Property of TiO2-15MgAl2O4 Electrical-Heating Coating Prepared by Atmospheric Plasma Spraying and Hydrogen Heat Treatment
Abstract
1. Introduction
2. Experimental Procedure
2.1. Experimental Materials
2.2. Preparation of Coating and Hydrogen Heat Treatment
2.3. Performance Characterization of Coatings
3. Results and Discussion
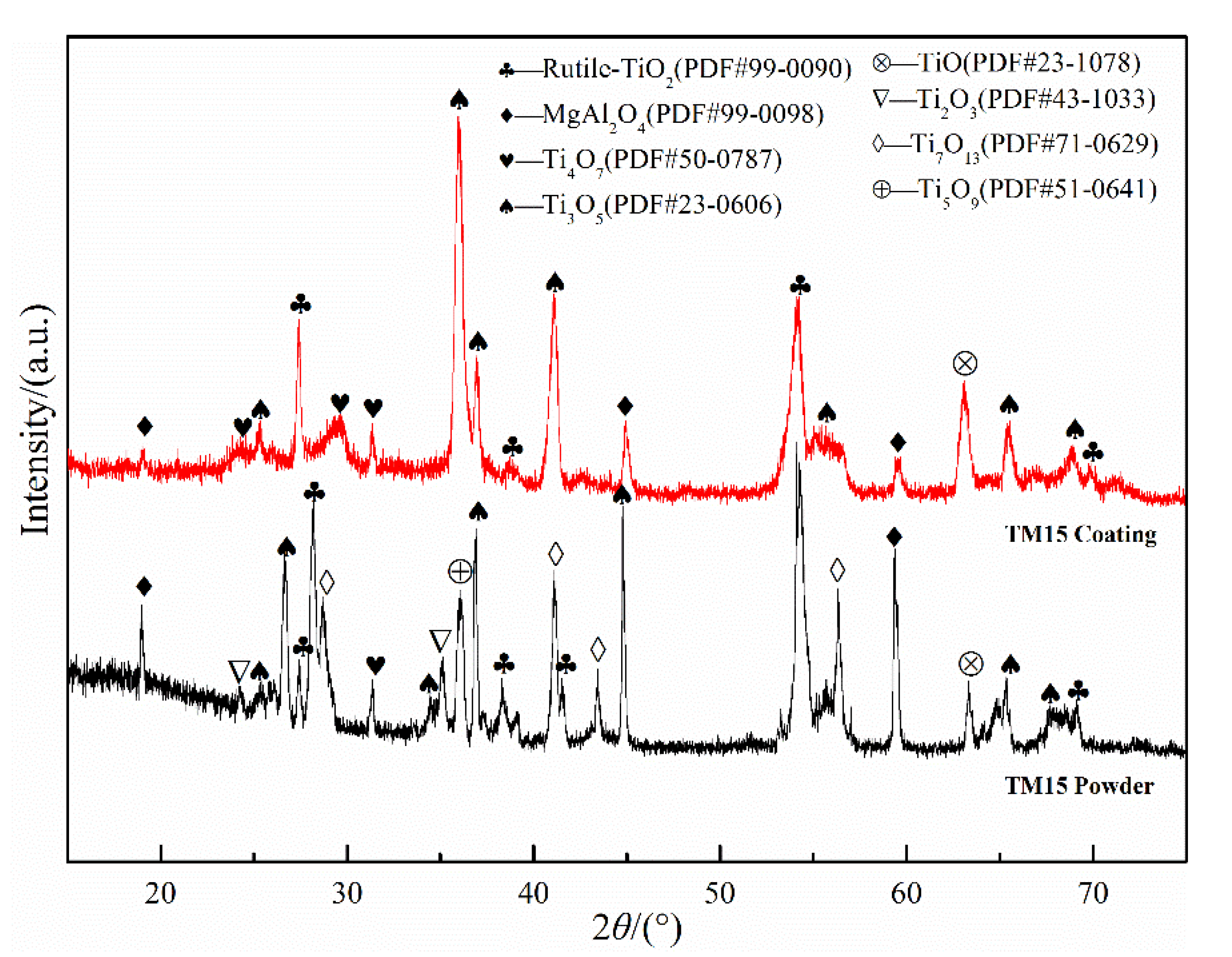
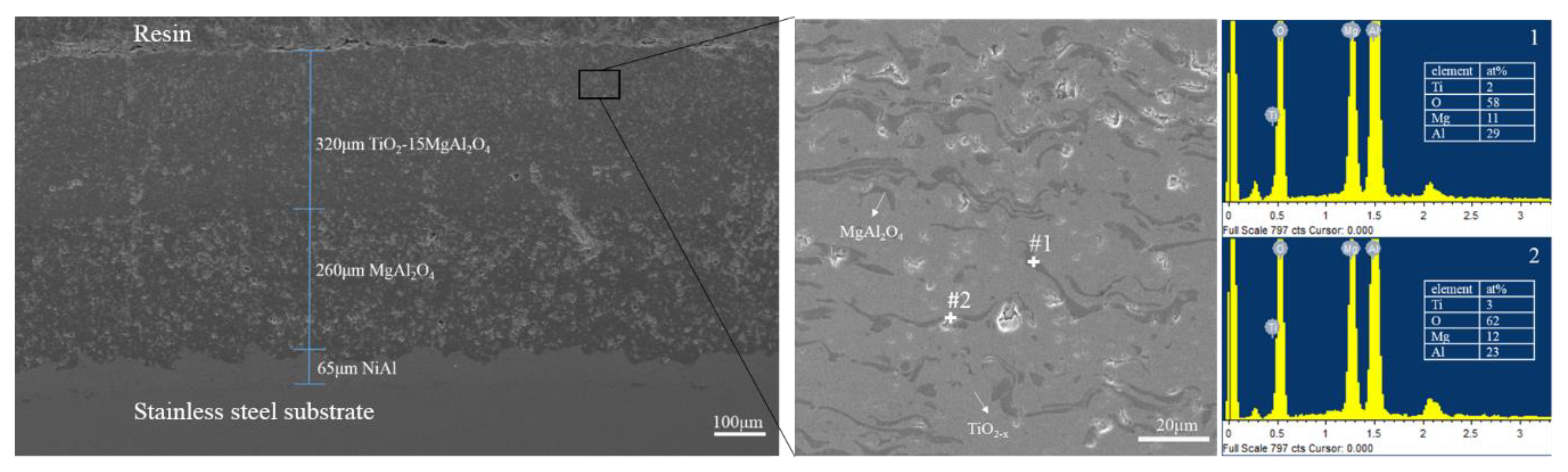
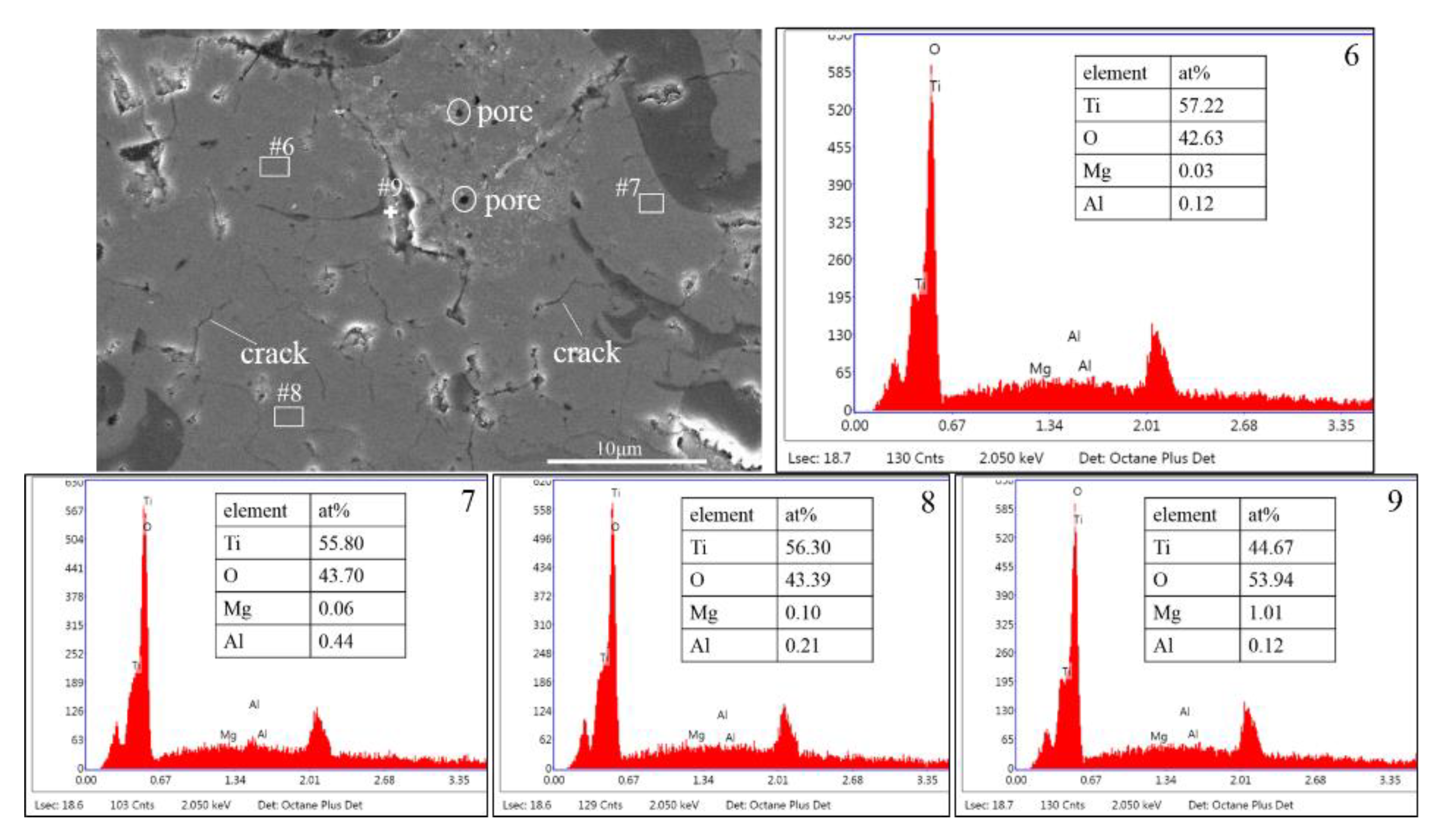
3.1. Phase Composition and Microstructure
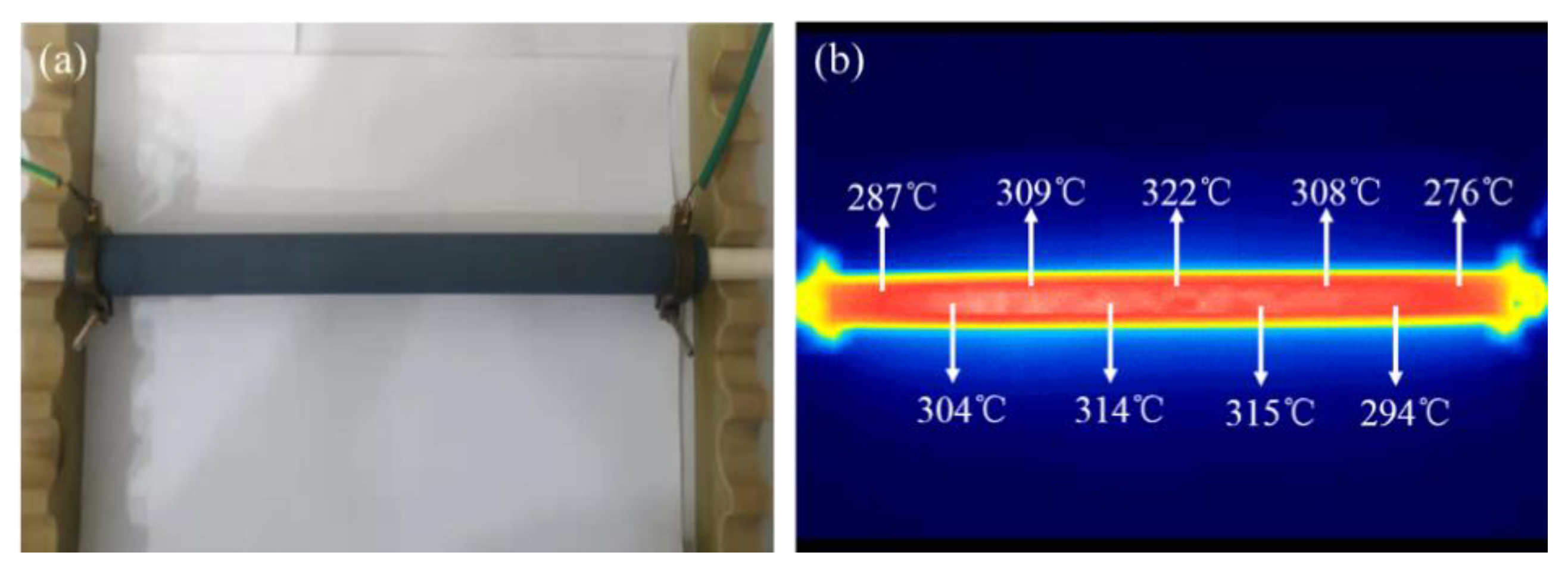
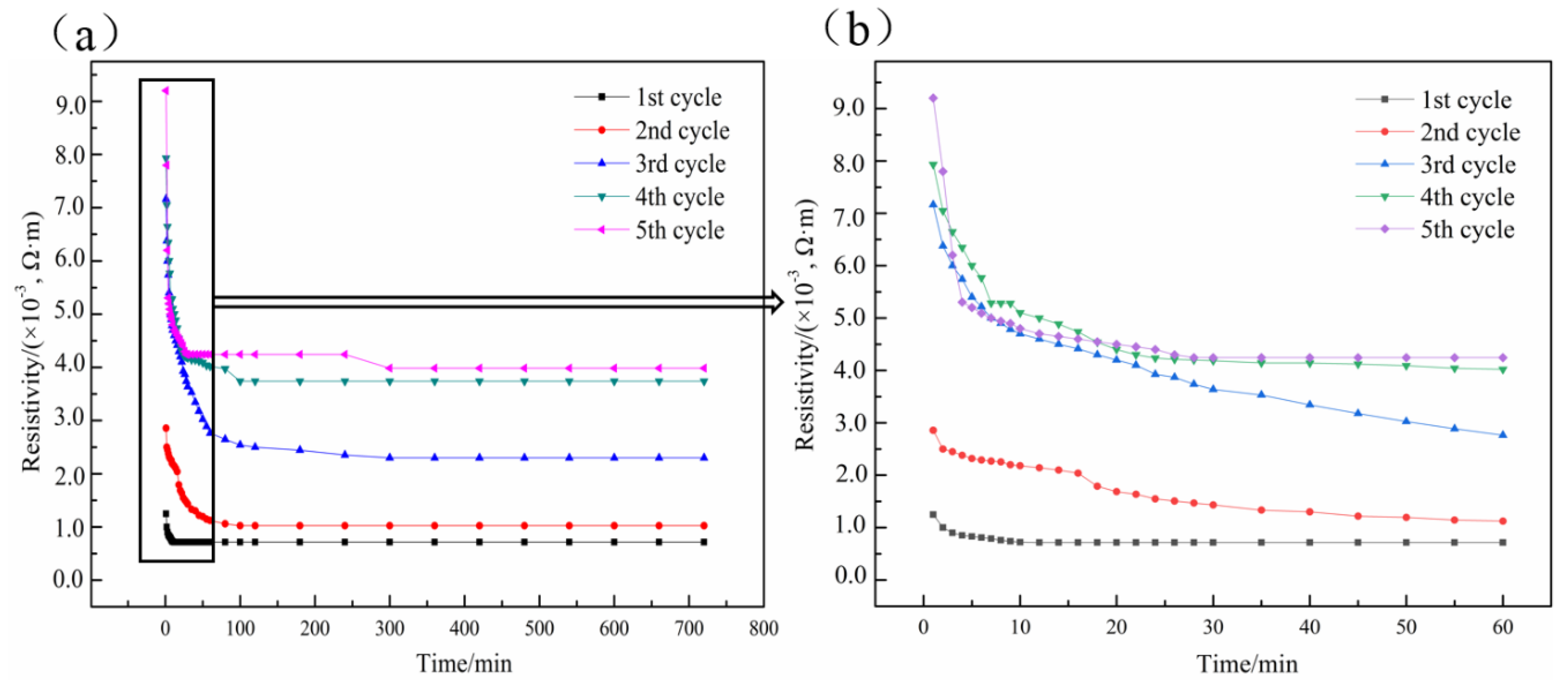
3.2. Electrical-Heating Properties
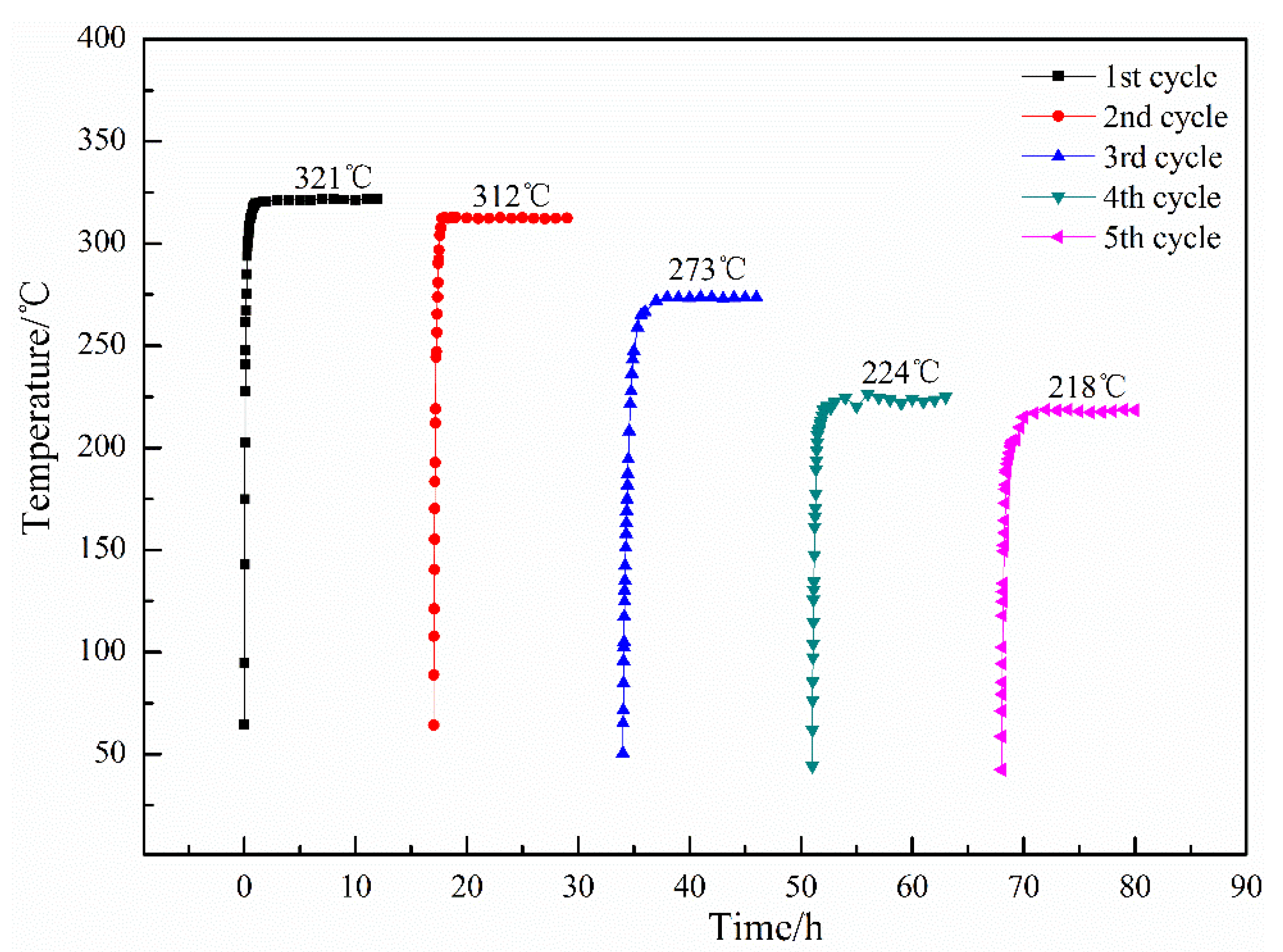
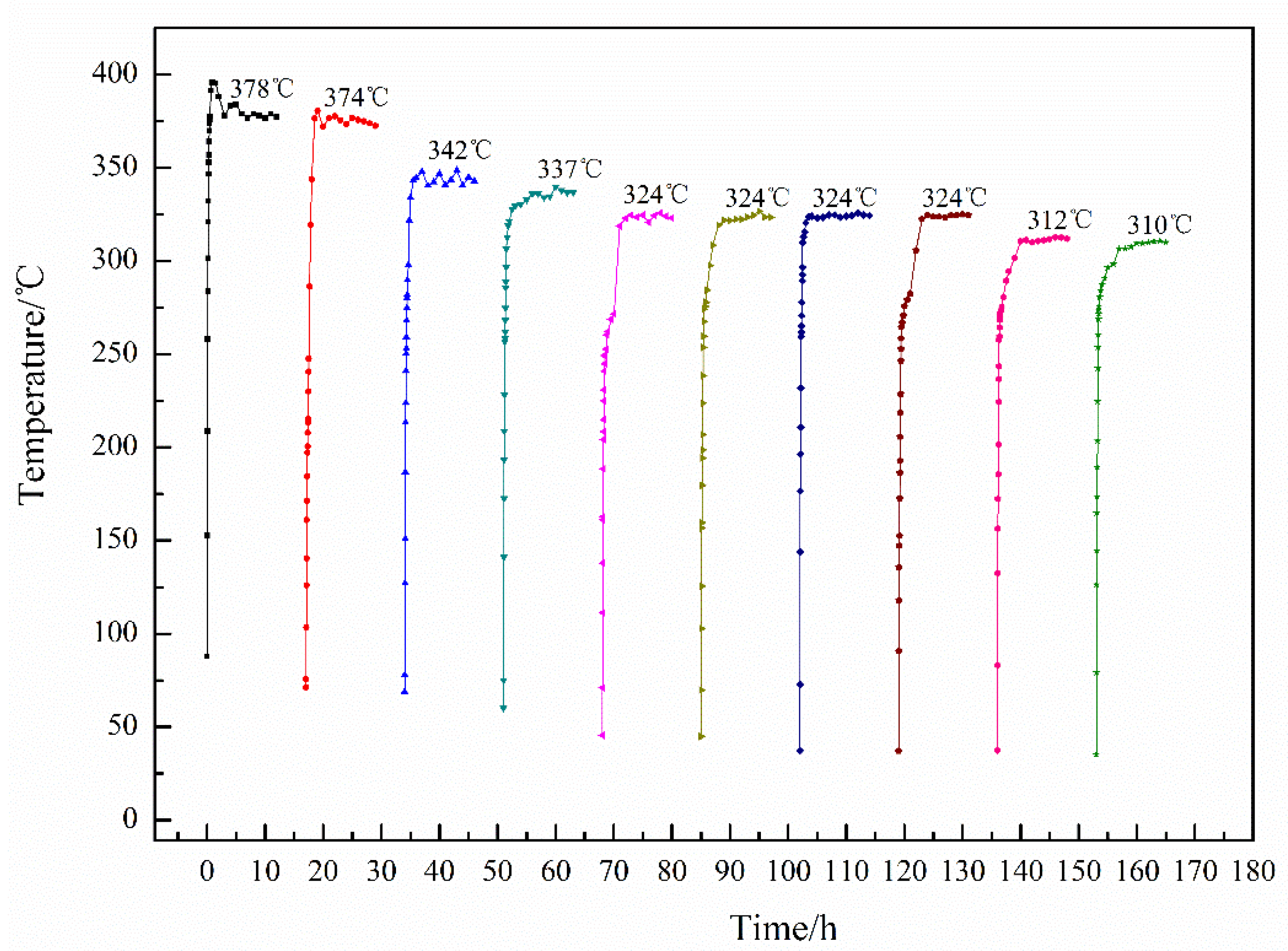
3.3. Effect of Hydrogen Heat Treatment on Coating Electrical-Heating Properties
4. Conclusions
- (1)
- The phases of atmospheric plasma sprayed TiO2-15MgAl2O4 coating include Ti3O5, rutile TiO2, Ti4O7, MgAl2O4, and TiO phase. The MgAl2O4 distributes uniformly in a wavy strip in the TiO2–x matrix.
- (2)
- The resistivity of electrical-heating coating rises and the available heating temperature declines during cyclic electrification tests. This is attributed to the increased pores, expanded micro-cracks, and re-oxidized TiO2–x.
- (3)
