode for High-Performance Asymmetric Supercapacitors. Chem. Sus. Chem. 2017, 10, 1771–1782. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Xu, G.; Li, H.; Dou, H.; Zhang, X. NiCo2S4 nanosheets grown on nitrogen-doped carbon foams as an advanced electrode for supercapacitors. Adv. Energy Mater. 2015, 5, 1400977. [Google Scholar] [CrossRef]
- Guo, S.; Chen, W.; Li, M.; Wang, J.; Liu, F.; Cheng, J.P. Effect of reaction temperature on the amorphous-crystalline transition of copper cobalt sulfide for supercapacitors. Electrochim. Acta 2018, 271, 498–506. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Kim, Y.; Cho, J. Nanostructured transition metal sulfides for lithium ion batteries: Progress and challenges. Nano Today 2014, 9, 604–630. [Google Scholar] [CrossRef]
- Gulen, M.; Sarilmaz, A.; Patir, I.H.; Ozel, F.; Sonmezoglu, S. Ternary copper-tungsten-disulfide nanocube inks as catalyst for highly efficient dye-sensitized solar cells. Electrochim. Acta 2018, 269, 119–127. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Cao, S.; Xue, H.; Pang, H. Transition metal sulfides based on graphene for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, X.; Zhu, X.; Liu, H.; Hou, L. Nickel cobalt sulfide double-shelled hollow nanospheres as superior bifunctional electrocatalysts for photovoltaics and alkaline hydrogen evolution. Acs Appl. Mater. Interfaces 2018, 10, 9379–9389. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Kang, H.; Jin, T.; Jiao, L. Design, synthesis, and energy-related applications of metal sulfides. Mater. Horiz. 2016, 3, 402–421. [Google Scholar] [CrossRef]
- Deng, C.; Yang, L.; Yang, C.; Shen, P.; Zhao, L.; Wang, Z.; Wang, C.; Li, J.; Qian, D. Spinel FeCo2S4 nanoflower arrays grown on Ni foam as novel binder-free electrodes for long-cycle-life supercapacitors. Appl. Surf. Sci. 2018, 428, 148–153. [Google Scholar] [CrossRef]
- Rajesh, J.A.; Park, J.H.; Quy, V.H.V.; Kwon, J.M.; Chae, J.; Kang, S.H.; Kim, H.; Ahn, K.S. Rambutan-like cobalt nickel sulfide (CoNi2S4) hierarchitecture for high-performance symmetric aqueous supercapacitors. J. Ind. Eng. Chem. 2018, 63, 73–83. [Google Scholar] [CrossRef]
- Liu, S.; Jun, S.C. Hierarchical manganese cobalt sulfide core–shell nanostructures for high-performance asymmetric supercapacitors. J. Power Sources 2017, 342, 629–637. [Google Scholar] [CrossRef]
- Gao, Y.P.; Huang, K.J. NiCo2S4 materials for supercapacitor applications. Chem. Asian J. 2017, 12, 1969–1984. [Google Scholar] [CrossRef]
- Li, B.; Zheng, M.; Xue, H.; Pang, H. High performance electrochemical capacitor materials focusing on nickel based materials. Inorg. Chem. Front. 2016, 3, 175–202. [Google Scholar] [CrossRef]
- Huang, T.; Song, X.Z.; Chen, X.; Chen, X.L.; Sun, F.F.; Su, Q.F.; Li, L.D.; Tan, Z. Carbon coated nickel–cobalt bimetallic sulfides hollow dodecahedrons for a supercapacitor with enhanced electrochemical performance. New J. Chem. 2018, 42, 5128–5134. [Google Scholar] [CrossRef]