- After hydrogen heat treatment, Ti3O5 of the electrical-heating coating increases obviously. The available heating temperature and the stability of coating resistivity are significantly improved.
Author Contributions
Funding
Conflicts of Interest
References
- Jang, H.-S.; Jeon, S.-K.; Nahm, S.-H. The manufacture of a transparent film heater by spinning multi-walled carbon nanotubes. Carbon 2011, 49, 111–116. [Google Scholar] [CrossRef]
- Cheng, C.; Ke, K.-C.; Yang, S.-Y. Application of graphene–polymer composite heaters in gas-assisted micro hot embossing. RSC Adv. 2017, 7, 6336–6344. [Google Scholar] [CrossRef]
- Heinz, C.-N.; Luigi, V.; Andre, S. Epoxy/MWCNT Composite as Temperature Sensor and Electrical Heating Element. IEEE Trans. Nanotechnol. 2011, 10, 688–693. [Google Scholar] [CrossRef]
- Sui, D.; Huang, Y.; Huang, L.; Liang, J.; Ma, Y.; Chen, Y. Flexible and Transparent Electrothermal Film Heaters Based on Graphene Materials. Small 2011, 7, 3186–3192. [Google Scholar] [CrossRef]
- Zhang, M.-C.; Wang, C.-Y.; Liang, X.-P.; Yin, Z.; Xia, K.; Wang, H.; Jian, M.; Zhang, Y. Weft-Knitted Fabric for a Highly Stretchable and Low-Voltage Wearable Heater. Adv. Electron. Mater. 2017, 3. [Google Scholar] [CrossRef]
- Li, D.-Q.; Tang, B.; Lu, X.; Li, Q.; Chen, W.; Dong, X.; Wang, J.; Wang, X. Simultaneous PAN Carbonization and Ceramic Sintering for Fabricating Carbon Fiber-Ceramic Composite Heaters. Appl. Sci. 2019, 9, 4945. [Google Scholar] [CrossRef]
- Prudenziati, M.; Gualtieri, M.L. Electrical properties of thermally sprayed Ni- and Ni20Cr-based resistors. J. Therm. Spray Technol. 2008, 17, 385–394. [Google Scholar] [CrossRef]
- Gadow, R.; Killinger, A.; Li, C. Product Development with Thermally Sprayed Functional Coatings on Glass and Glassceramics Substrates. Int. J. Appl. Ceram. Technol. 2005, 2, 493–503. [Google Scholar] [CrossRef]
- Prudenziatji, M. Development and the Implementation of High-Temperature Reliable Heaters in Plasma Spray Technology. J. Therm. Spray Technol. 2008, 17, 234–243. [Google Scholar] [CrossRef]
- Frenck, H.-J.; Kulisch, W.; Kuhr, M.; Kassing, R. Deposition of TiO2 thin films by plasma-enhanced decomposition of tetraisopropyltitanate. Thin Solid Film. 1991, 201, 327–335. [Google Scholar] [CrossRef]
- Killinger, A.; Gadow, R. Thermally Sprayed Coating Composites for Film Heating Devices. Adv. Sci. Technol. 2006, 45, 1230–1239. [Google Scholar] [CrossRef]
- Apkaryan, A.-S.; Khristyukov, V.-G.; Smirnov, G.-V. Heat insulation of high-temperature heating units for ceramic production. Glass Ceram. 2010, 67, 52–55. [Google Scholar] [CrossRef]