- Liu, W.; Niu, H.; Yang, J.; Cheng, K.; Ye, K.; Zhu, K.; Wang, G.; Cao, D.; Yan, J. Ternary transition metal sulfides embedded in graphene nanosheets as both the anode and cathode for high-performance asymmetric supercapacitors. Chem. Mater. 2018, 30, 1055–1068. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Wang, X.; Song, S.; Lou, X.W. Formation of onion-like NiCo2S4 particles via sequential ion-exchange for hybrid supercapacitors. Adv. Mater. 2017, 29, 1605051. [Google Scholar] [CrossRef]
- Kristl, M.; Dojer, B.; Gyergyek, S.; Kristl, J. Synthesis of nickel and cobalt sulfide nanoparticles using a low cost sonochemical method. Heliyon 2017, 3, e00273. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yu, J.; Xing, Z.; Guo, X.; Yu, X.; Tang, B.; Zou, J. Enhanced generation of H2O2 and radicals on Co9S8/partly-graphitized carbon cathode for degradation of bio-refractory organic wastewater. Electrochim. Acta 2016, 213, 341–350. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, L.; Hu, H.; Zhao, S. Co9S8 nanotubes synthesized on the basis of nanoscale Kirkendall effect and their magnetic and electrochemical properties. Cryst. Eng. Comm. 2010, 12, 1899–1904. [Google Scholar] [CrossRef]
- Tang, X.; Huang, J.; Feng, Q.; Liu, K.; Luo, X.; Li, Z. Carbon sphere@Co9S8 yolk-shell structure with good morphology stability for improved lithium storage performance. Nanotechnology 2017, 28, 375402. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Deng, J.; Zhu, J.; Bi, J.; Kan, E.; Wang, X. Cobalt sulfide/graphene composite hydrogel as electrode for high-performance pseudocapacitors. Sci. Rep. 2016, 6, 21717. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, S.; Chen, Y.; Han, L.; Zhou, J.; Wu, X.; Qin, W. Graphene/Co9S8 nanocomposite paper as a binder-free and free-standing anode for lithium-ion batteries. J. Mater. Chem. A. 2015, 3, 23677–23683. [Google Scholar] [CrossRef]
- Xie, S.; Deng, Y.; Mei, J.; Yang, Z.; Lau, W.M.; Liu, H. Facile synthesis of CoS2/CNTs composite and its exploitation in thermal battery fabrication. Composites Part. B. 2016, 93, 203–209. [Google Scholar] [CrossRef]
- Cai, X.; Shen, X.; Ma, L.; Ji, Z. Facile synthesis of nickel–cobalt sulfide/reduced graphene oxide hybrid with enhanced capacitive performance. Rsc Adv. 2015, 5, 58777–58783. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Yanagisawa, K.; Li, X.; Yan, X. Hydrothermal synthesis of highly crystalline RuS2 nanoparticles as cathodic catalysts in the methanol fuel cell and hydrochloric acid electrolysis. Mater. Res. Bull. 2015, 65, 110–115. [Google Scholar] [CrossRef]
- Gabold, H.; Luan, Z.; Paul, N.; Opel, M.; Müller-Buschbaum, P.; Law, M.; Paul, A. Structural and magnetic properties of cobalt iron disulfide (CoxFe1−xS2) nanocrystals. Sci. Rep. 2018, 8, 4835. [Google Scholar] [CrossRef]
- Li, M.L.; Xiao, K.; Su, J.; Li, N.; Cai, Y.P.; Liu, Z.Q. CuCo2S4 nanosheets coupled with carbon nanotube heterostructures for highly efficient capacitive energy storage. Chem. Electro. Chem. 2018, 5, 2496–2502. [Google Scholar]