- Akani, A.; Suryanarayanau, R.; Brun, G. Influence of Process Parameters on the Electrical Properties of Plasma-Sprayed Silicon. J. Appl. Phys. 1986, 60, 457–459. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Q.; Liu, W. Mechanical and oxidation resistance properties of 3d carbon fiber-reinforced mullite matrix composites prepared by sol–gel process. Ceram. Int. 2014, 40, 7203–7212. [Google Scholar] [CrossRef]
- Liang, Y.-H.; Zhou, B.-M.; Li, N.; Liu, L.; Xu, Z.; Li, F.; Li, J.; Mai, W.; Qian, X.; Wu, N. Enhanced dye photocatalysis and recycling abilities of semi-wrapped TiO2@carbon nanofibers formed via foaming agent driving. Ceram. Int. 2018, 44, 1711–1718. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.-C.; Villarreal-Chiu, J.-F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Lv, X.; Wang, T.-H.; Jiang, W. Preparation of Ag@AgCl/g-C3N4/TiO2 porous ceramic films with enhanced photocatalysis performance and self-cleaning effect. Ceram. Int. 2018, 44, 9326–9337. [Google Scholar] [CrossRef]
- Shi, J.-B.; Chen, G.-Q.; Zeng, G.-M.; Chen, A.; He, K.; Huang, Z.; Hu, L.; Zeng, J.; Wu, J.; Liu, W. Hydrothermal synthesis of graphene wrapped Fe-doped TiO2 nanospheres with high photocatalysis performance. Ceram. Int. 2018, 44, 7473–7480. [Google Scholar] [CrossRef]
- Roman, J.; Lech, P.; Francine, R.; Kozerski, S.; Petit, F. Characterization of mechanical properties of suspension plasma sprayed TiO2 coatings using scratch test. Surf. Coat. Technol. 2008, 202, 2644–2653. [Google Scholar] [CrossRef]
- Branland, N.; Meillot, E.; Fauchais, P.; Vaedelle, A.; Gitzhofer, F.; Boulos, M. Relationships between Microstructure and Electrical Properties of RF and DC Plasma-Sprayed Titania Coatings. J. Therm. Spray Technol. 2006, 15, 53–62. [Google Scholar] [CrossRef]
- Keshuukh, H.-P.; Shinde, P.-S.; Patil, P.-S. Structural, Optical and Electrical Characterization of Spray-deposited TiO2 Thin Films. Mater. Sci. Eng. B 2006, 130, 220–227. [Google Scholar] [CrossRef]
- Toma, F.-L.; Scheitz, S.; Puschmann, R.; Berger, L.-M. Development of ceramic heating elements produced by thermal spray technology. In Proceedings of the International Thermal Spray Conference, Dusseldorf, Germany, 27–29 September 2011; DVS Publishing House: Hamburg, Germany, 2012. [Google Scholar]
- Bobzin, K.; Oete, M.; Knoch, M.A.; Alkhasli, I. Temperature distribution on thermally sprayed heating conductor coatings. IOP Conf. Ser. Mater. Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Culha, O.; Celik, E.; Azem, N.-F.-A.; Birlik, I.; Toparli, M.; Turk, A. Microstructural, thermal and mechanical properties of HVOF sprayed Ni-Al-based bond coatings on stainless steel substrate. J. Mater. Process. Technol. 2008, 204, 221–230. [Google Scholar] [CrossRef]