- Xiao, J.; Wan, L.; Yang, S.; Xiao, F.; Wang, S. Design hierarchical electrodes with highly conductive NiCo2S4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett. 2014, 14, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, P.; Koltypin, Y.; Gofer, Y.; Diamant, Y.; Gedanken, A. Sonochemical synthesis of nanocrystallites of ruthenium sulfide, RuS1.7. J. Mater. Chem. 2000, 10, 2769–2773. [Google Scholar] [CrossRef]
- Singu, B.S.; Yoon, K.R. Synthesis and characterization of MnO2-decorated graphene for supercapacitors. Electrochim. Acta 2017, 231, 749–758. [Google Scholar] [CrossRef]
- Hu, C.-C.; Huang, Y.-H.; Chang, K.-H. Annealing effects on the physicochemical characteristics of hydrous ruthenium and ruthenium–iridium oxides for electrochemical supercapacitors. J. Power Sources 2002, 108, 117–127. [Google Scholar] [CrossRef]
- Patake, V.D.; Lokhande, C.D. Chemical synthesis of nano-porous ruthenium oxide (RuO2) thin films for supercapacitor application. App. Surf. Sci. 2008, 254, 2820–2824. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Veerasubramani, G.K.; Kim, S.-J. Mechanically delaminated few layered MoS2 nanosheets based high performance wire type solid-state symmetric supercapacitors. J. Power Sources. 2016, 321, 112–119. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, G.K.; Radhakrishnan, S.; Kim, S.-J. Preparation of copper sulfide nanoparticles by sonochemical method and study on their electrochemical properties. J. Nanosci. Nanotechnol. 2015, 15, 4409–4413. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Ambrosi, A.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Transition metal dichalcogenides (MoS2, MoSe2, WS2 and WSe2) exfoliation technique has strong influence upon their capacitance. Electrochem. Commun. 2015, 56, 24–28. [Google Scholar] [CrossRef]
- Chen, C.M.; Zhang, Q.; Yang, M.G.; Huang, C.H.; Yang, Y.G.; Wang, M.Z. Structural evolution during annealing of thermally reduced graphene nanosheets for application in supercapacitors. Carbon 2012, 50, 3572–3584. [Google Scholar] [CrossRef]
- Bolagam, R.; Boddula, R.; Srinivasan, P. Hybrid material of PANI with TiO2-SnO2: Pseudocapacitor electrode for higher performance supercapacitors. Chem. Sel. 2017, 2, 65–73. [Google Scholar] [CrossRef]
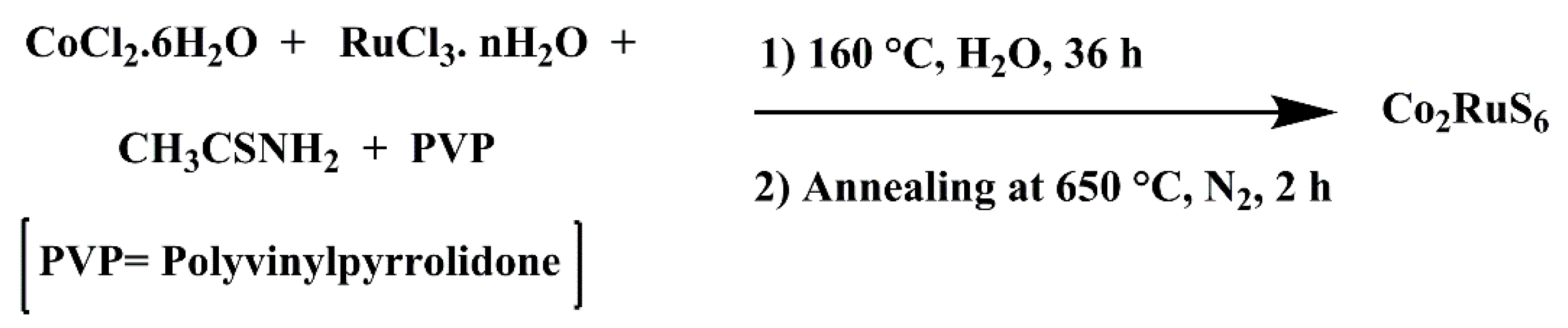
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolagam, R.; Um, S. Hydrothermal Synthesis of Cobalt Ruthenium Sulfides as Promising Pseudocapacitor Electrode Materials. Coatings 2020, 10, 200. https://doi.org/10.3390/coatings10030200
Bolagam R, Um S. Hydrothermal Synthesis of Cobalt Ruthenium Sulfides as Promising Pseudocapacitor Electrode Materials. Coatings. 2020; 10(3):200. https://doi.org/10.3390/coatings10030200
Chicago/Turabian StyleBolagam, Ravi, and Sukkee Um. 2020. "Hydrothermal Synthesis of Cobalt Ruthenium Sulfides as Promising Pseudocapacitor Electrode Materials" Coatings 10, no. 3: 200. https://doi.org/10.3390/coatings10030200
APA StyleBolagam, R., & Um, S. (2020). Hydrothermal Synthesis of Cobalt Ruthenium Sulfides as Promising Pseudocapacitor Electrode Materials. Coatings, 10(3), 200. https://doi.org/10.3390/coatings10030200