- Toma, F.-L.; Scheitz, S.; Berger, L.-M.; Sauchuk, V.; Kusnezoff, M.; Thiele, S. Comparative study of the electrical properties and characteristics of thermally sprayed alumina and spinel coatings. J. Therm. Spray Technol. 2011, 20, 195–204. [Google Scholar] [CrossRef]
- Lee, C. Photocatalytic properties of nano-structured TiO2 plasma sprayed coating. Surf. Coat. Technol. 2003, 173, 192–200. [Google Scholar] [CrossRef]
- Palmer, R.-A.; Doan, T.-M.; Lloyd, P.-G.; Jarvis, B.L.; Ahmed, N.U. Reduction of TiO2 with hydrogen plasma. Plasma Chem. Plasma Process. 2002, 22, 335–349. [Google Scholar] [CrossRef]
- Floristan, M.; Fontarnau, R.; Killinger, A.; Gadow, R. Development of electrically conductive plasma sprayed coatings on glass ceramic substrates. Surf. Coat. Technol. 2010, 205, 1021–1028. [Google Scholar] [CrossRef]
- Song, J.-B.; Zhang, X.-F.; Deng, C.-M.; Deng, C.; Liu, M.; Zhou, K.; Tong, X. Research of in situ modified PS-PVD thermal barrier coating against CMAS (CaO-MgO-Al2O3-SiO2) corrosion. Ceram. Int. 2016, 42, 3163–3169. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Deng, C.-M.; Deng, C.-G.; Liu, M.; Zhou, K. Mullite-modified ZrB2-MoSi2 coating for carbon/carbon composites to withstand long term ablation. Ceram. Int. 2018, 44, 4330–4337. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhou, K.S.; Deng, C.M.; Liu, M.; Deng, Z.Q.; Deng, C.G.; Song, J.B. Gas-deposition mechanisms of 7YSZ coating based on plasma spray-physical vapor deposition. J. Eur. Ceram. Soc. 2016, 36, 697–703. [Google Scholar] [CrossRef]
- Zhou, Y.-Z.; Liu, M.; Yang, K.; Zeng, W.; Song, J.-B.; Deng, C.-M.; Deng, C.-G. Microstructure and Property of MoSi2-30Al2O3 Electrothermal Coating Prepared by Atmospheric Plasma Spraying. J. Inorg. Mater. 2019, 34, 646–652. [Google Scholar] [CrossRef]
- Yao, J.; Shao, J.; He, H.; Fan, Z. Optical and electrical properties of TiOx thin films deposited by electron beam evaporation. Vacuum 2007, 81, 1023–1028. [Google Scholar] [CrossRef]
- Cronemeyer, D.-C. Electrical and Optical Properties of Rutile Single Crystals. Phys. Rev. 1952, 87, 876–886. [Google Scholar] [CrossRef]
- Akira, O.-M.; Masayuki, I.; Yoshiaki, A.; Park, K.; Inuzuka, M.; Inoue, K.; Iwamoto, N. Electrical conductivity of plasma-sprayed titanium oxide (rutile) coatings. Thin Solid Film. 1991, 201, 1–8. [Google Scholar] [CrossRef]
- Bartholomew Robert, F.; Frankl, D.-R. Electrical Properties of Some Titanium Oxides. Phys. Rev. 1969, 187, 828–832. [Google Scholar] [CrossRef]
- Marezio, M.; Mcwhan, D.-B.; Dernier, P.-D.; Remeika, J.P. Structural Aspects of the Metal-insulator Transition in Ti4O7. J. Solid State Chem. 1973, 6, 213–221. [Google Scholar] [CrossRef]







| Coating | Current/A | Voltage/V | Distance/mm | Flux of Ar L/min | Flux of H2 L/min | Powder Feed Rate g/min |
|---|---|---|---|---|---|---|
| Ni-Al | 600 | 70 | 110 | 45 | 8 | 25 |
| MgAl2O4 | 630 | 75 | 110 | 40 | 9 | 33 |
| TM15 | 580 | 79 | 110 | 41 | 11 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, K.; Wang, D.; Deng, C.; Zhou, Y. Property of TiO2-15MgAl2O4 Electrical-Heating Coating Prepared by Atmospheric Plasma Spraying and Hydrogen Heat Treatment. Coatings 2020, 10, 177. https://doi.org/10.3390/coatings10020177
Li X, Yang K, Wang D, Deng C, Zhou Y. Property of TiO2-15MgAl2O4 Electrical-Heating Coating Prepared by Atmospheric Plasma Spraying and Hydrogen Heat Treatment. Coatings. 2020; 10(2):177. https://doi.org/10.3390/coatings10020177
Chicago/Turabian StyleLi, Xiyang, Kun Yang, Dezhi Wang, Chunming Deng, and Yanzhe Zhou. 2020. "Property of TiO2-15MgAl2O4 Electrical-Heating Coating Prepared by Atmospheric Plasma Spraying and Hydrogen Heat Treatment" Coatings 10, no. 2: 177. https://doi.org/10.3390/coatings10020177
APA StyleLi, X., Yang, K., Wang, D., Deng, C., & Zhou, Y. (2020). Property of TiO2-15MgAl2O4 Electrical-Heating Coating Prepared by Atmospheric Plasma Spraying and Hydrogen Heat Treatment. Coatings, 10(2), 177. https://doi.org/10.3390/coatings10020177





